Nitrogen-bridged Ni(II) porphyrinoid trimers with a central quinodiimine unit
Kisheng Wng,Boyu Xio,Ling Xu,Mingo Zhou,Tkyuki Tnk,Atsuhiro Osuk,Jinxin Song,*
a College of Chemistry and Chemical Engineering,Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China),Key Laboratory of the Assembly and Application of Organic Functional Molecules of Hunan Province,Hunan Normal University,Changsha 410081,China
b Department of Chemistry,Graduate School of Science Kyoto University,Kyoto 606-8502,Japan
Keywords:Porphyrinoid trimer Aminoporphyrins Buchwald-Hartwig amination Reduction Charge transfer
ABSTRACT Buchwald-Hartwig amination of 5,15-dibromo and 5,10-dibromo Ni(II)porphyrins with 5-amino Ni(II)porphyrin gave linear and bent trimers 4Ni and 5Ni with a central quinodiimine-type Ni(II)porphyrinoid.The structures of 4Ni and 5Ni have been confirmed by X-ray diffraction analysis in both cases.The formation of unusual products 4Ni and 5Ni has been ascribed to facile oxidation of 5,15-and 5,10-amino Ni(II) porphyrin unit.Reduction of 4Ni and 5Ni under proper conditions gave NH-bridged Ni(II)porphyrin trimers 4Ni-2H and 5Ni-2H in high yields.Trimers 4Ni and 5Ni exhibit the lowest energy band as compared with 4Ni-2H and 5Ni-2H.Especially the bent trimer 5Ni exhibits a broad absorption tail beyond 1400 nm.
Porphyrin arrays are organic functional molecules with largeπconjugated systems and have potential applications in optoelectronic devices [1-11],sensors [12-15]and photodynamic therapy(PDT) [16-18].In the last decade,porphyrin arrays with alkynes[19,20],benzene [21]or heterocycles (such as thiophene [22],pyridine [23],pyrrole [24,25]) as bridging units have been intensively studied.Porphyrin dimers with a single carbon or heteroatom bridging unit have received much attention due to their unique photophysical properties,chemical properties,and characteristic electronic delocalization [26-37].In 2006,Arnoldet al.reported the first isolation ofmeso-mesonitrogen-bridged diporphyrinylamine 1,which showed a broadened Soret band and red shift Q bands,indicating substantial electronic interaction between the porphyrins [27].Ruppertet al.reportedmeso-meso,β-meso,ββ-nitrogen-bridged diporphyrinylamines [29],which were all synthesized by Buchwald-Hartwig amination.Later,Osukaet al.reported thatmeso-mesonitrogen-bridged Ni(II) porphyrin dimer was cleanly converted into aminyl radical 2 and nitrenium cation 3 by oxidation with PbO2and tris(4-bromophenyl)aminiumyl hexachloroantimonate (Magic Blue),respectively (Fig.1) [34].As an extension,we report here the synthesis of nitrogen-atom bridged Ni(II) porphyrin trimers.
First we attempted to synthesize linear NH-bridged porphyrin trimer 4Ni-2H by the similar Buchwald-Hartwig amination of 5,15-dibromo Ni(II)porphyrin 7Ni with 5-amino Ni(II)porphyrin 6Ni[34].A 4:1 solution of 6Ni and 7Ni in toluene was heated at 100 °C for 12 h in the presence of 0.4 equiv.Pd(OAc)2,0.4 equiv.BINAP,and 7 equiv.t-BuOK (Scheme 1).To our surprise,only a linear trimer 4Ni bearing a central quinodiimine-type porphyrinoid unit was obtained in 38% yield.The matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrum showed the parent ion of 4Ni atm/z2627.3453 [M]+(calcd.for(C172H192N14Ni3)+= 2627.3509) (Fig.S13 in Supporting information),which is smaller by two than the expected parent ion peak of 4Ni-2H.The structure of 4Ni has been revealed by X-ray diffraction structural analysis (Fig.2 and Fig.S17 in Supporting information).The bond lengths of C2meso-N (1.300(6) ˚A and 1.304(6) ˚A) are distinctly shorter than those of C1meso-N (1.412(6) ˚A and 1.395(7) ˚A).The1H NMR spectrum of 4Ni showed broadened signals at room temperature in CDCl3(Fig.S3 in Supporting information),which gradually changed to sharp peaks upon cooling down to-60 °C(Fig.S4 in Supporting information) [38],suggesting conformational motions at room temperature,which are comparable or faster than1H NMR timescale.It is noteworthy that four doublets due to theb-protons of the central quinodiimine unit were observed in the up-field shifted region at 7.77,6.76,5.70 and 3.99 ppm.
