Pancreatic acinar cell carcinoma: A comprehensive review
Luis Fernando Calimano-Ramirez,Taher Daoud, Dheeraj Reddy Gopireddy,Ajaykumar C Morani,Rebecca Waters, Kazim Gumus,Albert Russell Klekers,Priya R Bhosale,Mayur K Virarkar
Abstract Acinar cell carcinoma (ACC) is a rare pancreatic malignancy with distinctive clinical, molecular, and morphological features. The long-term survival of ACC patients is substantially superior to that of pancreatic adenocarcinoma patients.As there are no significant patient series about ACCs, our understanding of this illness is mainly based on case reports and limited patient series. Surgical resection is the treatment of choice for patients with the disease restricted to one organ; however, with recent breakthroughs in precision medicine, medicines targeting the one-of-a-kind molecular profile of ACC are on the horizon. There are no standard treatment protocols available for people in which a total surgical resection to cure the condition is not possible. As a result of shared genetic alterations, ACCs are chemosensitive to agents with activity against pancreatic adenocarcinomas and colorectal carcinomas. The role of neoadjuvant or adjuvant chemoradiotherapy has not been established. This article aims to do a comprehensive literature study and present the most recent information on acinar cell cancer.
Key Words: Acinar cell carcinoma; Pancreas; Imaging; Immunohistochemical stains;Molecular features; Surgery; Chemotherapy
INTRODUCTION
The rising prevalence of abdominal imaging has resulted in an uptick in historically rare pancreatic cancer cases. The exocrine pancreas primarily comprises acinar and ductal cells[1]. Although acinar tissue accounts for most of the pancreatic organ, pancreatic neoplasms that mainly exhibit acinar differentiation are rare. The expression of various pancreatic enzymes, such as chymotrypsin, trypsin, and lipase, leads to acinar differentiation in the exocrine pancreas[1]. Most acinar neoplasms are solid,malignant, and not well-recognized[2]. Acinar cell cystadenoma is the sole benign acinar neoplasm documented; uncommon benign cystic, malignant cystic, and mixed carcinomas also exist.
Acinar cell carcinoma (ACC) of the pancreas is a rare, malignant tumor composed of cells with morphological resemblance to acinar cells and with evidence of exocrine enzyme synthesis by the neoplastic cells, accounting for 1%-2% of all pancreatic neoplasms[2-4]. ACC of the pancreas is a highgrade malignancy with no apparent site preference; it can occur in any section of the pancreas, but the head is the most common location.
Currently, there is no definitive course of treatment for ACC; however, vigorous surgical resection with negative margins has been linked to improved long-term survival[5]. Particularly with the use of computerized tomography (CT) and magnetic resonance imaging (MRI), as well as with the confirmation of fine needle aspiration (FNA) biopsies, early recognition and diagnosis are of particular relevance. Knowledge of these lesions' morphologic and immunohistochemical characteristics is also essential for a precise clinical diagnosis. In addition, extensive molecular analyses of acinar neoplasms have identified genomic alterations that may be amenable to specific therapies, thereby increasing the likelihood of widespread clinical molecular testing of these tumors.
This article aims to do a comprehensive literature study and present the most recent information on acinar cell cancer.
EPIDEMIOLOGY
ACC of the pancreas accounts for 1%-2% of all adult exocrine pancreatic neoplasms and 15% of pediatric neoplasms. ACC occurs most frequently in white males and is usually bimodal in age, with peaks in childhood at 8-15 years of age and adulthood with a peak incidence at 60 and is rare between the ages of 20 and 40. ACC has a 3.6 male-to-female ratio. No known racial relationships exist[1,6,7].
CLINICAL FEATURES
Most ACC s exhibit vague symptoms, which include weight loss (45%), abdominal pain (60%), back pain (50%), nausea and vomiting (20%), melena (12%), weakness, anorexia, and diarrhea (8%) are typical non-specific symptoms of ACC[8,9]. In distinction to ductal adenocarcinoma, ACC infrequently obstructs the bile duct and causes jaundice only in 12% of patients. ACC causes a mass effect and displaces those organs but does not infiltrate[10].
Some patients experience paraneoplastic disease known as "Schmid's Triad" or lipase hypersecretion syndrome due to increased lipase levels reaching over 10000 U/dL[11,12]. Elevated lipase may be the first sign of ACC. Patients may present with multiple nodular foci of subcutaneous fat necrosis,polyarthralgia may occur due to fat necrosis in the cancellous bone, and may have peripheral blood eosinophilia[13,14]. On imaging, fat necrosis within the bone can appear as a focal area of sclerosis.Paraneoplastic syndrome may occur following tumor recurrence. Most patients have hepatic metastases at presentation; however, some may present with only a bulky pancreatic mass. The serum lipase levels may normalize after surgical resection of the tumor, and patients may have symptomatic relief. Lipase may be used as a tumor marker in these patients. Alpha-fetoprotein (AFP) blood levels can be elevated in young patients[15].
