Interaction between gut microbiota and COVID-19 and its vaccines
John S M Leung
Abstract The whole world has been continuously afflicted by the coronavirus disease 2019(COVID-19) pandemic for the past 3 years. Many countries have tried many methods to control this virus infection with varying successes and failures. The gut microbiota is a biosystem spanning the entire length of the digestive tract and playing important roles in health and disease. It is much affected by COVID-19. In return it also substantially impacts infection. In particular, the gut microbiota has established a bidirectional interaction with the COVID-19 vaccines, enhancing or reducing vaccine efficacy by virtue of its varying components. Conversely,COVID-19 vaccines also make a substantial impact on the gut microbiota, reducing its overall population and biodiversity. It is hoped that by exploring and harnessing this bidirectional interaction we may break new ground and develop new methods to prevent and treat this formidable virus infection.
Key Words: Gut microbiota; COVID-19 vaccines; Vaccine interactions; Gut microbiota alterations
INTRODUCTION
The gut microbiota is a highly important and intriguing biosystem extending throughout the alimentary tract, covering an area of 400 square meters and with a biodiversity spanning over 2000 species,including protozoa, fungi, bacteria and viruses[1]. These organisms could be intraluminal or attached to the linings of the gut. Some even occupy an intracellular or subepithelial intercellular residence, while others enter the tissue fluid, the lymphatics and even the blood stream[1].
With such a background, the gut microbiota is constantly engaged in interactions with the various systems of the host and plays an important part both in health and in disease. In health, it is an integral part of digestion, absorption and nutrition. The contribution of gut microbiota in the production of vitamins of the B family and vitamin K has become common knowledge. More recently, it has been shown that gut microbiota, with their rich endowment of enzymes, could digest far more varieties of carbohydrates than their host could do alone[2]. Gut microbiota could also synthesize essential amino acids from inorganic nitrogen so that the host could survive even on a protein-free diet.
In fat metabolism, some bacteria are able to synthesize long chain fatty acids, contributing to the host energy supply as well as obesity, while many others, typically the strict anaerobes, ferment carbohydrates into short chain fatty acids (SCFAs). SCFAs inhibit histone deacetylase and activate Gprotein coupled receptors with benefits in antioxidant, anti-inflammatory, antitumorigenic and antidegenerative functions[3]. Indeed, research has shown the potential of fecal SCFA content as a marker of intestinal health, being at a lower level in colon cancervshealthy controls[4]. Further studies of serum free fatty acid profiles especially the SCFA profiles show distinct patterns for healthy colon, colonic adenomatous polyps, colon cancer and coeliac disease reflecting the differences of gut microbiota among these conditions[5].
It is beyond the scope of this review to cover all aspects of the gut microbiota in health and disease but rather to focus on the intriguing interaction between the gut microbiota and coronavirus disease 2019 (COVID-19). The COVID-19 pandemic has been continuously ravaging the whole world for the past 2.5 years. Up to August 22, 2022, the World Health Organization’s statistics showed the cumulative number of infected cases exceeded 211 million and was still increasing at 4.5 millionperweek, while the cumulative number of deaths had exceeded 4.4 million and was still increasing at around 68000perweek[6]. Various well-established methods of infection control have been tried, mostly with only partial success, and complete control remained elusive. Meanwhile, the phenomenon of “pandemic fatigue”has set in, and people become less inclined to adhere to control measures instead of tightening infection control measures[7]. In this review, aspects of gut microbiota that are relevant to the pandemic and its potential contribution to pandemic control was the focus.
GUT MANIFESTATION IN COVID-19 AND MICROBIOTA ALTERATIONS
The earliest report of COVID-19 in The Lancet on January 24, 2020 on the first 41 cases in Wuhan stated that diarrhea was the presenting symptom in only 1 case[8]. Three months later, when a New York center reported its first 393 cases of COVID-19[9], diarrhea was the presenting symptom in 23.7%. The incidence of diarrhea in the two districts remained almost unchanged over the next year with China at 3.80% diarrhea among 1141 cases[10]and New York at 20.14% diarrhea among 278 cases[11]. The difference is obvious and significant. Conceivably the diet in the Wuhan population is quite different from that in New York. It is probable that the gut microbiota in these two localities would also have a considerable difference, offering a plausible explanation of the increased diarrhea among the New York patients. In addition a Western diet, rich in processed meat but deficient in microbiota accessible carbohydrates, would have lower biodiversity[12]and less favorable to health. Unfortunately for the research investigator, most of these early studies did not report details of gut microbiota status, and the opportunity to study the influence of the gut microbiota on the early phase of the pandemic was lost.
