A trefoil-shaped macrocycle with [12]-imidazolium cations
Le Zhang,Li-Ying Sun,Jin-Ping Chang,Hui-Yu Xie,Ya-Wen Zhang,Yi-Fan Zhang,Ying-Feng Han
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education,College of Chemistry and Materials Science,Northwest University,Xi’an 710127,China
Keywords:Trefoil-shaped macrocycle N-Heterocyclic carbenes Photochemical reaction Imidazolium
ABSTRACT A trefoil-like two-dimensional (2D) C3v symmetric organic [12]-imidazolium cation H12-2(PF6)12 featuring three [4]-imidazolium macrocycles was synthesized in three steps.The reaction of a dodecakis H12-1(PF6)12 imidazolium salt with Ag2O resulted in the formation of a hexanuclear AgI dodecacarbene assembly [Ag6(1)](PF6)6.Upon UV irradiation,the photodimerization of the cinnamic ester pendants of[Ag6(1)](PF6)6 led to the generation of a trefoil-like complex [Ag6(2)](PF6)6 containing three closed metallacycles.Removal of metal ions allowed for the synthesis of the target molecule.All complexes were fully characterized by NMR spectroscopy (1H,13C{1H} and 2D NMR) and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS).
The discrete macrocyclic compounds,which contain both artificial metal-macrocycles (triangles,rhomboids,squares,hexagons,etc.) [1-12]and non-metal-macrocycles (crown ethers,cyclodextrins,cyclophanes,pillararenes,etc.) [13-18],have made significant progress in the application of host-guest chemistry,selective absorption/recognition,molecular electronic devices and supramolecular catalysis fields over the past decades [19-24].Among these macrocyclic compounds,the polyimidazolium cyclophanes have drawn considerable attention as unique receptors due to the ionic hydrogen bond (C-H)+···X-between the imidazolium units and guests recently [23,25-31].The polyimidazolium cyclophanes can also be utilized as precursors for the formation of functionalized assemblies bearingN-heterocyclic carbenes (NHCs) [32-47].
So far,attempts to make [n]-polyimidazolium-based (n>4) cyclophanes by one-pot macrocyclization or stepwise macrocyclization processes have proved unsuccessful [48-50],probably due to the multiple incorporations of the dihaloalkanes and bis-imidazole precursors with required connectivity becomes more difficult as reactive sites increases.For example,Beer and co-workers isolated an organic polyimidazolium cation featuring 8 internal imidazolium units only as few crystals incidentally [48].To overcome this limitation,our group developed a metal-carbene template approach and a series of three-dimensional [n]-imidazolium (n=6,12,16 or 18) cages were controllably constructed [51-55].
In addition,most of the known polyimidazolium cationic compounds only have one macrocyclic unit of polyimidazolium.It should be noted that the increase in the number of macrocycles can bring potential properties [56-60].For example,Stoddart and co-workers reported a trefoil-like rotaxane,which was used for a molecule elevator [57].Therefore,the design and synthesis of the polyimidazolium-based compounds featuring more than one macrocyclic unit are imminent and challenging.
Based on these results,we attempted to construct a trefoillike 2D C3vsymmetric organic [12]-imidazolium cation featuring three [4]-imidazolium macrocycles.Unfortunately,direct synthesis of compound IV by the one-pot method using the olefin-modified dodecakisimidazolium salt I as the precursor failed after various trials.Here,we reported an alternative method by using the precursor I to react with Ag2O to generate the hexanuclear AgIassembly II.Then photochemically induced [2+2]cycloaddition reaction was performed with complex II,thereby leading to a trefoil-like complex containing three closed metallacycles.Finally,all AgIions in complex III could be eventually removed to provide the target compound IV (Scheme 1).
