Thiocytochalasins A-D,four sulfur-containing cytochalasans from an endophytic fungus Phoma multirostrata XJ-2-1
Xiaogang Peng,Jinling Chang,Ying Gao,Fangfang Duan,Hanli Ruan
School of Pharmacy,Tongji Medical College,Huazhong University of Science and Technology,Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation,Wuhan 430030,China
Keywords:Phoma multirostrata XJ-2-1 Sulfur-containing cytochalasans Thiocytochalasins A-D Cytotoxicity Cell cycle
ABSTRACT Four unprecedented sulfur-containing cytochalasans,thiocytochalasins A-D (1-4),were isolated from an endophytic fungus Phoma multirostrata XJ-2-1.Thiocytochalasins A (1) and B (2) feature a novel 5/6/14/5 tetracyclic scaffold,which are the first examples of cytochalasan containing a thiophene moiety.Thiocytochalasins C (3) and D (4) are epimeric cytochalasan homodimers formed via a thioether bridge.Their structures with absolute configurations were established by detailed analysis of the HRESIMS,NMR,and X-ray crystallography.The plausible biogenetic pathway of 1-4 was postulated.Compounds 3 and 4 exhibited significant cytotoxicity against CT26 cells with IC50 values of 0.85 and 0.76 μmol/L,respectively.
Sulfur-containing natural products are a large class of multifunctional molecules existing in both terrestrial and marine organisms.To date,more than 1000 sulfur-containing natural products have been reported,many of which exhibit potent biological activities and pharmacological properties [1].For centuries,sulfur has continued to maintain its status as the dominating heteroatom integrated into a set of 362 sulfur-containing FDA approved drugs(besides oxygen or nitrogen) [2].Sulfonamides,thioethers,sulfones,and penicillin are the most common scaffolds in sulfur containing drugs,which are well studied both on synthesis and application during the past decades [2-4].
Cytochalasans are a group of fungal-derived polyketidenonribosomal peptide synthetase (PKS-NRPS) natural products characterized by a perhydro-isoindolone core fused with a macrocyclic ring,exhibiting high structural diversity and a broadspectrum of bioactivities.They are majorly produced by the generaAspergillus,Chaetomium,Xylaria,Helminthosporium,Penicillium,Zygosporium,Metarhizum,Rosellinia,Ascochyta,Hypoxylon,Spicaria,andPhoma[5-8].In recent decades,more than 500 cytochalasans have been reported [5,9-15],which are generally classified into six groups,namely cytochalasins,chaetoglobosins,pyrichalasins,aspochalasins,alachalasins,and trichalasin [5,6].Cytochalasins,a group of cytochalasans with a phenylalanine unit incorporated into the polyketide backbone,is the largest group of cytochalasans [5].
Phomaspecies are a rich source of bioactive secondary metabolites including cytochalasins [16],and the first cytochalasan was isolated from a culture broth of aPhomasp.in 1966 [17].Phoma multirostrataXJ-2-1,an endophytic fungus,was isolated from the medicinal plantParasenecio albuscollected from Xinning County,Hunan Province of China.In the previous study,we reported ergocytochalasin A,a merocytochalasan consisting of one cytochalasin moiety and one ergosterol moiety with a 5/6/14/6/5/6/6/6 fused octacyclic ring system,and nine new cytochalasins multirostratins B-J,fromP.multirostrataXJ-2-1 [9,10].To search for more novel metabolites with unique structures and significant bioactivities,the fungusP.multirostrataXJ-2-1 was further investigated,which led to the identification of four unprecedented sulfur-containing cytochalasans,thiocytochalasins A-D (1-4) (Fig.1).To the best of our knowledge,only five sulfur-containing cytochalasans have been reported before [14,18],of which bisaspochalasins B and C are cytochalasan dimers connected by thioether bond [14].Thiocytochalasins A (1) and B (2) feature an unprecedented 5/6/14/5 tetracyclic scaffold,which are the first examples of cytochalasan fused with a thiophene moiety.Thiocytochalasins C (3) and D (4) are epimeric cytochalasan homodimers formedviaa thioether bridge.Herein,the details of the isolation,structure elucidation,plausible biogenetic pathway,as well as biological activities of thiocytochalasins A-D (1-4) are reported.