GUT MICROBIOTA ALTERATIONS AND COVID-19 SEVERITY
By 2021, it became obvious that gut involvement and diarrhea were associated with greater severity of COVID-19[13]. An investigation in Hong Kong further demonstrated that certain components of the gut microbiota with immune-modulatory potential were depleted in severe and long-lasting COVID-19,notablyFaecalibacterium prausnitzii,Eubacterium rectaleand bifidobacterial species[14]. The investigators proposed that depletion of these bacteria could be taken as biomarkers predictive of severe and prolonged COVID-19. It is tempting to suggest that further studies might even explore the therapeutic value of replenishing these organisms to mitigate the disease.
GUT MICROBIOTA AND THE IMMUNE SYSTEM AND IMMUNE THERAPY
The interaction between the gut microbiota and the immune system goes far beyond the three groups of bacteria mentioned in the last section. In fact, beginning with the first colonization of the gut at birth or even before birth, the gut microbiota continuously evolve and influence the development of the host immune system, fostering reactivity against pathogenic invaders and tolerance towards harmless colonizers or beneficial symbionts[15]. Such actions are mediated by both regulatory cytokines (like interleukin-10 and interferon-beta) and T regulatory cells. This not only promotes diversity and increases beneficial microbes but actually helps to reduce host autoimmune disorders.
On the other hand, antibiotics have been shown to reduce the gut microbiota in both quantity and diversity, with reduced efficacy of immune checkpoint inhibitors in immunotherapy of cancer, and fecal transplantation has been shown to successfully restore the immune therapeutic response in such patients[16].
GUT MICROBIOTA’S IMPACT ON COVID-19 VACCINES: EFFICACY AND SIDE EFFECTS
With the foregoing background we now come to the important consideration of controlling the ongoing COVID-19 pandemic. It is common knowledge that pandemic control rests on five pathways: (1)Isolation of patients at the infectious stage by quarantines and social distancing; (2) Blocking the routes of infection by masking, air filtering/exchanging and sanitation; (3) Building up resistance among the population with vaccination; (4) Development of effective medicines to cure the infected patients and eliminate the carrier status; and (5) Letting the pandemic run its natural course, eliminating all susceptible components of the population and leaving those with inborn or naturally acquired immunity to survive.
The first four measures at present run into many obstacles, including social, economic, political, even personal egocentric considerations and biased sentiments. What is left is the fifth choice, otherwise called “herd immunity,” a rather primitive, counter-intuitive and inhuman approach. Fortunately,amidst these dark looming clouds appears a silver lining. The gut microbiota might not only modify the COVID-19 disease but actually improve the efficacy and reduce the side effects of its vaccines, winning more skeptics to accept this highly important preventive measure.
This seminal work, a combined effort of the two universities in Hong Kong, was reported by Nget al[17]and published on February 9, 2022[17]. It showed that for vaccine recipients of the inactivated virus,CoronaVac, the relatively low induction of neutralizing antibodies could be increased with a higher level ofBifidobacterium adolescentis(B. adolescentis) in the gut microbiota, whileBacteroides vulgaris,Bacteroides thetaiotaomicronandRuminococcus gnavuswere enriched in low responders. Another vaccine,the viral spike protein-encoded messenger RNA, BNT-162b, under the brand name Comirnaty, although capable of eliciting high antibody levels, could be further improved by the abundance of flagellate and fimbriate bacteria likeRoseburia faecis. In addition, for both vaccines, enrichment ofPrevotella copriand twoMegamonasspecies led to fewer side effects, likely due to the anti-inflammatory influence of these organisms.
