Copper-catalyzed decarboxylative Se insertion coupling of indoles and propiolic acids
Ge Wu,Xueying Zhou,Cihong Wng
a School of Pharmaceutical Sciences,Wenzhou Medical University,Wenzhou 325035,China
b State Key Laboratory of Structural Chemistry,Fujian Institute of Research on the Structure of Matter,Chinese Academy of Sciences,Fuzhou 350002,China
Keywords:Se powder Copper catalysis Decarboxylation Multi-component reaction Propiolic acids
ABSTRACT A novel and efficient copper-catalyzed decarboxylative alkynylselenation of indoles with Se powder and propiolic acids has been developed.The outstanding advantages of this protocol not only nicely avoid the use of prefabricated arylselenation reagent and address the facile over-selention issues,but also enrich the chemistry of selenium powder.Importantly,this reaction could be extended to pyrrole,and the practical utility of this transformation has been demonstrated in gram-scale synthesis and late-stage indolylselenation of Clofibrate-derived propiolic acid.
The incorporation of Se atom into organic small molecules could substantially unfold their important biological functions in antioxidant defense,regulation of inflammatory responses and metabolism of thyroid hormones [1,2].In discovery chemistry,it has been realized that aryl alkynyl selenides are useful precursors for the preparation of multifunctional vinyl selenides by welldeveloped hydrofunctionalizations [3-6],and are valuable handles in cyclization [7]and cross-coupling reactions [8].In this case,it has been of great interest to disclose concise and efficient protocols for preparing these privileged compounds.Although numerous attractive avenues for the construction of new Csp-Se bonds have been developed,including the cross-coupling of alkynyl bromides with ArSeSeAr [9,10],terminal alkynes with ArSeBr [11-13],ArSeSeAr [14-19]or ArSeCN [20],and alkynylboronic acid [21,22]with ArSeSeAr through various mechanistic processes.The existing preparation of electrophilic arylselenation reagent,1 equivalent ArSe-waste of ArSeSeAr and harsh reaction conditions would dramatically inhibit the research and development of new seleniumcontaining pharmaceutical molecules.
Alkynyl carboxylic acids have been employed as terminal alkyne surrogates in transition-metal catalyzed decarboxylative C-X bonds coupling reactions [23-26].Recently,Pan reported coppercatalyzed decarboxylative arylselenation of aryl propiolic acids with electrophilic diaryl diselenides (Scheme 1A) [27].However,the chemo-selectivity of these reactions was not well controlled.The unsaturated bonds in the product are liable to electrophilic addition with the excess electrophilic arylselenation reagent,thus,over-selenation pitfalls are facile to occur [28-32].In addition,some heteroaryl selenating reagents are difficult to access mainly because of the lack of synthetic routes.On the other hand,the use of Se powder as imput linkage is attractive and fantastic,since it is bench-stable,inexpensive,and easy-to-handle.We assumed that the decarboxylative arylalkynylation of Se powder could be employed to avoid the above-mentioned limitations and insurmountable issues of over-arylselenation.As a rational design of this strategy (Scheme 1B),copper-catalyzed decarboxylative selenation of alkynyl carboxylic acids with Se powder to generate the nucleophilic alknylselenocopper intermediates,which was then coupled with nucleophilic heterocyclic compoundsviaanalogous Chan-Lam coupling.As a continuous study on the difunctionalization of Se powder [33,34],herein,we describe a new protocol for the preparation of arylalknyl selenides through copper-catalyzed decarboxylative alkynylselenation of indole with Se powder and propiolic acids with the formation of double C-Se bonds.Compared with traditional alkynyl sources [35],the prominent reactivity of propiolic acids shows a higher exploitability and flexible propensity for selective decarboxylative selenation.This transformation not only enriched the chemical properties of selenium powder,but also broadened the new application value of alkynyl carboxylic acids.
