Remarkable mechanochromism and force-induced thermally activated delayed fluorescence enhancement from white-light-emitting organic luminogens with aggregation-induced emission
Yito Zheng,Lingqi Zuo,Letin Zhng,Ziho Hung,Shufeng Li,Zhn Yng,Zhu Mo,Suilin Luo,Cong Liu,Fengqing Sun,Gung Shi,Zhenguo Chib,,Bingji Xu,*
a Key Laboratory of Theoretical Chemistry of Environment,Ministry of Education;School of Chemistry,South China Normal University,Guangzhou 510006,China
b State Key Laboratory of Optoelectronic Materials and Technologies (Sun Yat-sen University),Guangzhou 510275,China
c School of Chemistry,Sun Yat-sen University,Guangzhou 510275,China
d Shenzhen Institute of Advanced Electronic Materials,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,China
Keywords:White-light emission Thermally activated delayed fluorescence Aggregation-induced delayed fluorescence mechanochromism Force-induced emission enhancement
ABSTRACT The development of organic materials with white-light emission and thermally activated delayed fluorescence (TADF) properties in the solid state remain a challenge.Herein,a series of white-light-emitting organic luminogens have been developed and are found to show aggregation-induced delayed fluorescence (AIDF) characteristics.The AIDF emitters present dual-emission consisted of prompt fluorescence and TADF in the crystalline state.Their white-light emissions can be easily tuned by altering the chemical structure and connecting position of the heterocyclic aromatic substituent.Under the stimuli of mechanical force and solvent vapor,the compounds exhibit remarkable and reversible mechanochromism,in which their emission colors are switchable between white and yellow.Upon grinding,they also display linearly tunable luminescence colors,as well as force-induced TADF enhancement,which may be associated with the more compact molecular packing and the restriction of intramolecular motions.The results from time-resolved emission scanning and theoretical calculation suggest that the dual-emission of the AIDF luminogens likely results from the twisted intramolecular charge transfer transitions of the molecules,and the reversible mechanochromism properties probably stem from the interconversion of the quasi-axial and the quasi-equatorial conformations.
Organic materials with white-light emission have received tremendous attention in recent years due to their promising applications in displays,lighting,and sensing [1-3].However,most of the reported white-light-emitting organic materials base on transition-metal-containing complexes [4,5]or macromolecules polymerized by two chromophores with complementary emission colors (e.g.,blue and yellow) [6,7],which have complicated molecular structures.Lately,Anand et al.reports a review about recent advances in the development of pure organic white-light emitters and introduces several approaches to achieve efficient whitelight emission applying pure organic molecules [8].On the other hand,thermally activated delayed fluorescence (TADF) materials are widely used in organic light-emitting diodes (OLEDs) because they can harvest triplet excitonsviareverse intersystem crossing (rISC) to elevate the external quantum efficiencies [9,10].Nevertheless,conventional organic TADF luminophores usually suffer from aggregation-caused quenching (ACQ),largely confining their practical applications.In this regard,great efforts have been devoted to developing new TADF luminogens,especially those with aggregation-induced emission (AIE) properties,which can perfectly surmount the notorious ACQ effect [11,12].For example,Chi and Xu have developed a series of AIE-active TADF emitters with asymmetric structures by connecting two different donors to an acceptor [13].Despite these significant advances,organic TADF materials with white-light emission remain pretty limited [14-18].Moreover,studies on their luminescence mechanism are very insuffi-cient.Therefore,it is urgent to develop more white-light-emitting luminophores with TADF and AIE properties and elucidate their photophysical processes.
Notably,AIE luminogens with mechanochromism properties have attracted great interest in the past few years [19,20].In general,AIE compounds derived from tetraphenylethene (TPE) [21],acrylonitrile [22],9,10-distyrylanthracene (DSA) [23]etc.,are prone to show mechanochromism activities.However,in most cases,their luminescence can only be switched between two colors upon mechanical stimuli.Furthermore,when the AIE emitters are treated with force,most of them undergo a decrease in luminescence intensity [17,24-26],which is unfavorable to the improvement of their mechanochromism performance.In this context,organic AIE luminogens,which exhibit tunable emission color and remarkable luminescence enhancement simultaneously under the stimuli of mechanical force,are highly desirable [27-30].
Fig.1.Chemical structures of 4DF,4DT and 2DT.