Thiocytochalasin A (1),colorless needles,possessed a molecular formula of C31H35NO5S as determined by the HRESIMS ([M+Na]+,m/z556.2127,calcd.for C31H35NO5SNa,556.2134),requiring 15 double bond equivalents (DBEs),which was consistent with the13C NMR data.Its IR spectrum showed absorptions for hydroxy (3419 cm-1),ester carbonyl (1717 cm-1),conjugated aldehyde and/or amide (1668 cm-1),and phenyl (1604 and 1494 cm-1) groups.The1H NMR data (Table S1 in Supporting information) of 1 showed two doublet methyls atδH1.01 (d,3H,J=6.7 Hz) and 1.15 (d,3H,J=6.6 Hz);five olefinic protons atδH5.23 (br s,1H),5.47 (br s,1H),5.77 (ddd,1H,J=15.2,10.6,3.4 Hz),5.90 (ddd,1H,J=15.2,9.6,2.2 Hz),and 7.93 (s,1H);one monosubstituted phenyl group with five coupled aromatic protons atδH7.24 (d,2H,J=7.6 Hz),7.25 (m,1H),and 7.33 (t,2H,J=7.6 Hz);one aldehyde proton atδH10.05 (s,1H).The13C NMR data (Table S1),with the aid of DEPT and HSQC experiments,exhibited 31 carbon resonances,comprising three carbonyls (δC160.8,170.1,and 182.7),six aromatic carbons,eight olefinic carbons,one sp3quaternary carbons,six sp3methines,five sp3methylenes,and two methyls.These functionalities accounted for 11 out of the 15 DBEs,indicating four additional rings in the structure of 1.The aforementioned NMR data,along with the previous reports of the chemical investigation onP.multirostrataEA-12 [9,10],suggested that 1 was likely a cytochalasin.
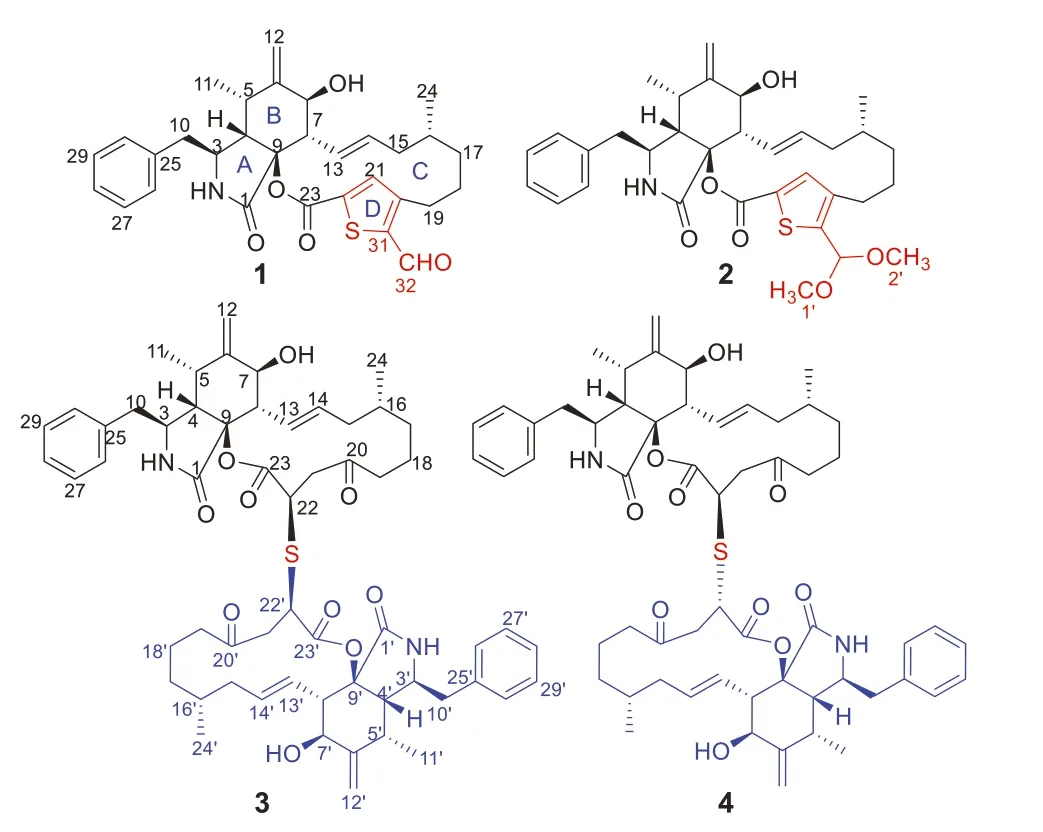
Fig.1.Chemical structures of compounds 1-4.