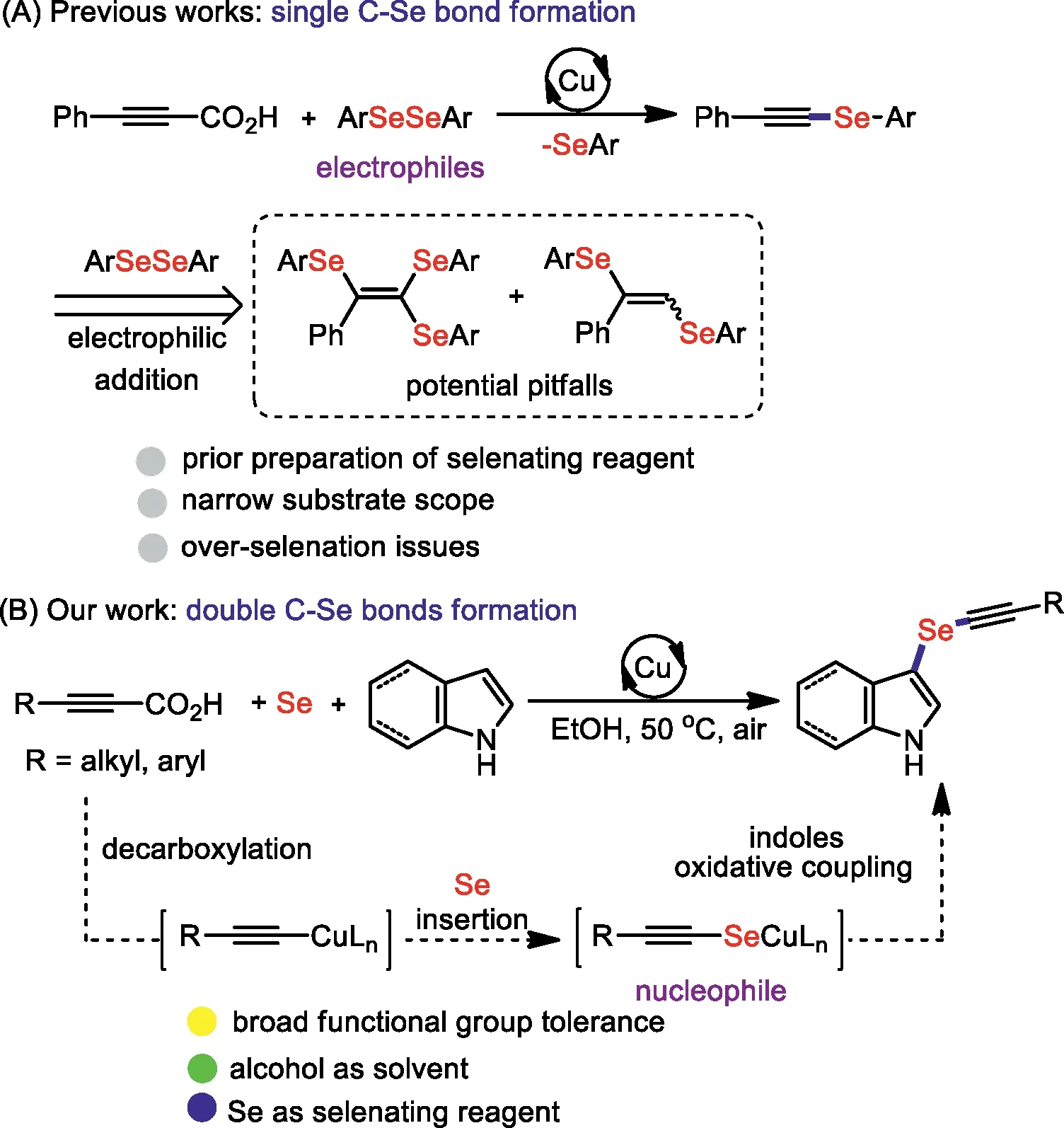
Scheme 1.Decarboxylative arylselenations.