In this communication,we present a series of white-lightemitting AIE luminogens with remarkable mechanochromism and force-induced TADF enhancement properties.Herein,benzophenone is employed as the electron acceptor,and phenothiazine and carbazole are selected as the electron donors.In the meantime,another donor,namely,dibenzofuran or dibenzothiophene,is attached to the carbazole unit to tune the white-light emission and mechanochromism performance of the luminogens.The resulting compounds show aggregation-induced delayed fluorescence (AIDF)characteristics and produce white-light emissions with different CIEx,ychromaticity coordinates in the solid state.Moreover,their emission colors are linearly tunable upon grinding,and the TADF can be dramatically intensified by force.In addition,a mechanism for the dual-emission of the compounds is also proposed [31,32].
The target compounds,i.e.,4DF,4DT,and 2DT,were successfully synthesizedviaFriedel-Crafts and Suzuki coupling reactions (Fig.1 and Scheme S1 in Supporting information).Their chemical structures and sample purity were fully confirmed by nuclear magnetic resonance spectroscopy (1H NMR and13C NMR),high-resolution mass spectrometry (HRMS),and high-performance liquid chromatography (HPLC) with satisfactory results (Figs.S28-S42 in Supporting information).All the compounds have similar UV-visible absorption spectra and photoluminescence (PL) spectra in THF solutions (Fig.S1 in Supporting information).The absorption bands at around 290 nm and 340 nm can be attributed to theπ-π*transitions and the intramolecular charge transfer (ICT) transitions of the molecules,respectively [13,33-35].It is found that the emission maxima of the luminogens are identical (λem,max=520 nm),indicating that their luminescence comes from similar excited states.
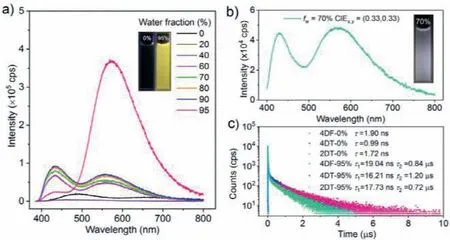
Fig.2.(a) PL spectra of 4DF in THF/H2O mixtures with different water fractions.The insets depict the fluorescent images of 4DF in THF/H2O mixtures with 0% and 95% water fractions,respectively.(b) PL spectra and the fluorescent image of 2DT in THF/H2O mixture with 70% water fraction.(c) Emission decay curves of the compounds in THF/H2O mixtures with 0% and 95% water fractions,respectively (Concentration: 0.1 mmol/L).
PL and UV-visible absorption spectra of the compounds in THF/H2O mixtures with different water fractions (fw,v/v) were then monitored to investigate their potential AIE properties(Fig.2a and Fig.S2 in Supporting information).Taking 4DF as an example,its luminescence signal is very weak in dilute THF solution.However,when thefwvalue reached 60%,the emission intensity dramatically increased.Further elevating thefwled to more intense luminescence.Meanwhile,Mie scattering effects were observed in the UV-visible absorption spectra of the mixtures with high water content,indicating the formation of nanoaggregates (Fig.S3 in Supporting information).Similar results were also obtained from 4DT and 2DT.Evidently,all the luminogens are AIE active [36].When the compounds are well dissolved in good solvents,the molecules can dissipate the energy of the excited statesviarotation and vibration,thus resulting in weak luminescence.As thefwincreases,their solubilities decrease,and the molecules gradually form nanoaggregates,which can rigidify the intramolecular motions to suppress the non-radiative decay channels,thereby enabling the AIE effects [37].Interestingly,dualemission was recorded in some of the mixtures with nanoaggregates.In particular,the CIEx,ychromaticity coordinates of 2DT in the THF/H2O mixture with 70% water fraction were determined to be (0.33,0.33) (Fig.2b and Fig.S4 in Supporting information),indicating that its nanoaggregates can emit pure white light,which has been seldom reported before [38,39].Thus,the emission color of 2DT in the mixture can be varied from green to pure white and then yellow by simply controlling the water fraction.
Transient emission decays of 4DF,4DT,and 2DT in the THF/H2O mixtures were then measured.As depicted in Fig.2c and Fig.S5(Supporting information),the compounds display single exponential decays with short lifetimes in both aerated and argon-degassed THF solutions,suggesting their prompt fluorescence attributes.By contrast,in the mixture with 95% water fraction,each of them shows a double exponential decay,and the fitting result gives a short lifetime and a long one in microsecond order,implying the TADF characteristic.The corresponding lifetimes for the delayed components of 4DF,4DT and 2DT were estimated to be 0.84 μs,1.20 μs,and 0.72 μs,respectively.These experimental results fully demonstrate that all three compounds exhibit unique AIDF properties [13,40,41].