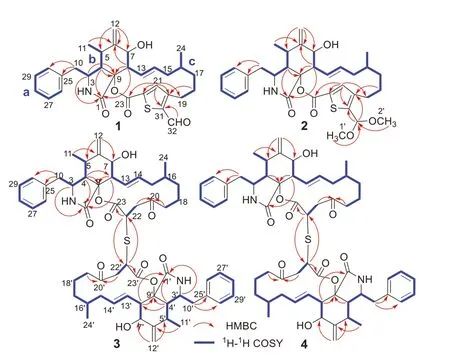
Fig.2.Key HMBC and 1H-1H COSY correlations of 1-4.
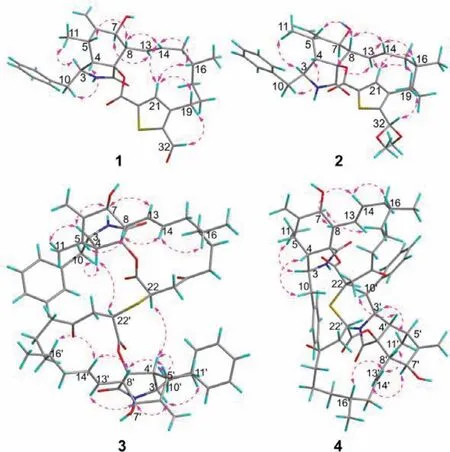
Fig.3.Key NOESY correlations of 1-4.
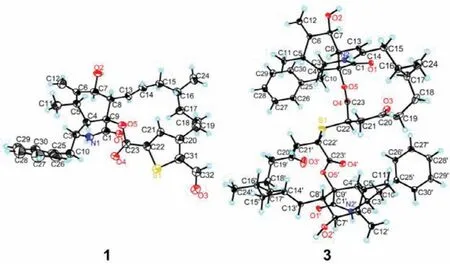
Fig.4.X-ray ORTEP drawing of compounds 1 and 3.Displacement ellipsoids are drawn at the 50% probability level.
The planar structure of 1 was established by interpretation of its 2D NMR spectra (Fig.2).Analysis of the1H-1H COSY spectrum of 1 revealed the presence of three spin coupling fragments a-c which are shown with blue bold bonds (Fig.2).Then,those fragments with the nonprotonated carbons and heteroatoms were assembled by examination of the HMBC spectrum to furnish the planar structure of 1.The HMBC cross-peaks of H2-10/C-25 and C-30 indicated the monosubstituted phenyl group attached to C-10.The HMBC correlations of H-3/C-1,C-9,H-4/C-1,C-9,H3-11/C-6,H2-12/C-5,C-6,C-7,H-8/C-1,C-6,C-9 delineated the perhydroisoindolone core (rings A and B).The construction of ring C was established by the HMBC networks of H2-18/C-20,H-21/C-19,C-22,C-23.The HMBC cross-peaks of H-21/C-20,C-22,C-31,H-32/C-31,allowed the establishment of the unique thiophene ring D,which bridged to the ring C at C-20 and C-22,and simultaneously suggested that the aldehyde group is attached to C-31.Thus,the gross structure of 1 was elucidated as shown in Fig.1.