MACROSCOPY (GROSS FEATURES)
On FNA, an abundance of cells with fluctuating degrees of acinar differentiation but no endocrine or ductal cells are seen. The aspirates consist primarily of cohesive fragments creating acini, cellular cords,or solid nests of neoplastic epithelium, composed of clusters of uniform cells with smoothly contoured,eccentric nuclei containing one or two prominent nuclei nucleoli[16,17].
ACCs can occur in any region of the pancreas and, on average, are 10 to 11 cm in size at the time of diagnosis[18-20]. The tumors are well circumscribed and have a soft, fleshy, tan to red appearance[2].They occasionally exhibit lobulated appearances, necrosis, and cystic degeneration[20]. On occasion, the neoplasm is found attached to the surface of the pancreas. Involvement of the duct system by ACCs can sometimes be observed grossly as polypoid or fingerlike projections extending from the primary tumor mass toward the duct[21].
PATHOLOGY
ACCs are cellular neoplasms with minimal stroma on microscopic examination. Multiple architectural patterns, including acinar (Figure 1A), solid (Figure 1B), glandular (Figure 1C), and trabecular(Figure 1D), can be observed. Solid and acinar patterns are the most prevalent. The acinar pattern is characterized by structures resembling normal acini, with small lumina and cells arranged in a monolayer with basally located nuclei. Large sheets of cells depict the solid pattern without lumina.Acinar structures with the dilated lumen characterize the less common granular pattern. The trabecular pattern, which resembles the architecture of well-differentiated neuroendocrine tumors, consists of more pronounced palisading with thinner, interlacing ribbons of cells. Multiple architectural patterns are visible within a single tumor.
IMMUNOHISTOCHEMISTRY
The immunohistochemical labeling of enzyme production from pancreatic cells aids in confirming the diagnosis of ACC (Table 1). There are commercially available antibodies for chymotrypsin, trypsin,lipase, and amylase, with the first three being the most frequently used in clinical settings (Figure 1E).These enzymes exhibit varying degrees of specificity and sensitivity. In approximately 95% of cases,trypsin and chymotrypsin reactivity can be demonstrated, and this combination is reportedly the most sensitive for diagnosing ACC[10,22].
ACC is also positive for CK8 and CK18, and as a result, they will label for AE1/AE3 and CAM5.2.CK7 (Figure 1F) and CK19, which are usually expressed in ductal adenocarcinomas, are generally missing in ACC but may be expressed in a subgroup of ACCs[15,23]. Focal labeling for chromogranin and synaptophysin is observed in a substantial proportion of ACCs, displaying the need to assess with further clear-cut acinar markers, especially in small samples, to avoid misidentifying an ACC as a neuroendocrine tumor or mixed acinar-neuroendocrine differentiation[10,24,25]. Recent research on histological and cytological samples has proven that the monoclonal antibody directed against the COOH-terminal region of the BCL10 protein, which identifies the COOH-terminal portion of CEL, is a highly specific and sensitive approach for detecting ACCs[26,27].
MOLECULAR FEATURES
The genetic alterations driving ACC have been thoroughly characterized and sequenced. The outcomes of these studies have emphasized the diversity of modified genes and pathways among various tumors,as well as the marked genomic instability in a subset of instances, including at the base pairs and chromosomes level. Several studies analyzed ACC for mutations in gene changed in pancreatic ductal adenocarcinoma (PDAC), the most prevalent subtype of pancreatic cancer, before the wide availability of whole genome and exome. Several of these studies reported uncommon mutations inTP53,KRAS,andSMAD4, in addition to the unusual loss of SMAD4 expression in acinar neoplasms, while others reported no modifications in these genes[28-33]. In these studies, the most prevalent ACC mutationsaffected WNT signalings, such as mutations in APC that rendered inactive and mutations in CTNNB1 that rendered it active; these alterations were found in roughly 20% of ACCs[30]. Recent sequencing studies of the entire exome have highlighted the diversity of mutated genes in the ACC[34,35]. Several other studies have shown substantial chromosomal gains and losses in ACCs and altered regions in various carcinomas; nevertheless, the target genes (if any) in these regions have not been detected[29,30,36,37].