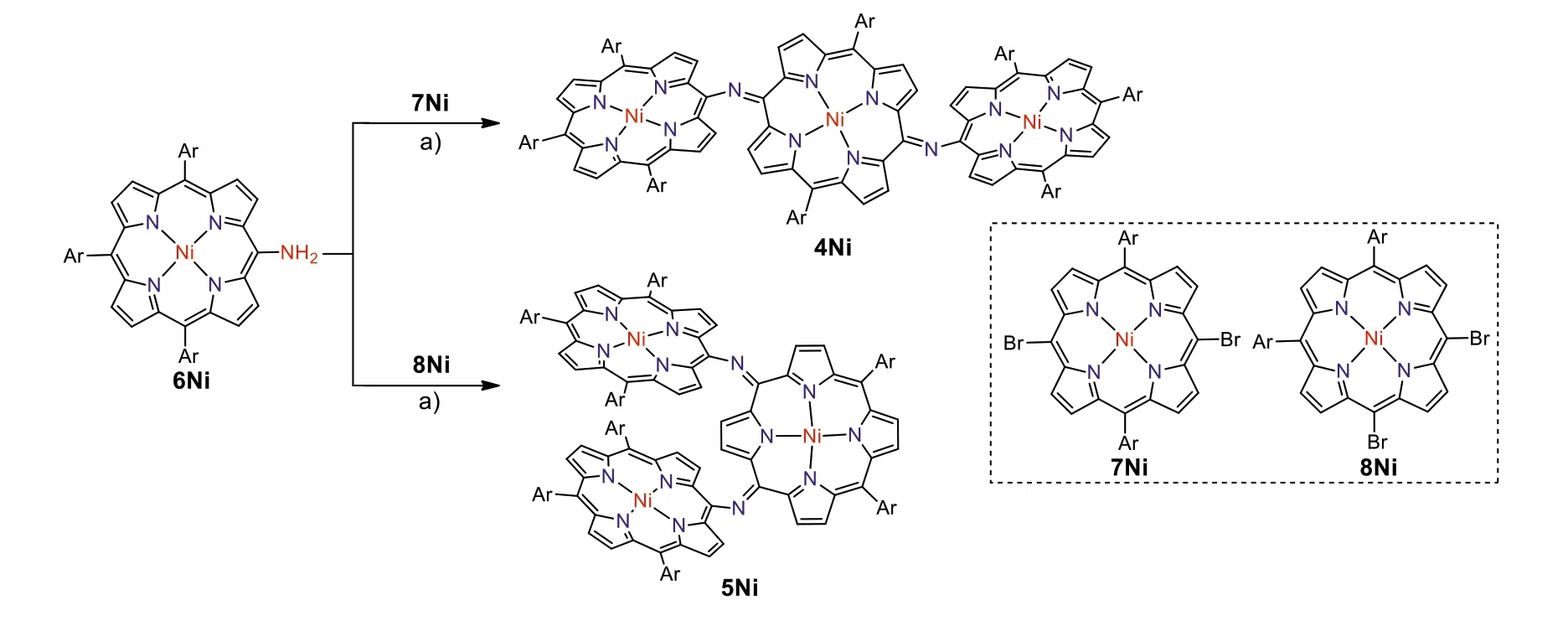
Scheme 1.Syntheses of meso-meso N-bridged porphyrinoid trimers.Conditions: a) Pd(OAc)2,BINAP,t-BuOK,toluene,100 °C,12 h.Ar = 3,5-di-tert-butylphenyl.