At the beginning of our study,we chose indole (1a),selenium powder and phenylpropiolic acid (2a) as model substrates to explore the optimal reaction conditions.As illustrated in Table 1,the reasonable choice of copper salt,solvent and base was found to be a critical success for the transformation.Among numerous copper catalysts,CuCl2displayed the highest catalytic activity,while CuBr2and Cu(OAc)2have relatively low performance (entries 1-3).It was found that using other alcohols solvent other than ethyl alcohol affected the transformation outcome (entries 4-6).In fact,common organic solvents such as DMSO and toluene could not realize the smooth conversion of the reaction (entries 7-11),and the target product was not detected.If the reaction does not add a phase transfer catalyst,the conversion efficiency decreases sharply (entry 12).When other carbonates were used instead of Cs2CO3,3a was not generated or the yield was significantly diminished (entries 13-15).We speculated that the suitable base plays an important role in the deprotonation of acetylenic acid and the activation of Se powder under these reaction conditions.The expected decarboxylative arylselenation reaction hardly occurred in the absence of copper catalyst and base (entries 16 and 17).In particular,the lack of 1,10-phen significantly reduced the yield of 3a (entry 18).Importantly,when the reaction is conducted under N2atmosphere or elevating the reaction temperature all decrease the conversion of substrates (entries 19 and 21).In addition,when the reaction is carried out under O2atmosphere,the reaction efficiency is also very good,but it is slightly lower than that under air (entry 20).
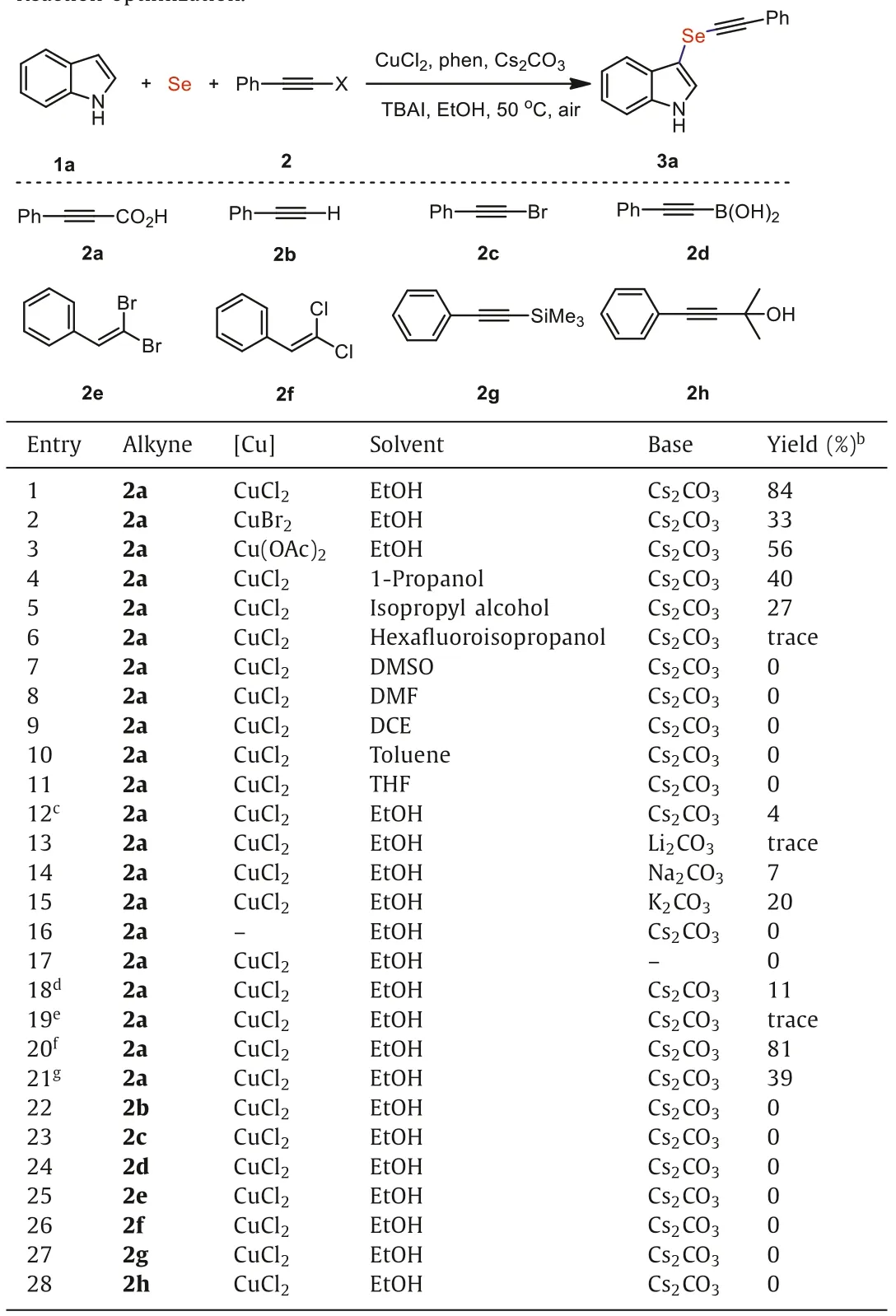
Table 1 Reaction optimization.a