The relative configuration of compound 1 was fixed by the analysis of the1H-1H coupling constants (Table S1) and the NOESY correlations (Fig.3).TheE-geometry ofΔ13double bond was deduced from the large coupling constant between H-13 and H-14(J13,14=15.2 Hz).The NOESY correlations of H-3 with H3-11 revealed that H-3 and H3-11 were positioned on the same side of the molecule and randomly assigned asα-oriented,while H-4,H-5,H-8 and H-16 were assigned to beβ-oriented by the NOESY crosspeaks of H-4/H2-10;H-8/H-5 and H14;H-14/H-16.Theαorientation of H-7 was determined by the NOESY correlation between H-7 and H-13.To confirm the structure assigned for compound 1 and determine its absolute configuration,quality crystals were successfully obtained from recrystallization in CH2Cl2/MeOH/H2O(10:10:1) at room temperature,and then subjected to single-crystal X-ray diffraction (XRD) analysis using Ga Kαradiation (Fig.4).The X-ray crystallography result not only secured the above structural assignment for 1 but also established its absolute configuration (3S,4S,5S,7S,8S,9S,16R) by the excellent Flack parameter [0.12 (2)].
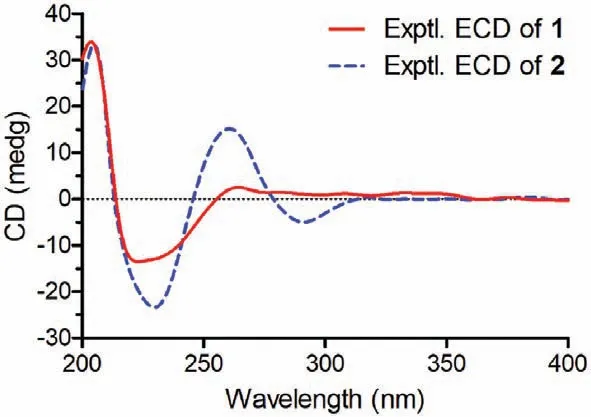
Fig.5.Experimental ECD spectra of 1 and 2.
Thiocytochalasin B (2) was obtained as white powder.Its molecular formula was deduced to be C33H41NO6S from (+)-HRESIMS ion peak atm/z602.2542 [M+Na]+(calcd.for C33H41NO6SNa,602.2552) and the13C NMR data (Table S1),indicating 14 DBEs.The IR spectrum revealed the presence of hydroxy (3383 cm-1),carbonyl (1711 cm-1),and terminal double bond (1640 and 972 cm-1).Detailed comparison of the NMR data between 1 and 2 (Table S1) indicated they had identical carbon skeleton.The main difference was that the C-32 aldehyde group in 1 was replaced by a dimethoxy methine group in 2,which was confirmed by the HMBC correlations (Fig.2) from H3-1′ (δH3.36)and H3-2′ (δH3.32) to C-32 (δC98.7).The NOESY correlations of 2 disclosed that this compound had the same relative configuration as that of compound 1 (Fig.3).Finally,the absolute configuration of 2 was identified by comparison of its ECD spectrum with that of 1 (Fig.5).