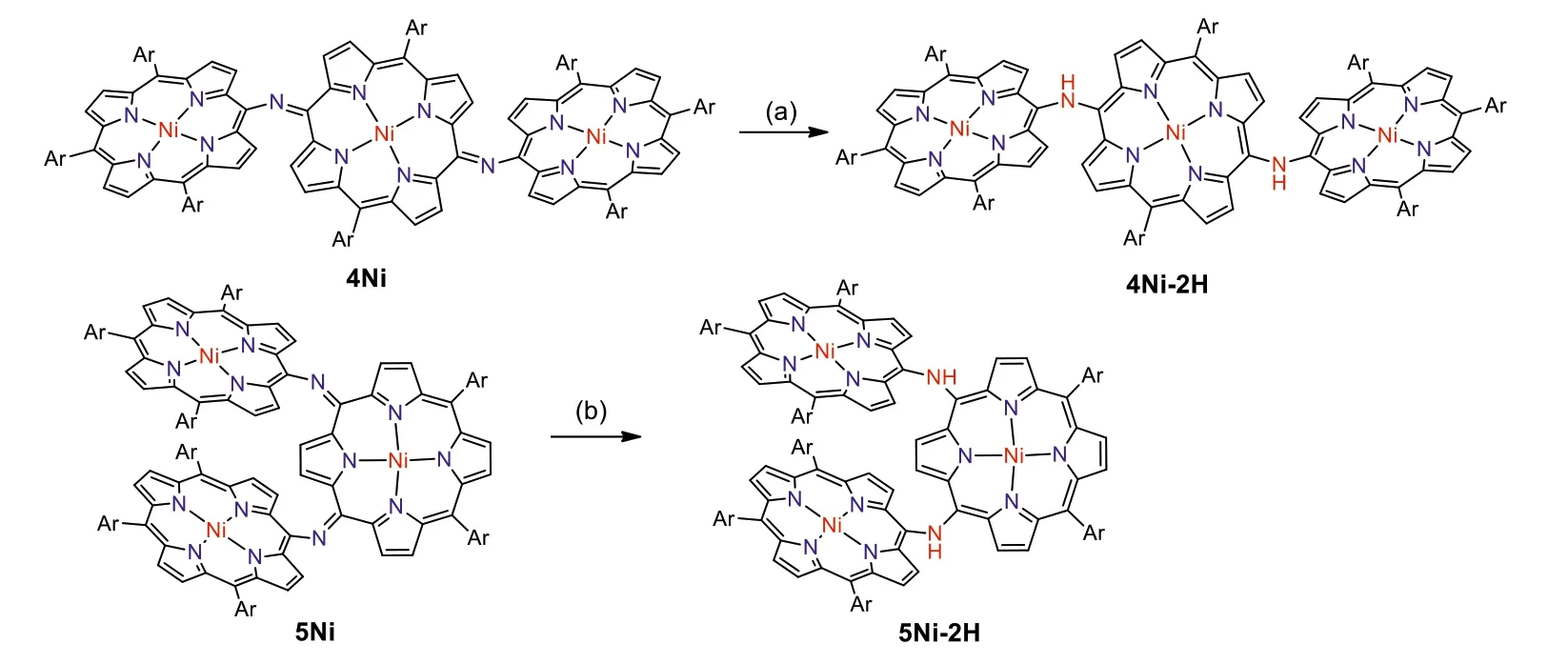
Scheme 2.Syntheses of meso-meso NH-bridged porphyrin trimers.Conditions: (a) NaBH4,Pd/C,CH2Cl2/CH3OH;(b) NH2-NH2.H2O,CH2Cl2.Ar = 3,5-di-tert-butylphenyl.
Similarly,Buchwald-Hartwig amination of 5,10-dibromo Ni(II)porphyrin 8Ni with 6Ni afforded L-shaped bent trimer 5Ni in 25% yield.The quinodiimine structure of 5Ni has been also confirmed by X-ray analysis.5Ni shows that the bond lengths of C2meso-N (1.299(5) ˚A and 1.302(6) ˚A) are shorter than those of C1meso-N bonds (1.399(5) ˚A and 1.413(6) ˚A) (Fig.2 and Fig.S18 in Supporting information).The1H NMR spectrum of 5Ni showed broadened signals at room temperature that became sharp and complicated signals at-60 °C in CDCl3(Figs.S5 and S6 in Supporting information).In line with the quinodiimine structure,the correspondingβ-protons were observed in the high field at 7.07,6.73,6.42,6.33,5.66,4.33,and 3.74 ppm.