The preparation of 1,2,3,4,5,6-hexakis(4-(1H-imidazol-1-yl)phenyl)benzene (L) andN-cinnamic-ester-appended dodecakisimidazolium precursor H12-1(PF6)12is shown in Scheme 2.Firstly,the compound L was obtained from imidazole and 1,2,3,4,5,6-hexakis(4-iodophenyl)benzene [61]by the solid-state reaction using an Ullmann coupling protocol (Fig.S1 in Supporting information) [62,63].Subsequently,compound L andN-pbromomethylbenzene-N’-cinnamic acid methyl ester imidazolium bromide was mixed to yield the corresponding dodecakisimidazolium precursor H12-1(Br)12,followed by the anion exchanged with NH4PF6in CH3OH to form hexafluorophosphate H12-1(PF6)12in 81% yield as a white solid.The hexafluorophosphate salt of H12-1(PF6)12is excellently soluble in DMSO,DMF,and CH3CN,whereas almost insoluble in ether and dichloromethane.
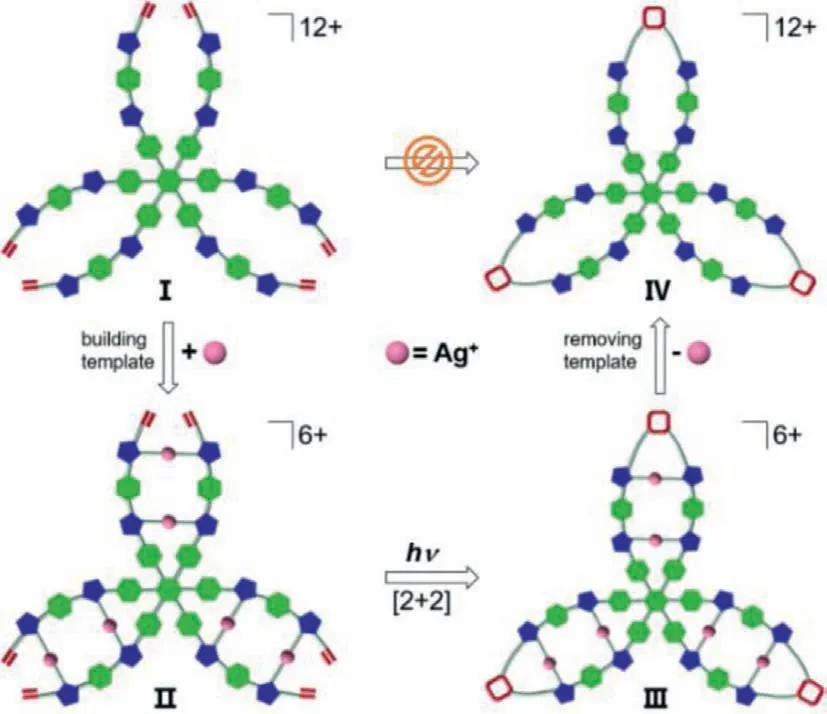