As shown in Fig.3a,the compounds exhibit dual-emission with PL maxima at around 440 nm and 560 nm in the solid state.The similar emission bands suggest that they probably undergo analogous luminescence processes.The CIEx,ychromaticity coordinates of 4DF,4DT and 2DT were determined to be (0.29,0.30),(0.33,0.38) and (0.36,0.39),respectively,illustrating the whitelight emission properties of the AIDF luminogens,which are potential for lighting and display applications [42].The distinct CIEx,ychromaticity coordinates also demonstrate that the emission colors of the compounds can be tuned by altering the substituent group on the carbazole unit.Subsequently,the transient emission decays of the pristine samples under different atmospheres were surveyed (Figs.3b and c,Figs.S6 and S7 in Supporting information).The emission bands in the blue-light region show short lifetimes(τ<1 ns),verifying their prompt fluorescence characteristics.In comparison,the ones in the yellow-light region simultaneously present prompt and delayed components in their decay curves,which suggest that they are composed of prompt fluorescence and TADF.This inference is also supported by the enhancement of their PL intensities under vacuum (Fig.S8 in Supporting information).To further validate the TADF properties of the compounds,measurements for the temperature-dependent transient emission decays were carried out on their pristine samples (Fig.3d and Fig.S9 in Supporting information).Taking 4DF as an example,the lifetime of its delayed component increases from 6.08 μs at 78 K to 6.91 μs at 100 K and then decreases to 0.99 μs at 300 K.Meanwhile,the delayed component continuously increases when the temperature rises [10,43,44].Similar results are also observed for 4DT and 2DT.Accordingly,the AIDF luminogens can show white-light emission composed of prompt fluorescence and TADF in the solid state [13].
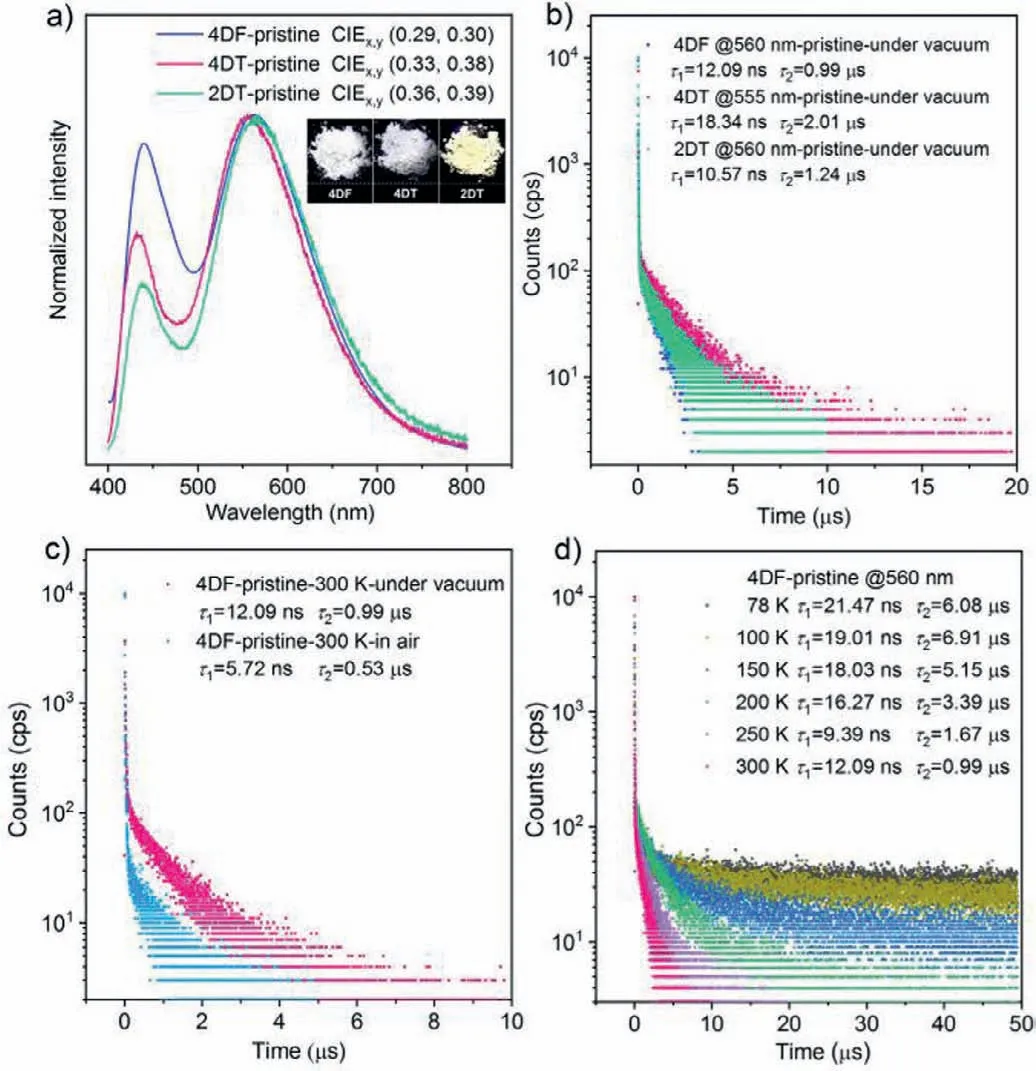
Fig.3.(a) PL spectra and CIEx,y chromaticity coordinates of the pristine samples.The insets depict the luminescence images of the pristine samples.(b) Emission decay curves for the yellow emission bands of the pristine samples under vacuum.(c) Emission decay curves for the yellow emission band of 4DF at 300 K under different atmospheres.(d) Emission decay curves for the yellow emission band of 4DF at different temperatures under vacuum.