Thiocytochalasin C (3) was isolated as colorless needles with the molecular formula of C58H72N2O10S from its HRESIMS ion peak atm/z1011.4811 [M+Na]+(calcd.for C58H72N2O10SNa,1011.4805),requiring 24 DBEs.The13C NMR (Table S2 in Supporting information) displayed only 29 resonances for 3,suggesting that compound 3 was a symmetrical cytochalasin dimer linked by a central bridged sulfur atom.Its IR absorption bands at 3359 and 1717 cm-1suggested the presence of hydroxy and carbonyl groups,respectively.Further analysis of the 1D NMR data (Table S2) revealed that the cytochalasin monomer in 3 closely resembled the known compound cytochalasin B6[19],with the only difference being the replacement of a methylene (δC29.0,C-22) in cytochalasin B6by a methine (δC42.1,C-22) in 3.This was confirmed by the1H-1H COSY correlation of H2-21/H-22,and the HMBC cross-peaks from H2-19 and H2-21 to C-20,and from H-22 to C-23 (Fig.2).Considering the symmetry of the molecule,this change led to the deduction that the two monomers were connected by virtue of a sulfur bridge between C-22 and C-22′,which was supported by the HMBC correlation of H-22/C-22′ (Fig.2).The relative configuration of 3 was determined by analysis of the NOESY spectrum (Fig.3).The NOESY correlations of H-3/H-7 and H3-11 indicated the assignment of theα-configuration for these protons.Theβ-configuration for H-4,H-8 and H3-16 was fixed according to the NOESY correlations of H-4/H-10;H-8/H-5,H-14;and H-14/H-16.However,the relative configuration of C-22 could not be resolved because of the absence of specific evidence.Fortunately,a suitable crystal was obtained from a solution of CH2Cl2/MeOH (1:1).The single-crystal Xray diffraction analysis using Cu Kαradiation (Fig.4) with Flack parameter [0.009 (10)]corroborated the structural assignment of 3 based on NMR and identified its absolute configuration as 3(3′)S,4(4′)S,5(5′)S,7(7′)S,9(9′)S,16(16′)R,22(22′)R.
Thiocytochalasin D (4),white powder,had the same molecular formula of C58H72N2O10S as 3,as deduced from its HRESIMS ion peak atm/z1011.4821 [M+Na]+(calcd.for C58H72N2O10SNa,1011.4805) and the13C NMR data (Table S3 in Supporting information).Its IR absorption bands at 3421 and 1713 cm-1suggested the presence of hydroxy and carbonyl groups,respectively.A detailed comparison of the NMR data of 4 and 3 (Tables S2 and S3) indicated that the two compounds are structurally related,except that the structural symmetry present in 3 was broken in 4,as the13C NMR of 4 showed 58 signals.Further analysis of the 2D NMR spectra (Fig.2) constructed the planar structure of 4,which was the same as that of 3.The only difference between 4 and 3 was the opposite configuration at C-22′,which was determined by comparison of their chemical shifts and coupling constants as H-22′ atδH3.91 (dd,2H,J=11.4,3.1 Hz) for 3 while H-22′ atδH4.14 (dd,1H,J=7.8,3.5 Hz) for 4.The relative configuration of other chiral centers in 4 was assigned as the same as that of 3 by their similar NMR data and NOESY correlations (Fig.3).As cytochalasins shared the same biogenetic pathway,the absolute configuration of 4 was assigned as 3(3′)S,4(4′)S,5(5′)S,7(7′)S,9(9′)S,16(16′)R,22R,22′S.The structure of 4 was hence established as shown in Fig.1.
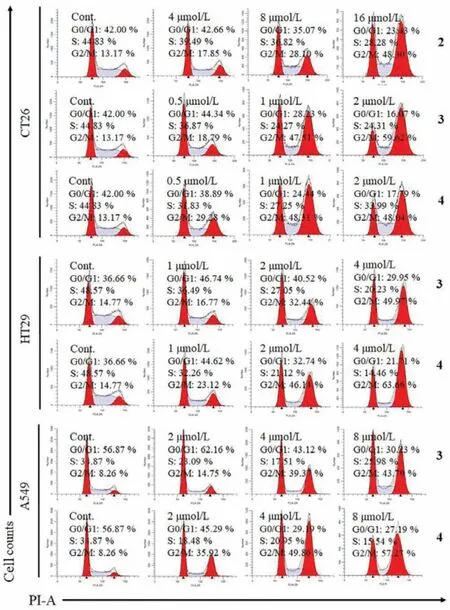
Fig.6.Cell cycle analysis of CT26,HT29 and A549 cells after 24 h treatment with compounds 2-4 at different concentrations using PBS as a negative control (Cont.).