Scheme 1.Cartoon presentation of the synthetic strategy for the construction of a trefoil-like dodecakisimidazolium cation.
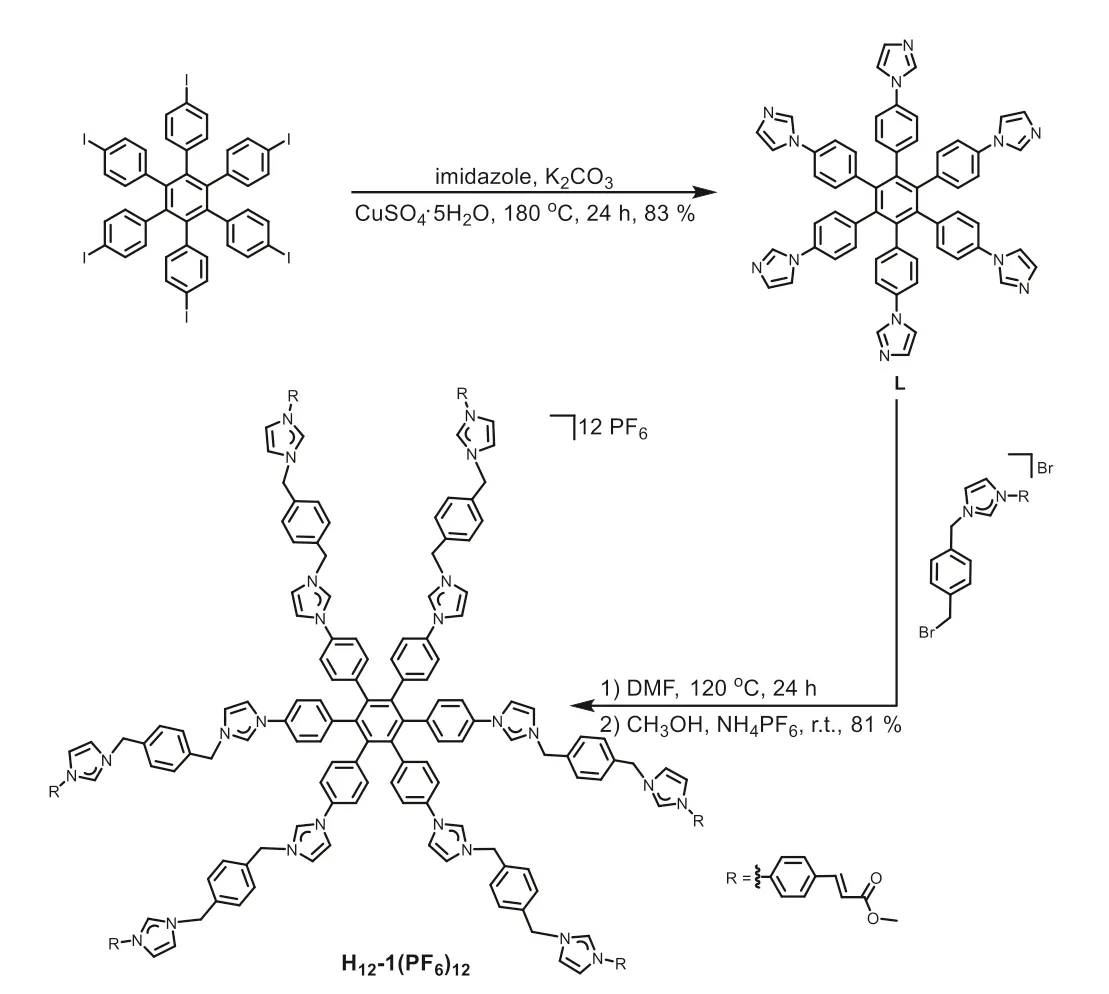
Scheme 2.Synthesis of compounds L and H12-1(PF6)12.
The formation of the dodecakisimidazolium precursor H12-1(PF6)12was verified by nuclear magnetic resonance (NMR) spectroscopy (1H,13C{1H},and 2D NMR) and high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) (Figs.S2-S7 in Supporting information).The1H NMR spectrum of H12-1(PF6)12in CD3CN reveals two characteristic resonances for the C2-H protons on imidazolium atδ9.00 and 8.84 ppm,respectively (Fig.1a).HR-ESI-MS spectrum provided further support for the formation of dodecakisimidazolium salt.The peaks of the highest intensity for H12-1(PF6)12were discovered atm/z632.4934 (calcd.for [H12-1+6PF6]6+632.5114),m/z1021.2212 (calcd.for [H12-1+8PF6]4+1021.2495).