It is worth noting that a small amount of 1,2-bis(phenylethynyl)diselane derived from the reaction of Se powder with propiolic acids and decarboxylative homocoupling of propiolic acids have always been observed [36],while over-selenation,decarboxylative addition or hydroamination of indole with propiolic acids byproducts did not detect during the optimization of reaction conditions [37].Finally,the alkynylselenation of indole with other typical arylacetylene sources,such as phenylacetylene(2b),1-bromo-2-phenylacetylene (2c),2-phenyl-1-ethynylboronic acid (2d),2,2-dibromostyrene (2e),(2,2-dichlorovinyl)benzene(2f),1-Phenyl-2-(trimethylsilyl)acetylene (2g) and 2-methyl-4-phenyl-3-butyn-2-ol (2h) were proved to be completely invalid under otherwise identical reaction conditions.These results suggested that CO2H group of propiolic acids plays a critical role in promoting the multi-component selenation reactions [38].It is presumable that the coordination of CO2H group with the copper center is beneficial to better substrate solubility and facilitates the insertion of selenium powder with the triple bonds.
Upon the optimization of feasible reaction conditions,a variety of functionalized indoles were used for the current threecomponent decarboxylative selenation (Scheme 2).Generally,various substituted indoles reacted smoothly.The substituting position and electronic nature of different substituents on the benzene ring of indoles showed little difference toward the transformation effi-ciency.It is worth noting that the nitro group is susceptible to reduction with Se powder,and can be tolerated on the indole ring to give 3g in 77% yield [39].The reaction efficiency was not affected by the different substituents (methyl and ester) at the 2-position of indole,and 3k,3m and 3n are generated with excellent yields.In addition,a substrate containing planar phenyl functional group(3l) can also be compatible and smoothly alkynylselenated as well.It was found that the free N-H bond of indole plays a critical role,andN-methylindole,N-acetylindole did not furnish the desired carboxylative alkynylselenation under our optimized reaction conditions.According to these experimental results,indoles bearing unprotected N-H bond for their generation the relatively stable carbanion for cross-coupling [40].