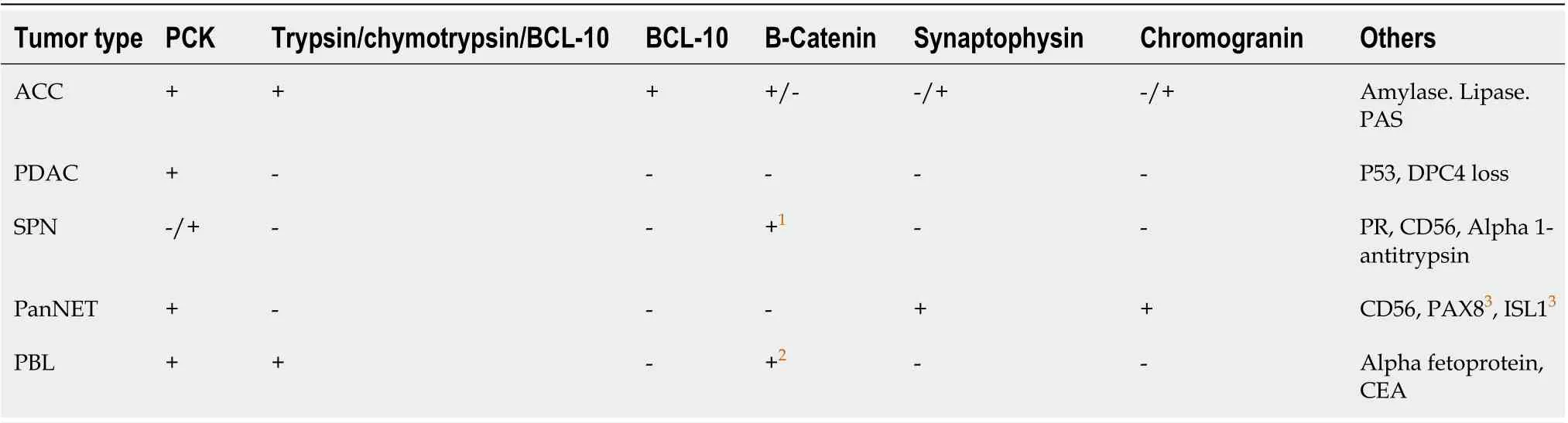
Table 1 Immunohistochemical profile from the differential diagnosis
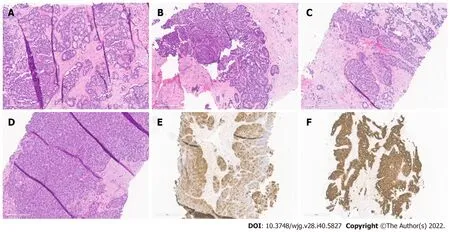
Figure 1 Histopathological patterns. A: Acinar cell carcinoma with the predominant acinar pattern. The acinar pattern is characterized by structures resembling normal acini, with small lumina and cells arranged in a monolayer with basally located nuclei; B: Acinar cell carcinoma with the predominant glandular pattern. Acinar structures with dilated lumina characterize the glandular pattern; C: Acinar cell carcinoma with the predominant trabecular pattern. The trabecular pattern is characterized by ribbons of cells resembling those of pancreatic neuroendocrine tumors; D: Acinar cell carcinoma with a predominant solid pattern. Large sheets of cells characterize the solid pattern without lumina; E: Acinar cell carcinoma, immunohistochemical staining for trypsin; F: Acinar cell carcinoma, immunohistochemical staining for cytokeratin 7.
Multiple potentially targetable genetic alterations in pancreatic ACC have been identified. A subset of cases exhibits genomic instability due to mutations in genes involved in DNA repairs, such asATM,BRCA1,BRCA2,PALB2, andMSH2[34,35]. According to various studies, microsatellite instability has been documented in fluctuating proportions of ACC, ranging from 7% to 14%[30,35,38]. Also, platinum agents and PARP inhibitors treat tumors with mutations in BRCA pathway genes[39,40]. Even though it has not yet been proven in major clinical trials, preliminary findings indicate that tumors with BRAF fusions react to MEK inhibitors, providing ACC with targeted therapy[41-43]. In a subgroup of ACCs,MYC abnormalities, including chromosome 8 polysomy and gene amplification, have been identified recently[44].

Table 2 Summary of pertinent differential diagnostic features
DIAGNOSIS
Laboratory analysis
Besides an increase in serum lipase levels associated with the syndrome of lipase hypersecretion, serum tumor markers are not consistently elevated in ACC. Even in the absence of lipase hypersecretion syndrome, lipase might be high. There have been numerous AFP increases, especially in younger patients[2]. Typically, Serum tumor markers such as carbohydrate antigen 19-9, alpha-fetoprotein, and carcinoembryonic antigen are expressed.
Radiological features (imaging)
In recent years, the incidence of uncommon pancreatic cancers has grown, likely due to the increased use of cross-sectional imaging[45]. In most instances, pancreatic cancers are incidentally found by CT.MRI is often used as a secondary test for further characterization. ACC of the pancreas is a well-defined,primarily oval or round exophytic mass in cross-sectional imaging[46,47]. Rarely have cystic variations been described. Typically, it manifests as a dense, largely solid tumor devoid of noticeable cystic alterations[46,48]. One-third of patients may exhibit calcifications, and the majority of the tumor increases constantly, but less so than the adjacent pancreatic parenchyma[49]. The tumor displays heterogeneous enhancement after intravenous infusion of contrast material[46]. Other studies also concluded that when confronted with a large mass with an ovoid shape, exophytic, well-circumscribed arising from the pancreas, with slight and persistent enhancement after contrast administration, and without appreciable biliary or pancreatic dilation, the diagnosis of ACC should be considered[18,47,50].Another study stated that MRI is better than CT for identifying tumor margination, intratumoral bleeding, tissue invasion, and ductal dilation[51]. On the other hand, CT is more sensitive to detecting central calcification and is commonly utilized due to its accessibility and quickness[50,51]. CT and MRI accurately depict most ACC imaging characteristics, and the two modalities correlate well[51].
