Improvement effect of acupuncture on locomotor function in Parkinson disease via regulating gut microbiota and inhibiting inflammatory factor release
WANG Qiang (王强), WANG Yuan (王渊), LIU Zhibin (刘智斌), GUO Jie (郭婕), LI Jie (李杰),ZHAO Yingqian (赵颖倩)
1 College of Acu-moxibustion and Massage, Shaanxi University of Chinese Medicine, Xianyang 712046, China
2 Shaanxi Key Laboratory of Acupuncture and Medicine, Xianyang 712046, China
Abstract
Keywords: Acupuncture Therapy; Parkinson Disease; Neurologic Manifestations; Gastrointestinal Microbiome; Tumor Necrosis Factor; Mice
Parkinson disease (PD) is a neurodegenerative disorder, characterized by the loss of dopaminergic neurons and the presence of Lewy bodies caused by the accumulation of α-synuclein in the substantia nigra(SN)[1]. Constipation serves as the most prominent gastrointestinal dysfunction of PD and is estimated as a premotor symptom before classical locomotor dysfunctions[2]. Functional and structural changes of gastrointestinal tissues have been found in PD patients,such as the aggregation of α-synuclein in the enteric nervous system[3], impaired colonic mucosa barrier function[4], and bacterial invasion caused by gut microbiota dysbiosis[5]. The specific pathogenesis of PD remains unclear. Nevertheless, dopaminergic neurodegeneration is closely related to microgliamediated neuroinflammation[6]. Aggregated α-synuclein can directly activate nuclear factor kappa-B (NF-κB) in the microglia through toll-like receptor (TLR) 2 and promote the release of inflammatory cytokines to accelerate the damage of neurons[7-8]. Excessive stimulation to the innate immune system resulting from gut microbiota dysbiosis may accelerate extensive α-synuclein aggregation in the gut.
BRAAK H,et al[9]found that the presence of Lewy pathology in the enteric nervous system is earlier than it presents in dopaminergic neurons of the brain and PD symptoms, and α-synuclein can spread from the gastrointestinal tract to the brain via gut-brain axis[10].Gut microbiota disorder causes destruction of intestinal barrier and increases intestinal permeability, which not only affects intestinal mucous immunity, but also interferes with behaviors and brain functions[11-13].Growing evidence shows that gastrointestinal and locomotor dysfunctions are robustly correlated with the changes of fecal microbiota in the composition, and gut microbiome perturbation contributes to the pathogenesis of PD[14-16]. Moreover, alterations in gut microbiota composition and microbial metabolites may be involved in the pathogenesis and clinical phenotype of PD[17-18]. Therefore, regulating the gut microbiota composition may be a novel therapeutic option to postpone the onset and following cascade of neurodegeneration via the gut-brain axis.
Levodopa, a standard drug for PD, can relieve the symptoms[19]. However, levodopa drugs cannot completely prevent the progression of PD and have toxic effects on neurons[20]. Acupuncture is the third most common alternative therapy for PD[21-22]. It is believed that disruptions of energy flow can be adjusted by the stimulation to specific points on the body and thus regain the patient’s homeostasis to promote health[23]. However, acupuncture trials for locomotor dysfunctions and specifically for PD are quite limited.Considering that the disorder of gut microbiota is the initial risking factor of PD, and will lead to a series of immune and neuroendocrine disorders, we speculate that acupuncture can regulate gut microbiota and improve neuroinflammation, thereby helping with the locomotor function of PD.
1 Materials and Methods
1.1 Animals and interventions
According to the report of SARKAR S,et al[24],32 8-week-old male C57BL/6 mice [18±2 g of body mass]were purchased from the Experimental Animal Center of Xi’an Jiaotong University (China). The production license number is SCXK (Shaanxi) 2020-001. The mice were maintained (3 mice/cage, 12 h/12 h light/dark cycle) under environmentally controlled conditions with room temperature at 20.0±2.0 ℃ and humidity of 50%-65%, and provided with free access to food and water. Then the mice were randomly divided into a control group, a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) group, a MPTP +acupuncture (MPTP+A) group, and a MPTP + madopar(MPTP+M) group, with 8 mice in each group. Except the control group, mice in other groups received intraperitoneal injection of 25 mg/(kg·bw) MPTP (Sigma Aldrich, USA) once daily for 5 d[24].