The structural data of 4Ni shows that lengths of C1meso-N bonds(1.412(6) ˚A and 1.395(7) ˚A) bond to the terminal porphyrin units are longer than C2meso-N (1.300(6) ˚A and 1.304(6) ˚A) attached to the central quinodiimine units.Similarly,5Ni shows that lengths of C1meso-N bonds (1.399(5) ˚A and 1.413(6) ˚A) bond to the terminal porphyrin units are longer than C2meso-N (1.299(5) ˚A and 1.302(6) ˚A) attached to the central quinodiimine units.The observed short C2meso-N bond lengths in 4Ni and 5Ni indicated its double bond characters significantly [34],which further proved the structure of 4Ni and 5Ni to beN-bridged (rather than NH-bridged)porphyrin trimer.The dihedral angles between the terminal porphyrins and terminal porphyrin,terminal porphyrin and central quinodiimine are 66.81(3)°,56.34(3)° and 58.06(3)° in 4Ni,and 6.83(3)°,42.67(3)° and 39.34(3)° in 5Ni (Fig.2 and Figs.S17 and S18 in Supporting information).
Electrochemical properties of 4Ni and 5Ni were examined by cyclic voltammetry and differential-pulse voltammetry in CH2Cl2against a ferrocene/ferrocenium ion couple (Table 1 and Table S4 in Supporting information).Reversible oxidation waves were recorded at 0.22 and 0.52 V for 4Ni,and at 0.12 and 0.23 V for 5Ni.Reversible reduction waves were observed at-1.02 and-1.11 V for 4Ni,and at-0.79 and-1.14 V for 5Ni (Figs.S20 and S21 in Supporting information).As a result,the electrochemical HOMO-LUMO gaps of 4Ni and 5Ni were determined to be 1.24 and 0.91 eV,respectively.The observed reversible reduction waves of 4Ni and 5Ni encouraged us to examine their chemical reduction.After many attempts,we found that reduction of 5Ni with aqueous hydrazine in CH2Cl2afforded 5Ni-2H quantitatively (Scheme 2).Curiously,4Ni was not reduced with aqueous hydrazine but was reduced quantitatively to give 4Ni-2H with NaBH4and Pd/C in CH2Cl2/CH3OH.1H NMR spectra of both 4Ni-2H and 5Ni-2H are very simple,reflecting their symmetric structures with signals of theβ-protons appearing in the range of 8-9 ppm (Fig.3 and Figs.S7 and S8 in Supporting information).The structure of 5Ni-2H has been confirmed by single crystal X-ray diffraction analysis (Fig.4 and Fig.S19 in Supporting information).In 5Ni-2H,the bond lengths of the C2meso-N bond and the C1meso-N bond are similar,being 1.409(8)˚A,1.406(8) ˚A and 1.393(7) ˚A,1.434(11) ˚A,respectively,in line with the assigned structures.In addition,the dihedral angles between the terminal porphyrins and the central porphyrin are 58.29(7)°and 58.15(7)°,which are larger than those on 5Ni (42.67(3)° and 39.34(3)°).
The unexpected formation of 4Ni and 5Ni may be ascribed to the facile oxidation of 4Ni-2H and 5Ni-2H under the amination reaction conditions.These trimers have the central electron-rich Ni(II) porphyrin bearing 5,15 or 5,10-aminoporphyrin units.Thus,we examined the electrochemical properties of 4Ni-2H and 5Ni-2H (Table 1 and Table S4 in Supporting information).Actually,the reversible oxidation waves were observed at-0.09 and 0.17 V for 4Ni-2H,and at 0.11,0.25 and 0.41 V for 5Ni-2H (Figs.S22 and S23 in Supporting information).It is thus conceivable that 4Ni-2H and 5Ni-2H are oxidized under the amination conditions with air.So,when we try to oxidized them with PbO2and Magic Blue,neither aminyl radical nor nitrenium cation was found.The possible reason may be that the quinodiimine unit is more stable than other species.