Differential diagnosis
Pancreatic endocrine neoplasms that are well-differentiated, solid pseudopapillary neoplasms (SPNs),mixed acinar neoplasms, pancreatoblastoma (PBL), and PDACs should be included in the differential diagnosis (Table 2). Well-differentiated endocrine neoplasm, also commonly known as pancreatic neuroendocrine tumors (PanNET), is the entity most widely confused and misdiagnosed[2]. Both neoplasms typically contain a high number of cells and little amount of stroma, are capable of forming cell sheets and can consist of medium-sized, spherical, monomorphic cells with a microglandular or solid pattern of growth[2]. In addition, the red cytoplasmic zymogen and neurosecretory granules from ACC and PanNETs, respectively, are similar with appropriate differentiation. PanNETs are associated with a more favorable prognosis, making differentiation crucial. ACCs are distinguished from PanNETs by their primary acinar development, cytoplasmic granulation, basally oriented nuclei, and individual conspicuous nucleoli. However, hyalinized stroma, plasmacytoid cell appearance, and a "salt and pepper" chromatin pattern are indicative of PanNET[6]. Immunohistochemistry is advantageous, but it must be utilized with care. Using general neuroendocrine markers such as synaptophysin and chromogranin A alone may be unsafe because several ACCs contain neuroendocrine cells, which are particularly abundant in 30% of cases, as stated before[10,24]. Therefore, the demonstration of acinar cell products is mandatory if an ACC is suspected.
SPNs present as massive, dense pancreatic masses that are clinically identical to ACC; however, SPNs virtually entirely affect women of young age[52]. Cytological smears are characterized by delicately branched vasculature with lightly adherent cells generating pseudopapillary structures, a homogenous population of microscopic cells with cuboidal formation, and a unique hyaline myxoid matrix[52].Additionally, cytoplasmic hyaline globules are occasionally visible. Moreover, the SPNs nuclei are often normochromic, with numerous indentations, and lack the conspicuous nucleoli found in acinar cells[53]. Typically, SPNs exhibit nuclear immunoreactivity for β-catenin and high expression of CD10,which are also detectable in roughly 10% and 60% of ACCs, respectively. As a result, these two markers must be utilized in conjunction with markers specific to acinar cells, such as trypsin and BCL10[53-55].
PBL is another entity with stroma-poor acinar differentiation that must be evaluated in the differential diagnosis. Similar to ACCs, upon the appearance, PBLs are typically large, with an average size of 10 cm ranging from 1.5 to 20 cm[56]. This uncommon tumor shares nearly all of the cytologic and clinical characteristics of ACC, notably in the population of younger age, where it is frequently observed[2,57]. Cytologically, the neoplasms may be indistinguishable because they both display a predominant acinar-cell differentiation[2]. Heterologous components and squamous nests, such as osseous and cartilage development, characterize PBL[2]. In addition to immunochemistry and clinicopathologic data,the heterogeneous cytologic elements of the types of the tumor-cell present, including a combination of squamoid, mesenchymal, and glandular cells, are a general clue to diagnosing PBL[58]. Histologically,the presence of squamoid nests in PBL is the distinguishing characteristic of the two entities.
ACCs typically lack the characteristic cytomorphologic features of PDACs, allowing these two tumors to be distinguished easily. PDACs typically exhibit "drunk honeycomb" and "tombstone cells"arrangements alongside nuclear heterogeneity and nuclear silhouette abnormalities[59]. This is in contrast to the relatively homogeneous polygonal cells of ACCs, which are arranged in threedimensional arrays and tissue fragments[1]. ACCs lack the large desmoplastic stroma that describes PDACs and display an overall solid and acinar architecture, whereas PDACs feature a prominent desmoplastic stroma[10,59,60].
STAGING FOR ACINAR PANCREATIC CARCINOMA
The staging system for acinar pancreatic cancer is undergoing continuous development. Clinical staging is determined by resectability, which is heavily impacted by surgical expertise. The agreed guidelines for the resectability of surgical procedures continue to be refined (e.g., National Comprehensive Cancer Network, MD Anderson Cancer Center, American Hepato-Pancreato-Biliary Association, and International Hepato-Pancreato-Biliary Association). Traditionally, they are categorized as resectable,borderline resectable, locally progressed, and metastatic. To begin with, resectable tumors lack vascular involvement. Next, the borderline resectable tumors involve vascular, local structures, or other signs indicating a high risk of R1 resection. Local invasions (particularly vascular involvement) prevent surgical intervention for locally progressed malignancies. Lastly, metastatic cancer has spread to other organs beyond the main pancreatic tumor. The American Joint Committee on Cancer (AJCC) has classified staging by TNM (tumor, node, metastasis) classification using the consensus guidelines for resectability (Table 3 and Figure 2)[61]. Imaging cases with different AJCC TNM staging were added in chronological order (Figures 3 - 7) to appreciate the differences in changes.