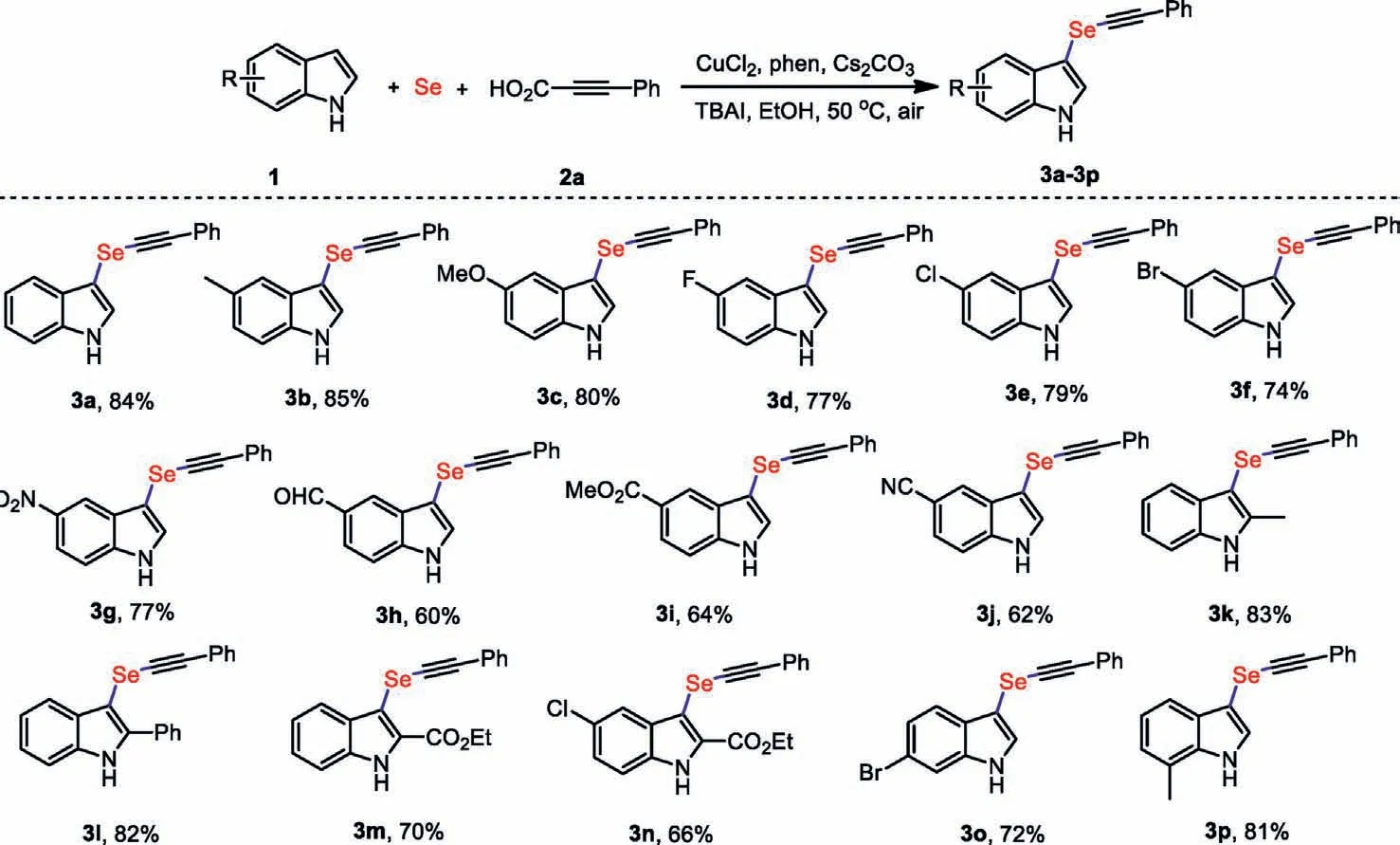
Scheme 2.Scope of indoles.Reaction conditions are the same as entry 1 in Table 1.Isolated yields after column chromatography are given.
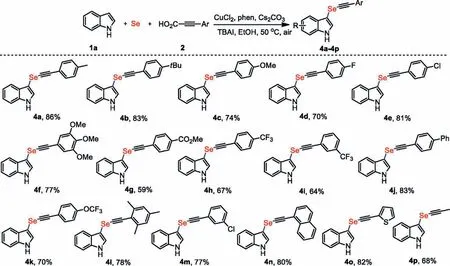
Scheme 3.Scope of propiolic acids.Reaction conditions are the same as entry 1 in Table 1.Isolated yields after column chromatography are given.
Next,a wide range of representative aryl-substituted propiolic acids were resoundingly converted into the expected indolyl alkynyl selenides in moderate to excellent yields (Scheme 3).Current mild reaction condition showed remarkable functional group compatibility,such as alkyl (4a,4b,4l),methoxyl (4c),halogen (4d,4e,4m),ester (4g),trifluoromethyl (4h,4i) and trifluoromethoxy(4k) had little effect on the reaction efficiency.Importantly,3,4,5-trimethoxyl,an important pharmacophore in biologically active molecules,proceeded the transformation smoothly (4f).Remarkably,the sterically hindered substrate was a competent substrate and afforded 4l with good efficiency.In addition to aryl ring substituents,naphthalene-substituted propiolic acid (4n) is also suitable for decarboxylative indolylselenation,and shows the broad substrate scope of this method.Moreover,thiophene-substituted propiolic acid worked as a viable substrate for decarboxylative arylselenation,providing the corresponding product 4o in a stepeconomic manner.The preparation of selenides bearing heteroaromatic compounds is of great significance to the discovery and development of new drugs.Intriguingly,alkyl-substituted propiolic acid is feasible,which commonly displays low reactivity in previous copper-catalyzed cross-coupling reactions [41],and provided 4p in good yield,which highlights the versatility of this protocol.