Interestingly,the pristine samples of the compounds exhibit remarkable mechanochromism under the stimulation of mechanical force.For 4DF,the prompt fluorescence emission band in the bluelight region gradually disappeared and the TADF one in the yellowlight region showed a slightly bathochromic shift upon grinding(Fig.4a).As a result,its emission color gradually changed from white to yellow (Fig.4b).It is found that the CIEx,ychromaticity coordinates for the ground samples of 4DF lie on a straight line with an excellent correlation coefficient of 0.993.The straight line crosses the white region and hits the point of (0.33,0.34),which is very close to standard white-light emission (Fig.4c).Similar mechanochromism properties were also observed for 4DT and 2DT(Figs.S10 and S11 in Supporting information).More importantly,the ground samples of the AIDF luminogens can turn back to the pristine ones upon fuming with DCM vapor (Fig.4d and Fig.S12 in Supporting information),indicating that the mechanochromism of the compounds is reversible.Therefore,the emission colors of 4DF,4DT,and 2DT can be easily tuned in a wide range by applying appropriate mechanical stimuli.It is noteworthy that the intensity ratio between the blue and the yellow emission bands decreases from 4DF to 4DT and then to 2DT,which may be associated with the enhancement of delayed fluorescence (Table S1 in Supporting information).Consequently,the variation of emission color of 4DF is more obvious than those of the other two luminogens under the stimuli of mechanical force.These results demonstrate that the mechanochromic properties of the AIDF luminogens can also be manipulated by simply altering the substituent group on the carbazole moiety besides the CIEx,ychromaticity coordinates of the white-light emission,and the introduction of dibenzofuran unit is favorable for achieving organic luminogen with better mechanochromism performance in comparison with that of dibenzothiophene.