PROGNOSIS, FACTORS OF PREDICTION, SPREAD, AND METASTASES
ACCs are exceptionally malignant neoplasms; relatively 50% of patients have metastasis during diagnosis[62]. Initial studies indicate that the prognosis for ACCs is better than that for ductal carcinomas but worse than that for PanNETs[5,63,64]. Previous research using population-based cancer databases and epidemiological methods indicated that individuals diagnosed with ACCs have a median overall survival time of roughly 47 mo for patients with localized disease and 14 mo for patients with metastatic disease, with a 5-year survival rate ranging from 36.2% to 72.8% for resected cancers[5,64,65].A recent study shows that ACC has a better prognosis than adenocarcinoma. They found that the prognosis was deteriorating with a decrease of 25% in a 5-year survival rate to previously reported rates[66]. However, data from a few studies published and anecdotal reports hint that some patients with ACCs have a greater survival rate than expected; unfortunately, no parameters are available to identify this group of patients[67,68].
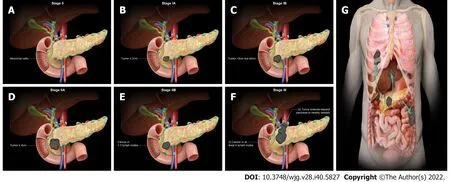
Figure 2 American Joint Committee on Cancer 8th Edition cancer staging for exocrine pancreatic tumor such as pancreatic acinar cell carcinoma. A: Stage 0 (TNM: Tis, N0, M0). Tis = Carcinoma in situ. N0 = No regional lymph node metastases. M0 = No distant metastasis; B: Stage IA (TNM: T1,N0, M0). T1 = Tumor ≤ 2 cm in greatest dimension. N0 = No regional lymph node metastases. M0 = No distant metastasis; C: Stage IB (TNM: T2, N0, M0). T2 =Tumor > 2 cm and ≤ 4 cm in greatest dimension. N0 = No regional lymph node metastases. M0 = No distant metastasis; D: Stage IIA (TNM: T3, N0, M0). T3 = Tumor> 4 cm in greatest dimension. N0 = No regional lymph node metastases. M0 = No distant metastasis; E: Stage IIB (TNM: T 1/2/3, N1, M0). T 1/2/3 = Tumor ≤ 2 cm >4 cm in greatest dimension. N1 = Metastasis in one to three regional lymph nodes. M0 = No distant metastasis; F: Stage III (TNM: T 1/2/3, N2, M0 or T4, Any N, M0).T 1/2/3 = Tumor ≤ 2 cm > 4 cm in greatest dimension. N2 = Metastasis in four or more regional lymph nodes. T4 = Tumor involves celiac axis, superior mesenteric artery, and common hepatic artery, regardless of size; G: Stage IV (TNM: Any T, Any N, M1). M1 = Distant metastasis. T = Primary tumor; N = Regional lymph node;M = Distant metastasis.

Figure 3 Stage IA (T1, N0, M0). A 77-year-old male patient with acinar cell carcinoma. Axial post-contrast portal-venous phase computed tomography image shows a solid mass (short arrows) measuring 1.8 cm × 1.6 cm and involving the region of the pancreatic head/body. Incidental findings are calcifications seen throughout the pancreas (likely related to changes in chronic pancreatitis) and mild dilatation of the biliary tree with pneumobilia (long arrow).
Rarely do patients with ACC are diagnose with lung, cervical lymph nodes, and ovary metastases.Metastatic illness is typically detected in regional lymph nodes and the liver[69]. Patients presenting as their first disease symptom with distant metastases are infrequent. It has been proposed that ACCs in patients younger than 20 may be less aggressive than in adults[10]. The most significant prognostic indicator is the tumor stage, with patients without lymph nodes or distant metastases having a better prognosis[5,70]. In addition, a poor prognosis is also associated with being male, being over 60, and having a tumor larger than 10 cm[5].
TREATMENT
Current, surgical, radiotherapeutic, and chemotherapeutic treatments used for PDAC are typically used to treat ACC (Table 4). Surgery is regarded as the optimal treatment modality for ACCs that areregionally circumscribed and resectable. Holenet al[8]found that median survival for surgically resected cases was 36 mo, compared to 14 mo for those without surgical resection[8]. Another systematic research by Glazeret al[71]indicated that the total median survival rate for patients with ACC who had resection was approximately 47 mo[71]. More aggressive therapeutic regimens must be sought when surgery is unavailable for locally advanced and metastatic diseases.

Table 3 American Joint Committee on Cancer 8th Edition cancer staging for exocrine pancreatic tumor such as pancreatic acinar cell carcinoma

large blood vessels2 Any N NX = Regional lymph nodes cannot be assessed N0 = No regional lymph node metastases N1 = Metastasis in one to three regional lymph nodes N2 = Metastasis in four or more regional lymph nodes M0 No distant metastasis IV Any T TX = Primary tumor cannot be assessed T0 = No evidence of primary tumor Tis = Carcinoma in situ1 T1 = Tumor is ≤ 2 cm in any direction T1a = Tumor is ≤ 0.5 cm in any direction T1b = Tumor is > 0.5 cm and < 1 cm in any direction T1c = Tumor is 1-2 cm in any direction T2 = Tumor is > 2 cm and ≤ 4 cm in any direction T3 = Tumor is > 4 cm in any direction T4 = Regardless of tumor size, the cancer has grown outside the pancreas, into the nearby large blood vessels2 Any N NX = Regional lymph nodes cannot be assessed N0 = No regional lymph node metastases N1 = Metastasis in one to three regional lymph nodes N2 = Metastasis in four or more regional lymph nodes M1 Distant metastasis 1Included in this category are high-grade pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm with high-grade dysplasia,intraductal tubulopapillary neoplasm with high-grade dysplasia, and mucinous cystic neoplasm with high-grade dysplasia.2Celiac axis, superior mesenteric artery, and/or common hepatic artery are involved.T: Primary tumor; N: Regional lymph node; M: Distant metastasis.