Interestingly, the role ofB. adolescentisin CoronaVac seems very specific. So far, according to these researchers, any other species of the sameBifidobacteriumgenus tested would not work. As the speciesB.adolescentisis not present in commonly available health food or probiotic preparations, there seems no way to simply make an off-the-counter purchase of “health foods” to obtain such benefit. By contrast,the requirements of BNT-162b forRoseburia faecisseem less fastidious, and various bacteria with flagella and fimbriae might also impart benefit. EvenBacteroides thetaiotaomicron, known to be associated with low antibody production in CoronaVac, joins the company of antibody-enhancers for BNT-162b. This list may also include a minimal existence ofB. adolescentisbecause the only BNT-162b recipient who failed to develop adequate antibody level was entirely devoid ofB. adolescentis.
IMPACT OF COVID-19 VACCINES ON GUT MICROBIOTA: A BIDIRECTIONAL INTERACTION
Nget al[17]not only showed the impact of gut microbiota on vaccine efficacy but also showed the impact of vaccination on gut microbiota 1 mo after delivering two doses[17]. For CoronaVac, onlyBacteroides caccaewas increased. For BNT-162b2, bothB. caccaeandAlistipes shahiiincreased. Common to both vaccines, a large number of species were diminished includingAdlercreutzia equolifaciens,Asaccharobacter celatus,Blautia obeum,Blautia wexlerae,Dorea formicigenerans,Dorea longicatena,Coprococcus comes,Streptococcus vestibularis,Collinsella aerofaciensandRuminococcus obeum[17]. There seemed to be a substantial loss of biodiversity, but no further elaboration on the clinical and pathological significance was mentioned. With such substantial changes in the gut microbiota one would expect some alterations in bowel habits after vaccination. On a theoretical basis, vaccine-induced loss of diversity wouldincrease the opportunity of pathogens to thrive in the intestine. There would be less competition for nutrition and for the niche of bacteria habitat, with less antagonistic factors produced by healthy bacteria such as bacteriocin and SCFAs to discourage the growth of pathogenic organisms[18]. With the proliferation of pathogenic organisms, the chance of diarrhea would be increased. Minor changes in bowel habits, however, tend to be under-reported, and severe diarrhea would tend to be so uncommon that it is often underpowered to establish a statistically significant conclusion. Table 1 was constructed from data published online by the Centers for Disease Control and Prevention (United States).
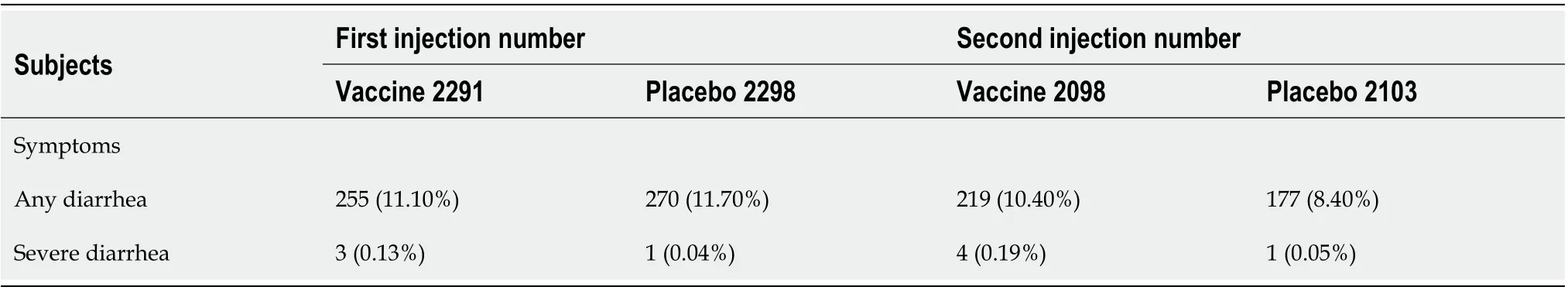
Table 1 Diarrhea in persons aged 19-55 years after Pfizer-BioNTech coronavirus disease 2019 vaccination based on The Centers for Disease Control online published table[23] under the title “Vaccines and Immunizations”
While there is no significant difference in mild diarrhea between recipients of vaccine and placebo, a signal of increased severe diarrhea among vaccine recipients seems to show up in the bottom row for both first and second injections, possibly reflecting the increase in severe diarrhea by 3.2 times (for first injection) to 3.8 times (for second injection) as a result of diminished diversity of gut microbiota. This table has two limitations. First, the actual number of severe diarrhea cases are too small for statistically significant computation. Second, no information is given for the composition of the microbiota of these patients, and it is not possible to relate the diarrhea to any particular organism or to the vaccine itself.