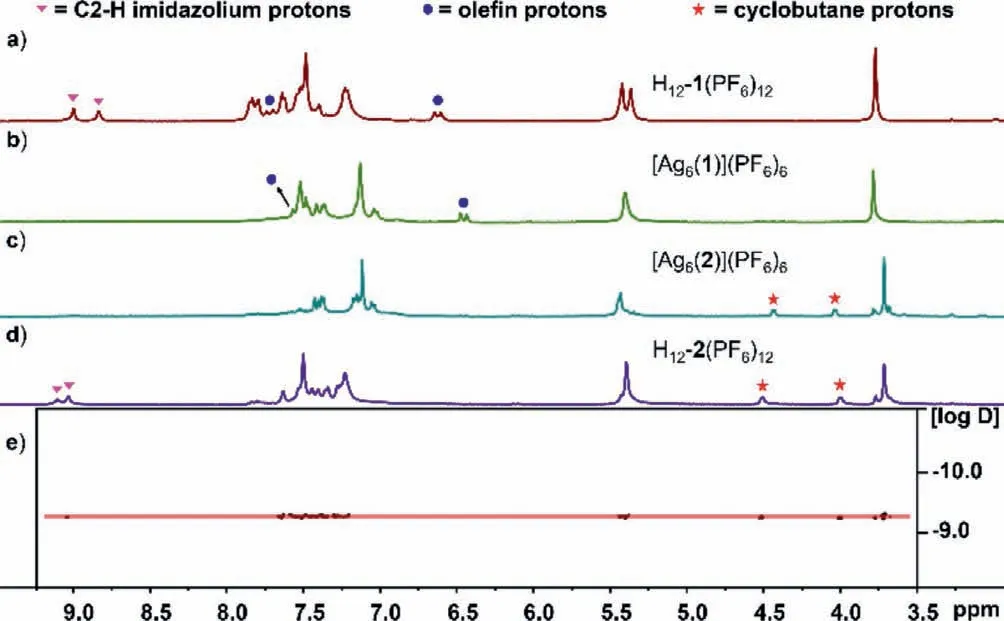
Fig.1.Partial 1H NMR spectra (400 MHz,298 K) in CD3CN of (a) dodecakisimidazolium salt H12-1(PF6)12;(b) complex [Ag6(1)](PF6)6 before irradiation;(c) complex [Ag6(2)](PF6)6 obtained after irradiation;(d) dodecakisimidazolium salt H12-2(PF6)12;(e) 1H DOSY spectrum of H12-2(PF6)12.
The reaction of H12-1(PF6)12with an excess of Ag2O generated the 2D C3vsymmetric hexanuclear AgIdodecacarbene assembly [Ag6(1)](PF6)6in 79% yield (Scheme 3),the compound[Ag6(1)](PF6)6contains two triangular metal silver ions form an inner Ag3triangle inserted inside a larger outer Ag3arrangement.The reaction was achieved in CH3CN under the exclusion of light,and the carbene complex was separated as a white solid after workup.The formation of the hexanuclear silver complex was unambiguously confirmed by NMR and HR-ESI-MS studies (Figs.S8-S14 in Supporting information).In the1H NMR spectra of complex [Ag6(1)](PF6)6,the characteristic resonance of the C2-H protons of H12-1(PF6)12atδ9.00 and 8.84 ppm were found to have disappeared (Fig.1b).The13C{1H} NMR spectra in CD3CN showed the two expected CNHCsignals atδ182.1 and 180.7 ppm,accompanied by two slightly different imidazolium rings.These values are within the normal range compared with previously reported for such derivatives [64-88].Diffusion-ordered NMR spectroscopy(DOSY) confirmed the formation of a signal configuration.All proton signals of complex [Ag6(1)](PF6)6displayed a closely related diffusion coefficient forD=6.46×10-10m2/s (logD=-9.19).The HR-ESI-MS spectrum further demonstrated the formation of hexanuclear silver complex.For example,the HR-ESI-MS spectrum for [Ag6(1)](PF6)6appeared two peaks atm/z593.4065 (calcd for [Ag6(1)]6+593.4365),m/z741.0843 (calcd for [Ag6(1)+PF6]5+741.1168),these theoretical isotopic distributions are consistent with the experimental peaks (Fig.S14 in Supporting information).Unfortunately,all attempts to obtain single crystals of the hexanuclear silver complex [Ag6(1)](PF6)6used for X-ray analysis failed.However,based on the related assemblies [56-60],the 2D C3vsymmetric structure [Ag6(1)](PF6)6could be speculated in Scheme 3.
The photochemical reaction of theN-cinnamic-ester-appended hexanuclear AgIdodecacarbene assembly [Ag6(1)](PF6)6was subsequently investigated (Scheme 3).The complex [Ag6(1)](PF6)6was treated with a Philips high-pressure mercury lamp (λ=365 nm)in CD3CN solvent at ambient temperature converted exclusively into the organometallic complex [Ag6(2)](PF6)6.The photochemical[2+2]cyclization reaction was finished within 24 h and the conversion was easily demonstrated by1H,13C{1H} NMR spectroscopy.