Table 4 Acinar cell carcinoma treatment options

Figure 4 Stage IB (T2, N0, M0). An 89-year-old male patient with acinar cell carcinoma. Axial post-contrast computed tomography arterial phase image shows a large mass in the body of the pancreas (arrow) measuring 3.3 cm × 3 cm. No regional adenopathy is identified.
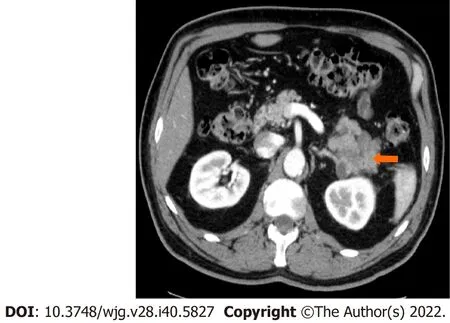
Figure 5 Stage IIA (T3, N0, M0). A 66-year-old patient with acinar cell carcinoma. Axial post-contrast computed tomography image in the arterial phase shows a solid lobulated mass measuring 6.5 cm × 5.4 cm arising from the tail of the pancreas (arrow). The mass is heterogeneous in density with areas of low-density and solid-enhancing areas. No regional or distant lymphadenopathy was detected.
Due to the rareness of ACC and the absence of sufficient randomized trials comparing different treatment approaches, the role of adjuvant therapy remains debatable. Table 5 enlists characteristics of studies on various regimes used for treating ACC. In a study at Memorial Sloan-Kettering Cancer Center, systemic chemotherapy for ACC was determined to be ineffective[8]. Following surgical resection, there are no clear treatment guidelines, and most adjuvant therapies are individualized with variable response rates. Lack of prospective data to create therapy guidelines and genetic variation in theAPCgene/β-catenin pathway observed in pancreatic acinar cells, ACC patients are frequently treated with chemotherapeutic agents known to be active against PDAC or colorectal cancer[72]. Most study participants received combination chemotherapy protocols based on gemcitabine or fluoropyrimidine[11,73]. If the ACC cannot be resected, the patient should undergo neoadjuvant or palliative 5-FU chemotherapy[70,74]. Combination fluoropyrimidine-based chemotherapies have increased disease control rates[8,11,73,75-77]. Glazeret al[71]systematic review showed that patients with a high-performance status typically receive folinic acid/fluorouracil/oxaliplatin or folinic acid/fluorouracil/irinotecan. In contrast, patients with a lower performance status typically receive gemcitabine/protein-bound paclitaxel[71].
Conventional fractionation and hypofractionated stereotactic body radiotherapy are used to "downstage" or convert a borderline resectable tumor to one that is resectable[73]. Patients who could potentially benefit from resection can be identified through the absence of ACC development following neoadjuvant radiotherapy. Several studies reported a "major response" rate from 25% to 35% of these individuals[8,11].