The plausible biosynthesis pathway of thiocytochalasins A-D(1-4) is proposed in Scheme 1.On the basis of the systematic report of enzymatic carbon-sulfur bond formation in natural product biosynthesis [20],cysteine is considered to be the sulfur source of thiocytochalasins A-D (1-4).And cytochalasin B,a main cytochalasin from this strain [9],is postulated to be the precursor.First,cytochalasin B was reduced to give a vital intermediate Iviaan oxidation reaction,which subsequently involved a nucleophilic addition to C-22 in theα,β-unsaturated 1,4-diketone moiety of intermediate I with mercaptopyruvate to produce the thio intermediate II.The intermediate II underwent a series of reactions including addition,oxidation,elimination,and decarboxylation to generate thiocytochalasin A (1) with a 2,3,5-trisubsituted thiophene moiety.Then,thiocytochalasin B (2) was produced by an addition of 1.The cysteine could conduct a nucleophilic addition to intermediate I,leading to an intermediate VI which could generate a sulfur anion to couple with another monomer intermediate I to produce a pair of epimeric cytochalasan homodimers thiocytochalasins C (3) and D (4) decorated with a thioether bridge.
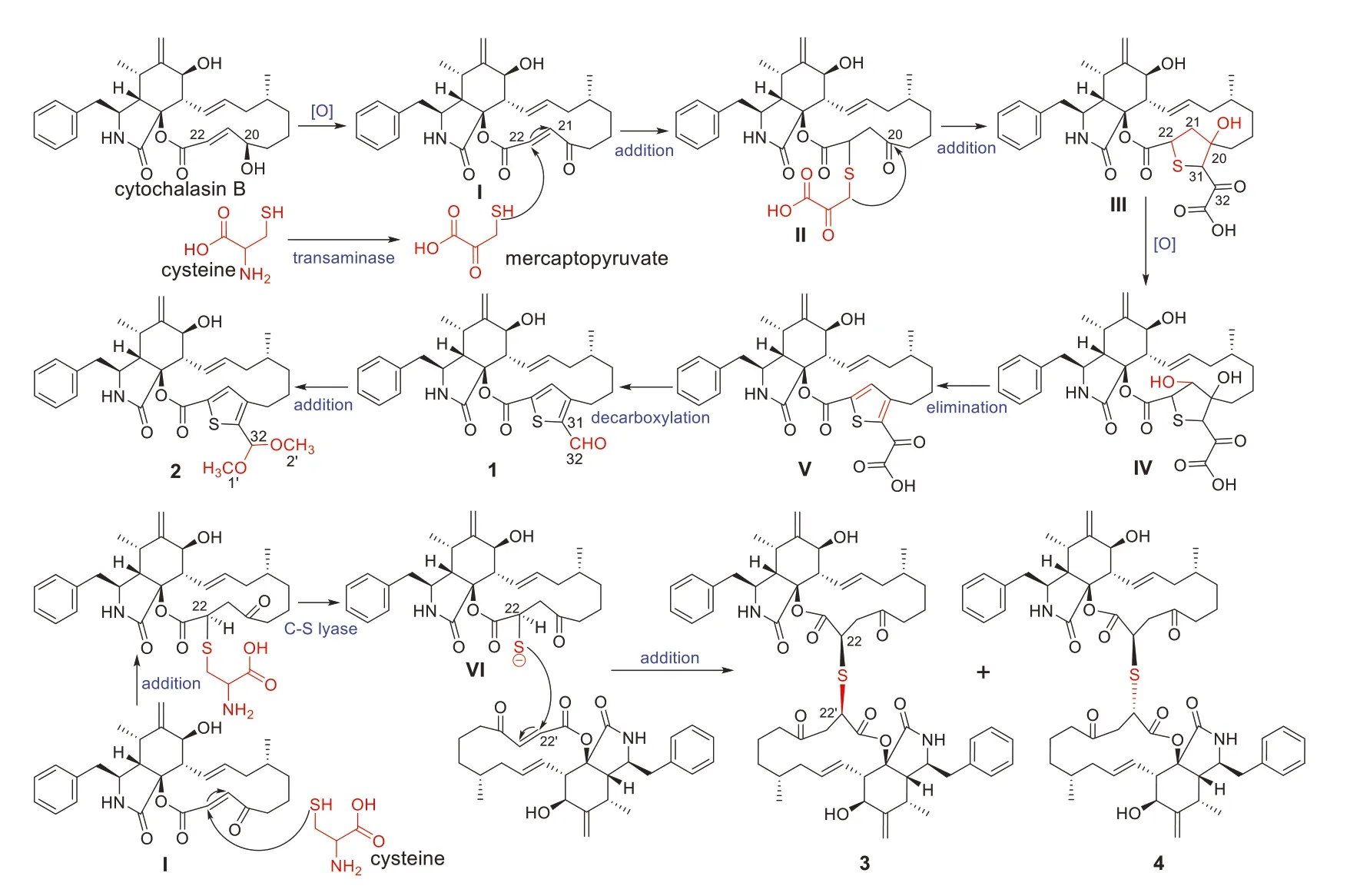
Scheme 1.Plausible biosynthetic pathway of compounds 1-4.
Compounds 1-4 were evaluated for their cytotoxicity against MCF-7 human breast adenocarcinoma,HepG2 human hepatocellular carcinoma,CT26 murine colon carcinoma,HT-29 human colon carcinoma,and A549 human lung carcinoma cell lines using MTT method.As a result,compounds 1 and 2 showed modest cytotoxicity against the tested cell lines with IC50values of 4.17-23.41 μmol/L (Table S4 in Supporting information).Compounds 3 and 4 displayed strong cytotoxicity against four cell lines with IC50values from 0.76 μmol/L to 7.52 μmol/L,and exhibited moderate cytotoxicity against HepG2 cells (Table