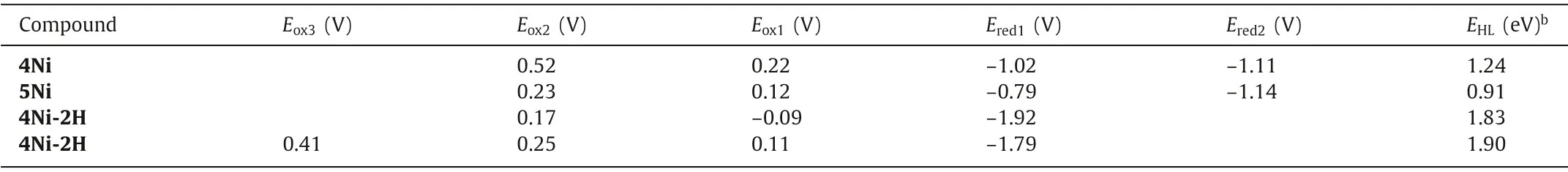
Table 1 Electrochemical measurement of 4Ni,5Ni,4Ni-2H and 5Ni-2H performed in CH2Cl2 at room temperature.a
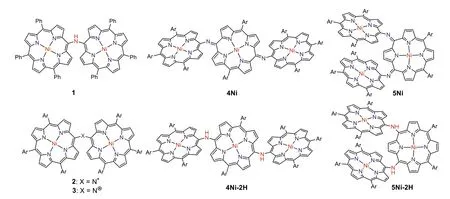
Fig.1.N-Bridged porphyrin oligomers.Ar = 3,5-di-tert-butylphenyl.
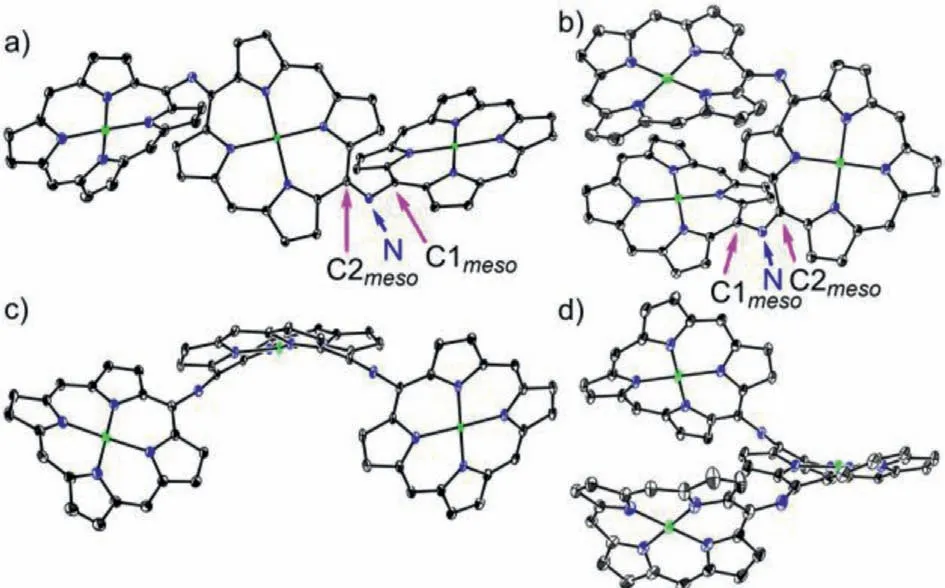
Fig.2.X-ray single crystal structure of 4Ni and 5Ni.(a) Top view and (b) side view of 4Ni,(c) top view and d) side view of 5Ni.The thermal ellipsoids are on 30%probability level.Solvent molecules,3,5-di-tert-butylphenyl groups,and hydrogens are omitted for clarity.
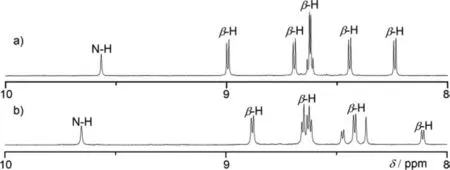
Fig.3.Partial 1H NMR spectra of (a) 4Ni-2H and (b) 5Ni-2H.