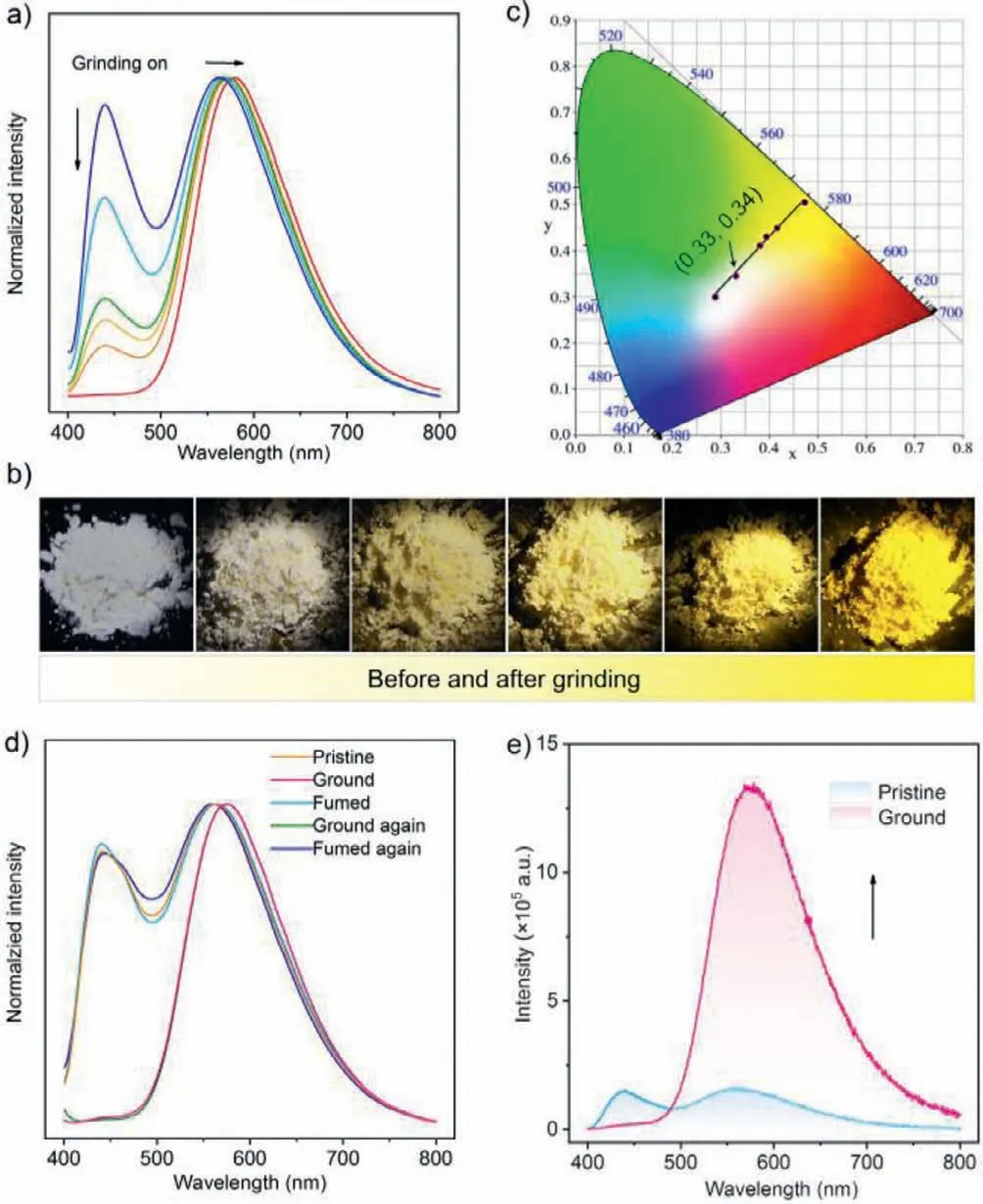
Fig.4.Mechanochromic properties of 4DF.(a) Changes of PL spectra of 4DF upon grinding.(b) Luminescent images of 4DF under the illumination of 365 nm UV light before and after grinding.(c) Changes of CIEx,y chromaticity coordinates of 4DF upon grinding.(d) Emission switching of 4DF realized by grinding and fuming treatments.The ground sample of 4DF were fumed in DCM vapor for about 20 min.(e)Force-induced emission enhancement of 4DF.