The practical utility of the copper-catalyzed decarboxylative Se insertion coupling reactions was directly demonstrated in an efficient gram-scale synthesis (Scheme 4A),and the target product 3a was isolated in a 77% yield.Pyrrole derivatives,as nitrogencontaining heterocyclic compounds,widely exist in a series of bioactive molecules and solar materials [42].Simple pyrrole could be successfully alkynylselenated under the slightly adjusted reaction conditions (Scheme 4B).More importantly,Clofibrate-derived propiolic acid,a hypolipidemic agent clinically used for atherosclerosis (Scheme 4C),was examined under the standard reaction condition and provided the desired product 5b in useful yield.To examine the synthetic elaboration of alkynyl selenide a product(Scheme 4D),3a compound was selected to evaluate the knownN-methylation obtained the 5c.Upon treatment of 5c with thiophenol in the presence of CsOH [43],the hydrothiolated product 5d was isolated in 91%.
Encouraged by the novel reactivity of propiolic acids underlying this multi-component coupling,and we wanted to gain reliable insight into this mechanism and elucidate the role of each substrate (Scheme 5).At first,1.0 equiv.of TEMPO was added(Eq.1),the yield of 3a was dramatically reduced,while phenylpropiolic acid was completely consumed,and no TEMPO-captured product was detected by HRMS and NMR.This result suggested that a radical pathway could be excluded,and the reason for the steep decrease in the product yield was that the coordination of TEMPO with copper salt mainly inhibited the Se insertion reaction [44].Subsequently,the reaction of phenylpropiolic acid with selenium powder under the standard reaction conditions (Eq.2),1,2-bis(phenylethynyl)diselane and 1,4-diphenylbuta-1,3-diyne were detected by HRMS.However,the reaction between indole and selenium powder was not observed,and 92%of the raw indole was recovered (Eq.3).The sluggishness of this reaction indicates that decarboxylative selenation of propiolic acids followed by the alkynylselenation of indoles is likely to be a possible pathway for the current transformation.This proposed reaction path could be supported by the following stepby-step reaction (Eq.6).In addition,we also made two controlled experiments to explain the effect of IBAI.We prepared alkynyl iodide and tried a three-component reaction,but there was no product except a large amount of indole raw material and homo-coupling of (iodoethynyl)benzene byproduct (Eq.4).Under standard reaction conditions,the reaction of phenylpropargylic acid and tetrabutylammonium iodide could not generate (iodoethynyl)benzene (Eq.5).These experimental results indicate that(iodoethynyl)benzene is not a key intermediate.Finally,when stoichiometric (phenylethynyl)copper was used as substrate in the absence of CuCl2,the expected product 3a was isolated in 42% (Eq.7).Meanwhile,a catalytic amount of (phenylethynyl)copper was used as catalyst under the optimized reaction conditions,the reaction was carried out smoothly (Eq.8).These results clearly show that alkynylcopper is a competent intermediate in current transformation.