BIDIRECTIONAL INTERACTION BETWEEN GUT MICROBIOTA AND IMMUNE ACTIVITIES BEYOND COVID-19
Therapeutic agents, including vaccines, may have therapeutic value well beyond their originally intended effects. One of the best-known examples is the anti-tuberculosis BCG vaccine, whose role in protection against leprosy is well studied and documented[19]. For over 40 years it has also played a role in the treatment of non-muscle-invasive bladder cancer[20]. Indeed, many vaccines have been actively studied as a platform for anticancer treatment[21]. In certain areas, like hepatocellular carcinoma and carcinoma of the cervix, anti-viral vaccines have successfully prevented the cancer by preventing infection of the respective oncogenic virus. In other cancers, the mRNA technology has played a pivotal role in stimulating the patient’s immune system to recognize and react against tumorassociated neo-antigens[22].
For three decades scientists have been struggling to iron out technical obstacles and the overall reluctance of recruiting the body’s immune cells to produce an antigen and provoke an immune reaction, which is like retracing the steps of autoimmune disorders (the same sentiment still prevails among COVID-19 vaccine doubters today). Consequently, the predominant form of cancer immunotherapy at present is immune checkpoint inhibition, which involves abolishing major mechanisms of evasion of cancer from the host immunityviaimmune checkpoints. Even in this context, certain components of the gut microbiota, notablyBifididobacterium longumandAkkermansia muciniphilaare found to be associated with good response to checkpoint inhibitors in melanoma and lung cancer,respectively, while lowered diversity and population of the gut microbiota under antibiotic treatment would have the opposite effect[16]. When the COVID-19 pandemic broke out, the mRNA-based vaccines, backed by years of research in previous anticancer immunotherapy, had a chance to be tested extensively and speedily with resounding success.
CONCLUSION
As mentioned earlier in the study of Nget al[17], certain gut microbes could enhance vaccine efficacy in antibody stimulation while other microbes have the opposite effect[17]. Conversely, COVID-19 vaccines could impact the gut microbiota, enhancing the growth of some microbes but suppressing the growth of many others[17]. This bidirectional interaction between gut microbiota and COVID-19 vaccine is highly reminiscent of that between gut microbiota and anticancer immunotherapy. Conceivably, modifying the gut microbiota might enhance the vaccine-induced therapeutic value for both infection and cancer. It will take further study to understand and possibly harness such interactions and convert their potential therapeutic value into reality.
FOOTNOTES
Author contributions:Leung JSM is the sole author and contributed to the entire article.
Conflict-of-interest statement:JSM Leung has received no fees for serving as a speaker, holds no paid position for any organization, received no funding from any source. Nor is he an employee of any company or establishment. He owns no stocks or shares in any commercial company, nor any patency in any form or context.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:John S M Leung 0000-0001-9283-2733.
S-Editor:Fan JR
L-Editor:Filipodia
P-Editor:Fan JR
 World Journal of Gastroenterology2022年40期
World Journal of Gastroenterology2022年40期
- World Journal of Gastroenterology的其它文章
- Improving the prognosis before and after liver transplantation: Is muscle a game changer?
- Management of liver diseases: Current perspectives
- Pancreatic acinar cell carcinoma: A comprehensive review
- Curcumin alleviates experimental colitis via a potential mechanism involving memory B cells and Bcl-6-Syk-BLNK signaling
- Liver transplantation is beneficial regardless of cirrhosis stage or acute-on-chronic liver failure grade: A single-center experience
- Expression of the methylcytosine dioxygenase ten-eleven translocation-2 and connexin 43 in inflammatory bowel disease and colorectal cancer