Notably,4DF showed significant emission enhancement after grinding (Fig.4e).Such mechanochromic behavior is distinctly different from typical AIE luminogens.The PL quantum yield (PLQY)of its well-ground sample at ambient condition was calculated to be 16.2%,much higher than that of the pristine one (ΦPL=1.8%).Similarly,4DT and 2DT also have force-induced emission enhancement properties,and their PLQYs change from 3.5% and 5.8%to 21.9% and 21.1% (Figs.S13 and S14 in Supporting information),respectively.The emission bands in the yellow-light region also present prompt and delayed components simultaneously in their decay curves,indicating that the well-ground samples remain TADF-active.Their TADF properties were further validated by the results from temperature-dependent transient emission decays and the changes of PL spectra under different atmospheres (Figs.S15 and S16 in Supporting information).Similar force-induced emission enhancement was observed for 4DF,4DT and 2DT under vacuum,and the PLQYs of their well-ground samples further increase to 20.6%,48.4%,and 29.8%,respectively.Consequently,all three AIE luminogens exhibit force-induced TADF enhancement characteristics.The TADF properties for the pristine and well-ground samples of the AIE luminogens probably originate from the small energy gaps (ΔEST) between the singlet (S1) and the triplet (T1) excited states (Fig.S17 in Supporting information) [45].TheΔESTvalues of the well-ground samples are smaller than those of the pristine ones.Furthermore,the rate constants of the delayed fluorescence (kDF),intersystem crossing (kISC),and rISC (krISC) of the wellground samples are all an order of magnitude higher than those of the pristine ones.These factors may be responsible for the forceinduced TADF enhancement properties of the compounds.Careful investigation reveals that the PLQY values and the quantum yields of delayed fluorescence of 4DT and 2DT are higher than those of 4DF,manifesting that the incorporation of dibenzothiophene is helpful for the elevation of luminescence performance.Among all the samples,the ground 4DT presented the highest PLQY,which may be ascribed to its smallerΔESTvalue as well as the largerkISC,krISCandkrconstants,leading to the more significant force-induced TADF enhancement.Thus,connecting dibenzothiopheneviaits 4-position to the chromophore may be prone to intensify the luminescence of the resulting organic luminogens.
To gain in-depth insight into the mechanochromism mechanisms of the AIDF luminogens,single-crystal analyses were then performed.The single crystals of 4DF (ΦPL=1.9%) and 2DT(ΦPL=4.2%) were isolated from the mixtures of DCM/n-hexane and DCM/acetonitrile,respectively.The PL spectra of the two single crystals also displayed dual-emission characteristics,similar to those of the pristine samples (Fig.S18 in Supporting information).The simulated XRD patterns from single-crystal data are in accordance with the experimental ones of the pristine powders (Fig.S19 in Supporting information),suggesting that the molecular stacking modes of the pristine samples are the same as those of the single crystals.The single crystal structures of 4DF and 2DT belong to the orthorhombic system with a space group ofPna21.In each unit cell,there are four highly twisted molecules (Fig.S20 in Supporting information).They take a head-to-tail molecular packing style and form a V-shape molecular arrangement.For 4DF,the bend angle of the phenothiazine unit is 130.37°,while the torsion angle between the phenothiazine and its adjacent benzene ring is determined to be 2.73°,suggesting aquasi-axial conformation(Fig.5a) [26].Similar twisted molecular conformation is observed for the molecules in the crystal structure of 2DT (Fig.S21 in Supporting information).It is found that C-H···S and C-H···πinteractions play primary roles in the crystals (Fig.S22 in Supporting information).Their strength and distribution were then simulated by the Multiwfn program [46-49].The green 3D isosurfaces and the smallδgintervalues (<0.015) fully demonstrate that the molecules build weak intermolecular interactions in the crystal structures(Figs.S23 and S24 in Supporting information).Moreover,few intermolecular interactions and ample space surround the dibenzofuran and the dibenzothiophene units (Fig.S25 in Supporting information).These results suggest that the AIDF luminogens have loose molecular stacking modes.Upon grinding,their crystal structures are prone to collapse,leading to changes of molecular conformations and more compact molecular packings.Consequently,the intramolecular motions are largely suppressed,which is verified by the significant reduction of the non-radiative transition constants(knrs) of the ground samples in comparison with those of the pristine ones,thereby resulting in the TADF enhancement of the compounds.
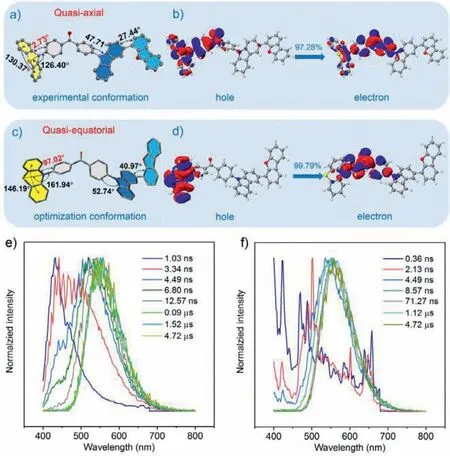
Fig.5.(a) Molecular conformation of 4DF in single crystal structure.(b) Natural transition orbitals of 4DF in quasi-axial conformation.(c) Molecular conformation of 4DF after optimization.The digits in (a) and (c) represent the bend and torsion angles.Taking the quasi-axial conformation as an example,the bend angle of the phenothiazine unit is 130.37°,while the torsion angle between the phenothiazine and its adjacent benzene ring is determined to be 2.73°.(d) Natural transition orbitals of 4DF in quasi-equatorial conformation.(e) Time-resolved emission spectra of the pristine sample of 4DF at 300 K under vacuum.(f) Time-resolved emission spectra of the ground sample of 4DF at 300 K under vacuum.