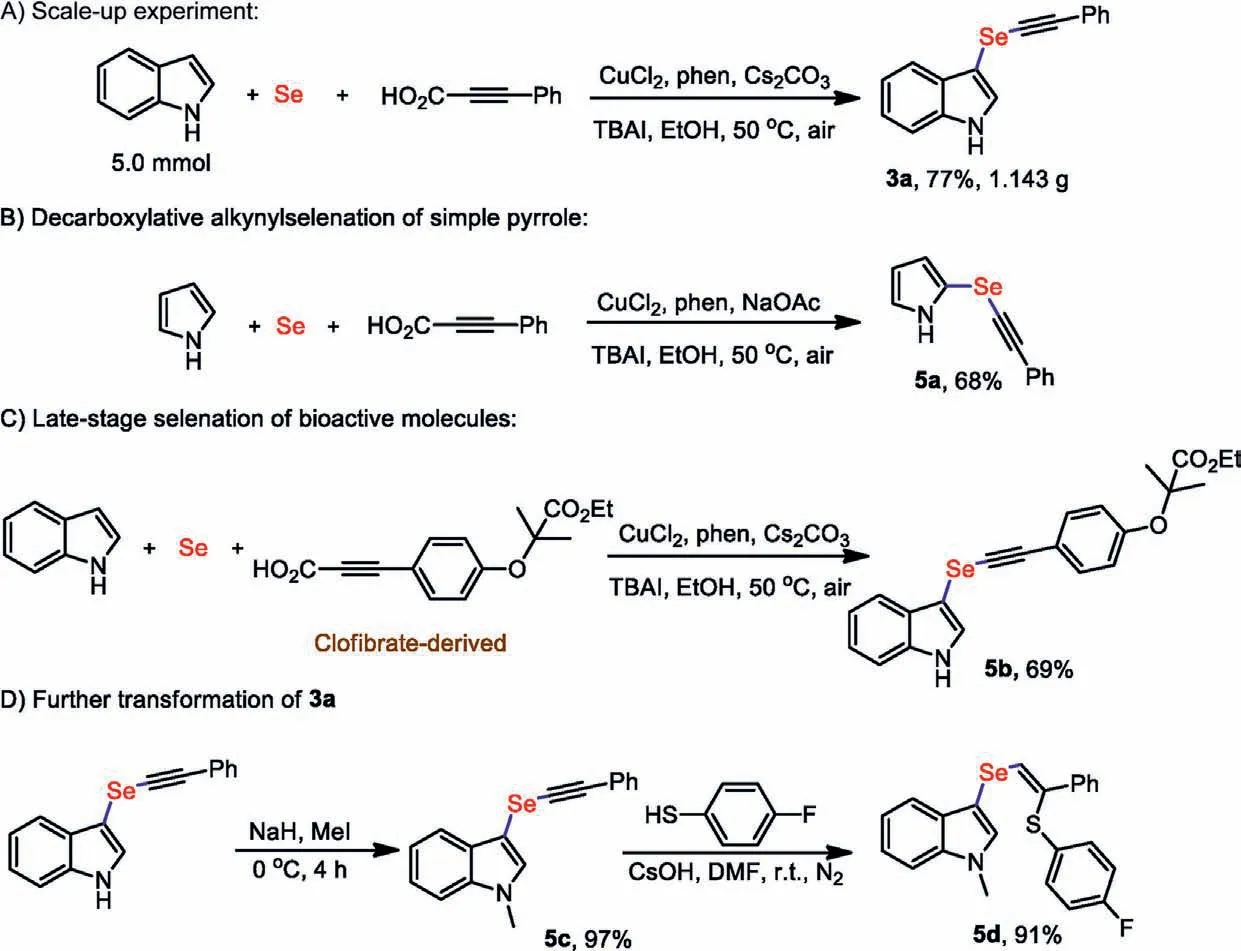
Scheme 4.Synthetic application.
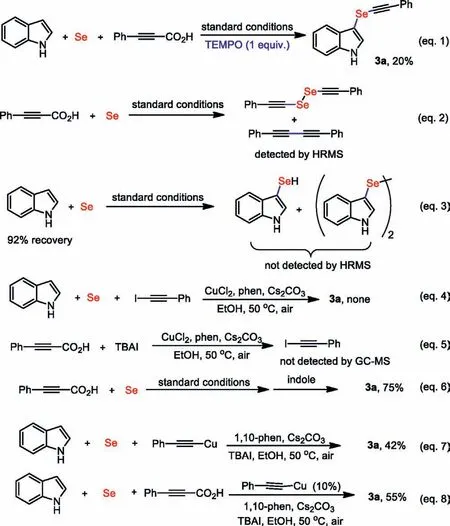
Scheme 5.Preliminary mechanism investigation.