S4).Subsequently,the effects of 2-4 on cell cycle progression of CT26,HT29 and A549 cells were investigated.The results showed that they could concentrationdependently induce G2/M cell cycle arrest in these cells,especially compounds 3 and 4 could significantly arrest cell cycle G2/M phase of CT26 cells at the concentration of 1 μmol/L (Fig.6).
In conclusion,the discovery of thiocytochalasins A-D (1-4)from an endophytic fungusP.multirostrataXJ-2-1 has expanded the scaffolds of cytochalasan with sulfur-containing moiety.In particular,identification of the thiocytochalasins A (1) and B (2),first examples of cytochalasans fused with a thiophene moiety,is of great importance in the research field of cytochalasans.It is noteworthy that two cytochalasan homodimers with thioether bridge have been reported fromAspergillus flavipesbefore,and thiocytochalasins C (3) and D (4) exhibit their differences on the type of cytochalasan monomer and non-selective addition at C-22′ in theα,β-unsaturated 1,4-diketone moiety compared to the reported compounds bisaspochalasins B and C [14].In addition,the significant cytotoxicity of compounds 3 and 4 against five cancer cell lines especially against CT26 cells with IC50values of 0.85 and 0.76μmol/L,respectively,is better than the previously reported cytochalasin monomer from this strain [9],which might provide an idea to boost the discovery of antitumor candidates for pharmaceutical chemists.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.22077041 and 31770380).We are grateful to Mr.Xianggao Meng from Central China Normal University for X-ray analysis.Thanks are also given to the staff at the Analytical and Testing Center of Huazhong University of Science and Technology for collecting the spectroscopic data.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.063.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