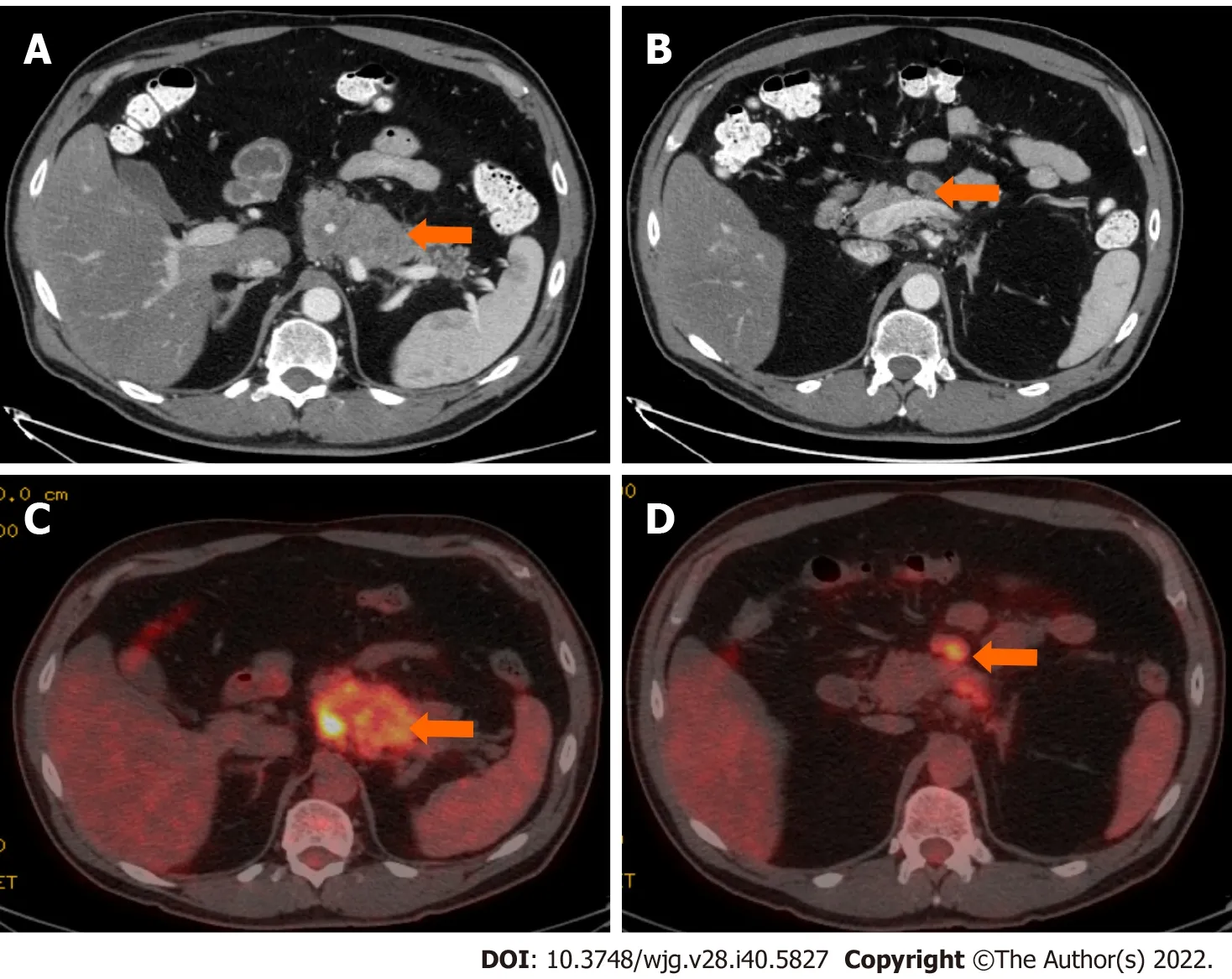
Figure 6 Stage IIB (T3, N1, M0). A 61-year-old patient with acinar cell carcinoma. A: Axial post-contrast computed tomography (CT) image in the portovenous phase shows an infiltrative mass arising from the pancreatic body and tail (arrow); B: axial post-contrast CT images in the portovenous phase shows an enlarged mesenteric lymph node (arrow) measuring 2.6 cm × 1.2 cm; C: Axial positron emission tomography/CT image in the portovenous phase shows hypermetabolic pancreatic body and tail mass (arrow); D: Axial post-contrast CT images in the portovenous phase shows hypermetabolic enlarged mesenteric lymph node (arrow).Pathology of the mass revealed Acinar cell carcinoma with a metastatic mesenteric lymph node.

Figure 7 Stage IV. A 64-year-old patient with acinar cell carcinoma. A: Axial post-contrast image port venous phase shows a pancreatic head mass measuring about 3.2 cm × 2.8 cm (arrow); B: Axial post-contrast image port venous phase shows multiple bilobar variable-sized hepatic metastatic lesions (arrows). An incidental finding is an area of splenic infarction (asterisk in image A).
CONCLUSION
Pancreatic acinar neoplasms are a molecularly and morphologically heterogeneous group of diseases far less prevalent than ductal neoplasms. Due to its low surgical resectability, high rate of recurrence, and high frequency of metastases at diagnosis, pancreatic ACC has been regarded as cancer with a poor prognosis. At least a subset of the neoplastic cells exhibits acinar differentiation, as defined morphologically (granular cytoplasm, single prominent nucleoli) or immunohistochemically. The molecular system underlying the advancement of ACCs has been elucidated, revealing potentially targetable mutations that offer optimism for developing additional curative options. ACC usually appears as a bulky mass that does not obstruct the pancreatic or bile duct and does not cause jaundice. Elevated lipase levels, subcutaneous nodules, and the presence of bone infarct should help clinch a diagnosis of ACC. Also, these tumors have large lymph nodes at presentation. In the future, molecular diagnostics to detect lesions susceptible to targeted therapy will likely play an important role in patient care.