A plausible reaction mechanism for copper-catalyzed threecomponent decarboxylative Se insertion coupling was proposed based on the above important experimental results and precedent literature (Scheme 6).Initially,the coordination of CuCl2with 1,10-phen to generate an active copper catalyst A,then ligand exchange with alkynyl carboxylic acids and decarboxylation to generate the alkynylcopper species C and releases CO2[45].Meanwhile,selenium anions and selenates are formed by the disproportionate reaction of selenium powder in the presence of base.The ligand exchange between intermediate C with Se2-afforded D,which was disproportionated with CuCl2or oxidation by O2to give Cu(III)species [46],and then reductive elimination to afford alkynylseleno anion E.Subsequently,the recombination of E with copper catalyst generates capable alknylselenocopper intermediate F.Then,the reaction of F with indoles produces the critical intermediate G in the presence of base [47,48].Finally,the reductive elimination of G provides the desired products in the presence of CuCl2and the generation of CuX catalyst to fulfill the catalytic cycle.
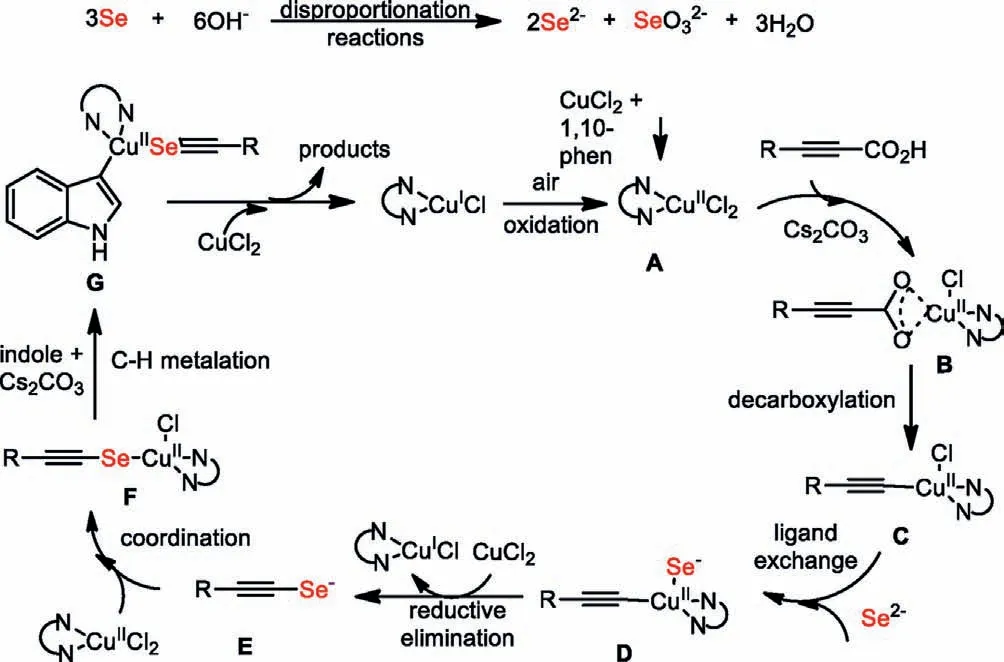
Scheme 6.Proposed mechanism.
In summary,we have developed a concise and straightforward copper-catalyzed decarboxylative alkynylselenation of indoles with propiolic acids using Se powder as selenating reagent.The key success of these multicomponent reactions was found to be the generation of nucleophilic alkynylseleno anions as the competent intermediate to suppress the unwished electrophilic polyselenation side reactions of propiolic acids.The transformation featured the use of air as an oxidant and alcohol as a greent solvent,prominent functional group compatibility and late-stage indolylselenation of small bioactive compounds,which would advance the research and development of selenium-containing drug molecules.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support from the National Natural Science Foundation of China (Nos.81803484,21602158),Zhejiang Provincial Natural Science Foundation (No.LY19B020011),Public Welfare Science and Technology Project of Wenzhou (Nos.Y20190132,Y20180233) are greatly appreciated.
 Chinese Chemical Letters2022年10期
Chinese Chemical Letters2022年10期
- Chinese Chemical Letters的其它文章
- An odyssey of lithium metal anode in liquid lithium-sulfur batteries
- Recent progress on preparation and applications of layered double hydroxides
- Two-dimensional transition metal chalcogenide nanomaterials for cancer diagnosis and treatment
- Emerging nanomedicine and prodrug delivery strategies for the treatment of inflammatory bowel disease
- Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment
- Recent advance of fluorescent probes for detection of drug-induced liver injury markers