Theoretical calculations based on density functional theory(DFT) were conducted for 4DF and 2DT at the B3LYP/6-311 G(d,p)level,and natural transition orbits (NTO) were subsequently simulated to evaluate their transition characteristics [48-50].As depicted in Fig.5c and Fig.S21c,the AIDF luminogens exhibitquasiequatorial conformations after optimization,different from those of thequasi-axial ones in the single crystal structures.When 4DF and 2DT are in thequasi-axial conformation,their hole and electron NTOs almost overlap,indicating that the emission mainly originates from localized excited state transitions (Fig.5b and Fig.S21b).However,in thequasi-equatorial conformation,the hole and electron NTOs of the molecules concentrate in the phenothiazine and benzophenone units,respectively,which suggest the ICT transitions (Fig.5d and Fig.S21d) [51].
Time-resolved emission scanning for the pristine and ground samples of the compounds was implemented to further elucidate their luminescence mechanisms (Figs.5e and f,Fig.S26 and S27 in Supporting information).For the pristine 4DF,its PL spectrum shows a single emission band with a maximum at 430 nm at the decay time of 1.03 ns.As time goes on,the emission band in the blue-light region decreases quickly and disappears in a few nanoseconds.On the other hand,an emission band at around 490 nm arises at 3.34 ns,and then it red-shifts to 550 nm rapidly.The yellow emission band keeps a constant profile after 0.09 μs and remains observable at 4.72 μs.These results suggest that the blue prompt fluorescence of the pristine sample probably originates from the localized excited state transitions of the molecules in thequasi-axial conformation.After excitation,the 4DF molecules gradually change into thequasi-equatorial conformation and allow a twisted intramolecular charge transfer (TICT) transition,enabling the ISC and rISC processes for the excitons to produce yellow TADF [32].The occurrence of TICT is probably associated with the loose molecular packing mode and weak intermolecular interactions of the compound.In the case of ground 4DF,the emission band in the blue-light region shows extremely low initial intensity and vanishes in 8.57 ns,while the one in the yellow region experience similar variations to that of the pristine sample.These results are reasonable because most of the molecules are likely in thequasi-equatorial conformation after grinding.Similar changes were also recorded for the pristine and ground samples of 4DT and 2DT (Figs.S26 and S27).Therefore,the dual-emission from the pristine samples of the AIDF luminogens may stem from the TICT transitions of the molecules,and the reversible mechanochromism is likely caused by the interconversion of thequasi-axial and thequasi-equatorial conformations [31,52-54].
In summary,three organic emitters with AIDF properties have been developed by attaching dibenzofuran or dibenzothiophene to the chromophore of (4-(10H-phenothiazin-10-yl)phenyl)(4-(9H-carbazol-9-yl)phenyl)methanone.The AIDF luminogens show unique dual-emissions,which are composed of prompt fluorescence and TADF,and produce white-light emissions with different CIEx,ychromaticity coordinates in the solid state.Moreover,they exhibit remarkable and reversible mechanochromism,in which the emission colors of the compounds are switchable between white and yellow under the stimuli of mechanical force and solvent vapors.Upon grinding,the luminogens also present linearly tunable emission colors,as well as force-induced TADF enhancement,which is likely associated with the restriction of intramolecular motions.The dual-emissions of the AIDF emitters may result from the TICT transitions of the molecules,and the reversible mechanochromism is probably caused by the interconversion of thequasi-axial and thequasi-equatorial conformations.These results demonstrate that the white-light emissive AIDF luminogens may have innovative applications in OLEDs,anti-counterfeiting,and sensing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.51603233),the Natural Science Foundation of Guangdong Province of China (Nos.2019A1515010550,2020A1515010439,2019A1515011389),the GDUPS (2019),the Opening Foundation of Key Laboratory for Polymeric Composite and Functional Materials of Ministry of Education (Sun Yat-sen University,No.PCFM-2019-05).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.059.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