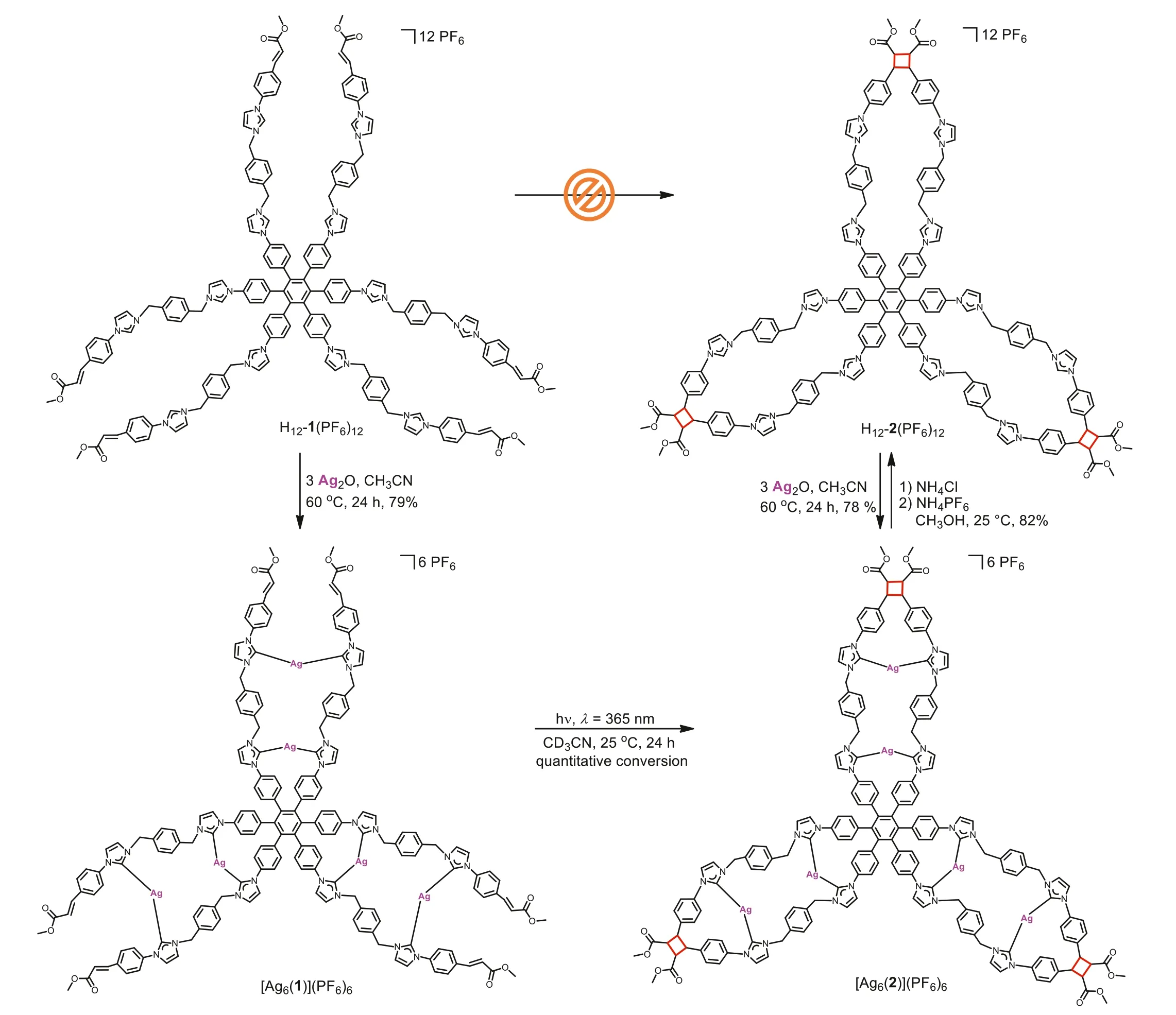
Scheme 3.Preparation of the hexanuclear AgI dodecacarbene assembly [Ag6(1)](PF6)6,of the organometallic complex [Ag6(2)](PF6)6 and of the dodecakisimidazolium cation H12-2(PF6)12.