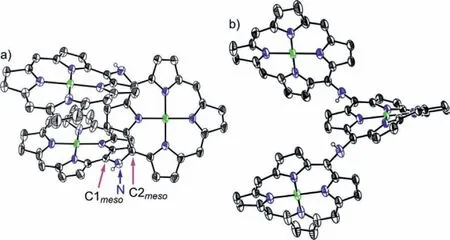
Fig.4.X-ray single crystal structure of 5Ni-2H.(a) Top view and (b) side view.The thermal ellipsoids are on 30% probability level.Solvent molecules,3,5-di-tertbutylphenyl groups,and hydrogens except those connected to N atoms are omitted for clarity.
The UV-vis-NIR absorption spectra of 4Ni,5Ni,4Ni-2H and 5Ni-2H in CH2Cl2are shown in Fig.5.4Ni shows two split Soret bands at 426 and 472 nm,a Q-band at 537 nm,and a broadened Q-like band at 915 nm.5Ni shows a Soret band at 429 nm,Q-bands at 540 and 581 nm,and a broadened Q-like band at 892 nm.Both 4Ni and 5Ni exhibit characteristic absorption spectra of quinonoidal porphyrinoid arrays [39-42].4Ni-2H shows a Soret band at 423 nm,and a Q-band at 627 nm.Similarly to 4Ni-2H,the absorption spectrum of 5Ni-2H shows a Soret band at 418 nm,and a Q-band at 664 nm.In particular,4Ni and 5Ni display the lowest energy band reaching to 1200 nm and 1400 nm,respectively.
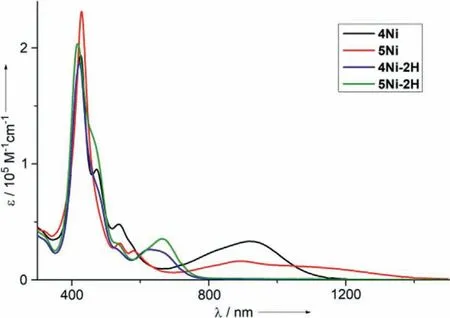
Fig.5.UV-vis-NIR absorption spectra of 4Ni (black line),5Ni (red line),4Ni-2H(blue line) and 5Ni-2H (green line) in CH2Cl2.
Density functional theory (DFT) calculations clearly indicated that both the HOMO of 4Ni and HOMO-1 5Ni were localized at terminal porphyrin units,whereas both LUMOs of 4Ni and 5Ni were localized at the central quinodiimine units (Figs.S28 and S29 in Supporting information).Time-dependent density functional theory (TD-DFT) calculations indicated that the absorption bands around 1000 nm of trimers 4Ni and 5Ni resulted from the transition from HOMO to LUMO of 4Ni and HOMO-1 to LUMO of 5Ni,respectively (Figs.S24 and S25 in Supporting information).These results show that both absorption bands around 1000 nm of 4Ni and 5Ni could be assigned to charge transfer (CT)band.
In summary,we synthesizedN-bridged porphyrinoid trimers 4Ni and 5Ni having the central quinodiimine through Buchwald-Hartwig amination,under which the oxidations of the NH-bridged porphyrin trimers 4Ni-2H and 5Ni-2H proceeded smoothly.The trimer 4Ni-2H was obtained by reduction with NaBH4and Pd/C,while 5Ni-2H was obtained by reduction with aqueous hydrazine.The structures of 4Ni,5Ni and 5Ni-2H were determined by Xray diffraction analysis.The UV-vis-NIR absorption spectra showed that the trimers 4Ni and 5Ni have the lowest energy band reaching to 1200 nm and 1400 nm,respectively.TheseN-bridged porphyrinoid trimers exhibited interesting spectral properties.Further exploration of cyclic or largerN-bridged porphyrinoid arrays is ongoing in our laboratory.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work at Hunan Normal University was supported by the National Natural Science Foundation of China (Nos.21772036,22071052,21602058,21702057),the Science and Technology Planning Project of Hunan Province (No.2018TP1017),and the Scientific Research Fund of Hunan Provincial Education Department (No.19A331),and Hunan Provincial Innovation Foundation for Postgraduate (No.CX20210473).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.061.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