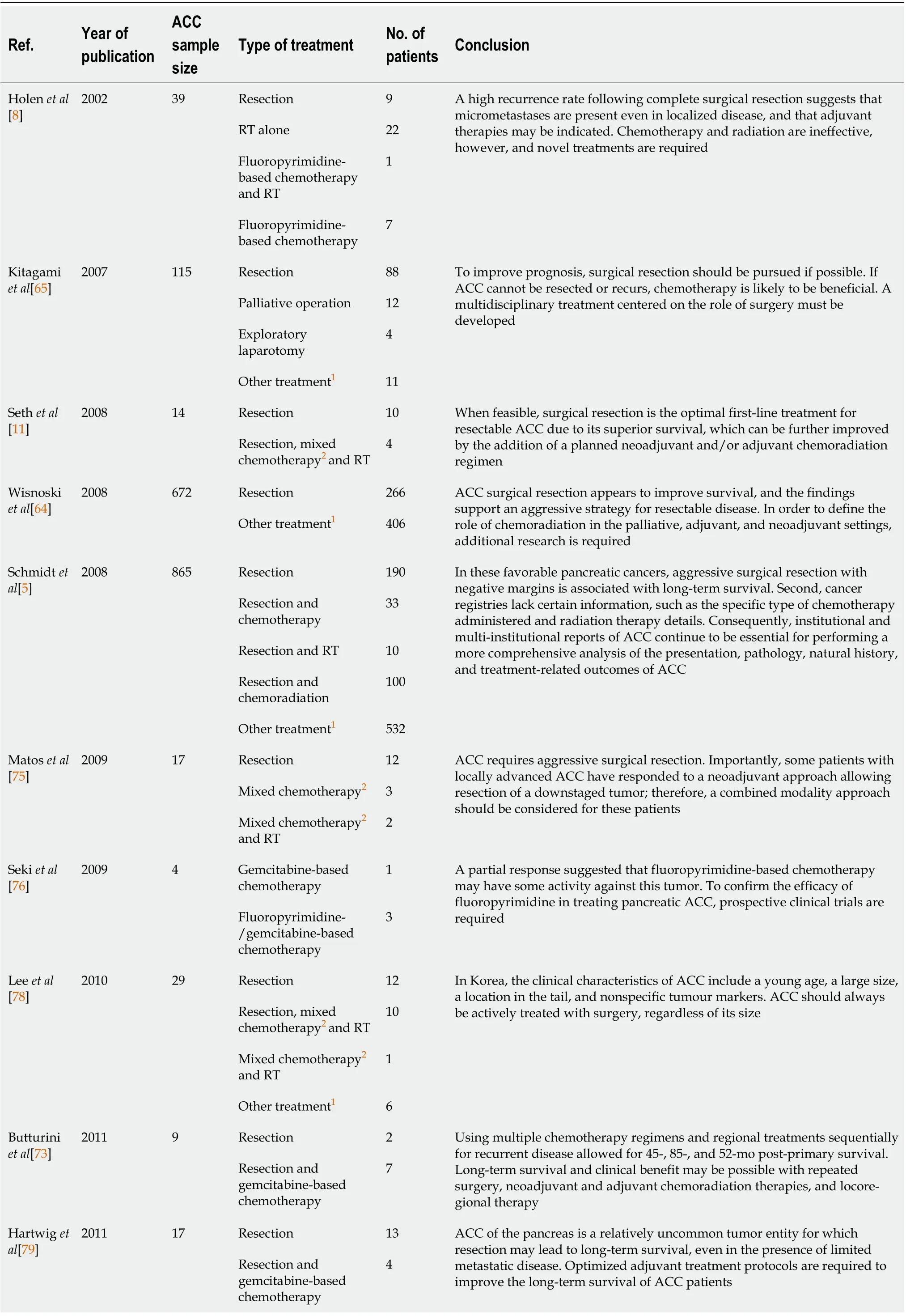
Table 5 Characteristics of studies on various acinar cell carcinoma treatment regimes

ACC: Acinar cell carcinoma; PDAC: Pancreatic ductal adenocarcinoma; RT: Radiotherapy; 5-FU: 5-fluorouracil; SOX: S1 + Oxaliplatin; AG: Albumin-bound paclitaxel + gemcitabine; FOLFIRNOX: 5-FU + oxaliplatin + leucovorin + irinotecan.
ACKNOWLEDGEMENTS
We thank Kelly Kage for the beautiful illustrations.
FOOTNOTES
Author contributions:All authors equally contributed to this paper with the conception and design of the study,literature review and analysis, drafting, critical revision, editing, and approval of the final version.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Luis Fernando Calimano-Ramirez 0000-0003-2599-3179; Taher Daoud 0000-0001-9161-1314; Dheeraj Reddy Gopireddy 0000-0001-8441-7804; Ajaykumar C Morani 0000-0002-2936-0291; Rebecca Waters 0000-0002-8163-7497; Kazim Gumus 0000-0002-1450-6868; Albert Russell Klekers 0000-0003-1202-7369; Priya R Bhosale 0000-0003-4014-4941; Mayur K Virarkar 0000-0002-5825-3102.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年40期
World Journal of Gastroenterology2022年40期
- World Journal of Gastroenterology的其它文章
- Heterogeneity of immune control in chronic hepatitis B virus infection: Clinical implications on immunity with interferon-α treatment and retreatment
- Expression of the methylcytosine dioxygenase ten-eleven translocation-2 and connexin 43 in inflammatory bowel disease and colorectal cancer
- Liver transplantation is beneficial regardless of cirrhosis stage or acute-on-chronic liver failure grade: A single-center experience
- Curcumin alleviates experimental colitis via a potential mechanism involving memory B cells and Bcl-6-Syk-BLNK signaling
- Management of liver diseases: Current perspectives
- Improving the prognosis before and after liver transplantation: Is muscle a game changer?