The formation of the hexanuclear complex [Ag6(2)](PF6)6featuring three cyclobutanes bridged was monitored by NMR spectroscopy (Figs.S15-S20 in Supporting information).Before irradiation,the1H NMR spectrum from the olefin protons of[Ag6(1)](PF6)6features characteristic two doublet resonances of the cinnamic ester groups atδ7.56 (3J=16.1 Hz,partly overlapped by other resonances) and 6.46 (3J=16.1 Hz) ppm (in CD3CN,Fig.1b).The1H NMR spectrum revealed that olefin protons resonances of H12-1(PF6)12(Fig.1a) slightly shifted upfield after producing to [Ag6(1)](PF6)6.The13C{1H} NMR spectrum of [Ag6(1)](PF6)6features signals for the C=C carbon atoms were observed atδ143.4 and 120.2 ppm (Fig.S9 in Supporting information).After UVirradiation (λ=365 nm) for 24 h,the intensity of the olefin doublet resonances completely vanished and two new multiplets for the cyclobutane protons of [Ag6(2)](PF6)6generated atδ4.43 and 4.03 (both d,3J=5.51 Hz) ppm (in CD3CN,Fig.1c),while two characteristic peaks for cyclobutane carbons were also found atδ52.7 and 46.1 ppm (Fig.S16 in Supporting information).Additionally,the formation of [Ag6(2)](PF6)6featuring three cyclobutanes have demonstrated by the HR-ESI-MS spectrum,showed peaks of highest intensity atm/z593.4272 (calcd for [Ag6(2)]6+593.4365),m/z741.1054 (calcd.for [Ag6(2)+PF6]5+741.1168) (Fig.S21 in Supporting information).Subsequently,UV-vis spectra also clearly revealed the disappearance of the characteristic absorption peaks at around 280 nm,attributed to the C=C double bonds (Fig.S29 in Supporting information).The photochemical [2+2]cycloaddition reaction of precursor H12-1(PF6)12was performed under the same experimental conditions.However,no target photoproduct was found except thetrans-cisphotoisomerization as indicated by1H NMR spectroscopy (Fig.S22 in Supporting information).
In our previous work,it has been confirmed that the newly generated terminal cyclobutanes can connect two poly-NHC ligands after removing the silver ions [51-55].Inspired by these results,the next generation of trefoil-like dodecakisimidazolium cation H12-2(PF6)12was prepared.The addition of excess NH4Cl in CH3OH successfully bring the liberation of the six Ag+ions from hexanuclear complex[Ag6(2)](PF6)6,followed by anion exchange with NH4PF6provided hexafluorophosphate H12-2(PF6)12in 82% yield as a white solid (Scheme 3).The trefoil-like dodecakisimidazolium cation H12-2(PF6)12was fully characterized by NMR spectroscopy and HR-ESI mass spectrometry (Figs.S23-S28 in Supporting information).In the1H NMR spectrum of H12-2(PF6)12,two resonances were observed atδ9.10 and 9.03 ppm (Fig.1d) because of imidazolium groups of dodecakisimidazolium salt H12-2(PF6)12have two different chemical environments.This is consistent with the dodecakisimidazolium precursor starting material.The intensity ratio of the cyclobutane protons and the imidazolium protons was checked to be 1:1:1:1,indicating the fully de-metalation of complex [Ag6(2)](PF6)6.All proton signals of H12-2(PF6)12showed a relatively narrow interval with a diffusion coefficient ofD=5.62×10-10m2/s (logD=-9.25),verifying that it is a single assembly (Fig.1e).Moreover,the HR-ESI-MS spectrum of compound H12-2(PF6)12(positive-ion mode) revealed peaks of highest intensity atm/z=438.1339 (calcd for [H12-2+4PF6]8+438.1424),m/z=521.4350 (calcd for [H12-2+5PF6]7+521.4434)(Fig.2).Furthermore,the addition of 3 equiv.Ag2O to H12-2(PF6)12resulted in regeneration of the hexanuclear cycloaddition product [Ag6(2)](PF6)6.This reaction was achieved after 24 h at 60 °C under the exclusion of light (Scheme 3).The HR-ESI-MS spectrum data were similar to those of [Ag6(2)](PF6)6obtained from the photochemical cycloaddition of [Ag6(1)](PF6)6(Fig.S21 in Supporting information).
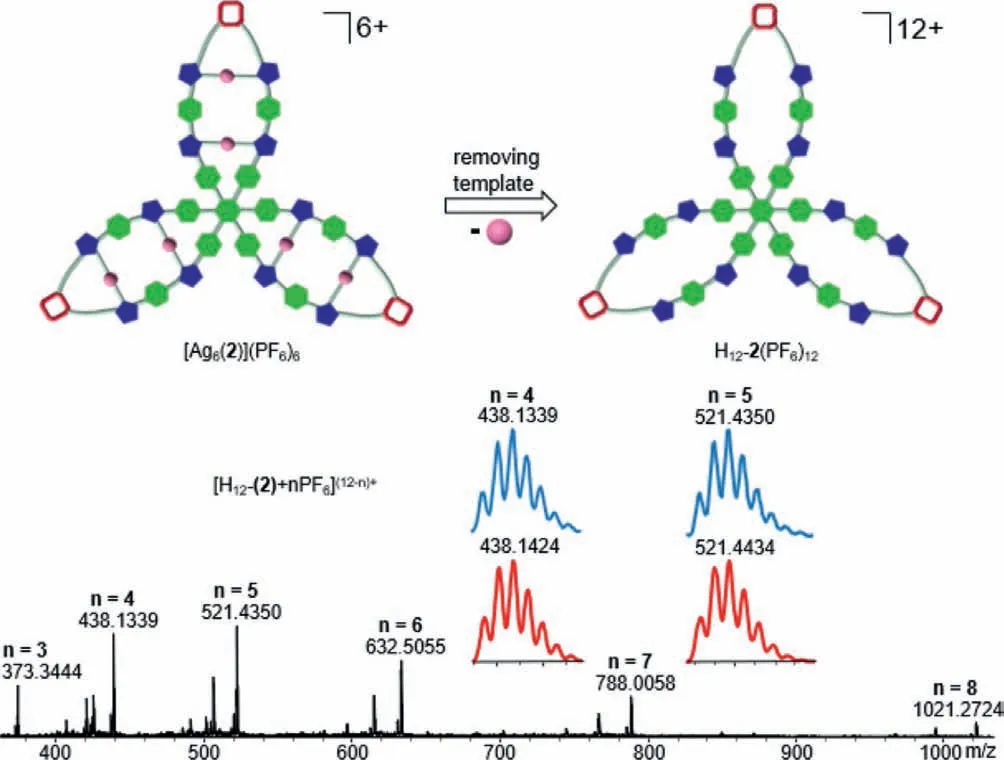
Fig.2.HR-ESI-MS spectrum (position ion mode) of H12-2(PF6)12,inset experimental(blue) and calculated (red) isotope distributions for peaks corresponding to [H12-2+4PF6]8+ and [H12-2+5PF6]7+.
In summary,based on the rational design of a hexagonal poly-NHC precursor,we described the synthesis of two different discrete 2D C3vsymmetric hexanuclear AgIdodecacarbene metallacycles and corresponding trefoil-like 2D C3vsymmetric organic [12]-imidazolium cation featuring three [4]-imidazolium macrocycles.To the best of our knowledge,this is the first example of 2D polyimidazolium derivative containing three [4]-imidazolium macrocycles.In addition,this approach potentially prepares newly functional two-dimensional polyimidazolium materials under study in our group.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (Nos.22025107,21722105,22101225),the National Youth Top-notch Talent Support Program of China,the Key Science and Technology Innovation Team of Shaanxi Province (Nos.2019TD-007,2019JLZ-02),and the FM&EM International Joint Laboratory of Northwest University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.064.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