To verify the hypothesis that acupuncture intervention may improve the SN of PD mice by adjusting the intestinal microbiota, the MPTPintervened mice were intervened by acupuncture once per day for 10 d. Acupuncture intervention parameters are shown in Table 1, and the treatment was performed with disposable sterile acupuncture needles (0.3 mm in diameter and 13 mm in length, Hwato Medical Instrument Company, China) inserted by 5 mm and retained for 5 min. In addition, another group of MPTP-intervened mice, as a positive intervention group,were intervened with levodopa + benserazide at a ratio of 4:1 [madopar, 125 mg/(kg·bw) per mouse] by gavage for 10 d.
The whole process followed the guidelines established by the Ethics Committee for the Care and Use of Laboratory Animals in the College of Acu-moxibustion and Massage, Shaanxi University of Chinese Medicine, and this experiment was approved with permit number AM2018-7041.
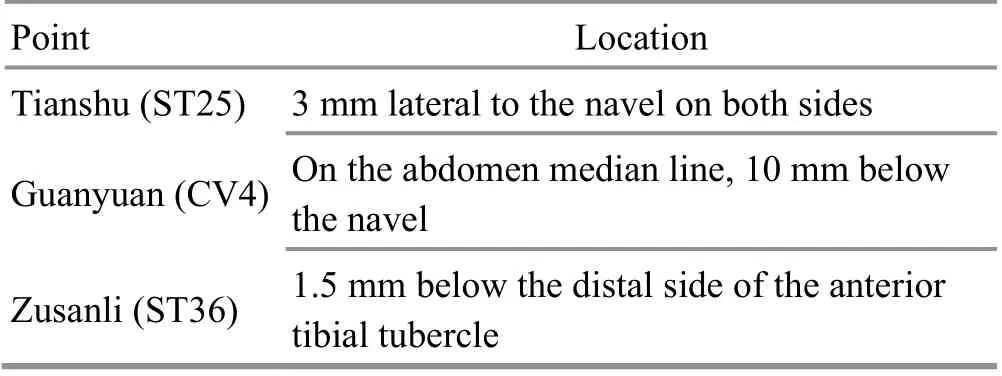
Table 1. Acupuncture intervention parameters[25]
1.2 Behavioral tests
On the 7th day of acupuncture or madopar intervention, the mice received behavioral training once daily for 3 d. The behavioral tests were performed on the first day after the last acupuncture or madopar intervention. The open-field test was employed to evaluate the spontaneous locomotor activities of animals[26]. The pole descent test was adapted from the protocol previously described[27]. The total time taken to climb down the pole was measured. Each animal performed 3 successive trials. The average of the 3 trials was calculated for each experiment.
1.3 Measurement of colon motility
One-hour stool frequency was measured in the initial status of MPTP-intervened mice, and after the intervention of acupuncture or madopar for 10 d.Assays were performed between 9 a.m. and 11 a.m.Each animal was removed from its home cage and placed in a clean and clear plastic cage without food or water. Stools were collected immediately after expulsion and placed in sealed 1.5 mL tubes. The total stools were weighed to obtain a wet weight, then dried overnight at 65 ℃ and weighed again to obtain a dry weight. The stool water content was calculated from the difference between the wet and dry stool weights.Results were normalized to body weight.
1.4 Sample collection and tissue preparation
Fecal samples were collected from MPTP-induced PD mice, madopar-intervened mice, and acupunctureintervened mice in empty autoclaved cages, where mice were allowed to defecate freely in the morning after the day of the last intervention. Samples were collected immediately and then frozen at -80 ℃, prior to DNA extraction. The mice were sacrificed after fecal collection for biochemical and histological tests. The tissues were quickly dissected and immediately stored at -80 ℃.
1.5 Real-time fluorescence quantitative polymerase chain reaction (RT-qPCR)
The inflammatory cytokines [tumor necrosis factor(TNF)-α, inducible nitric oxide synthase (iNOS),interleukin (IL)-1β and IL-6], TLR2, and lipopolysaccharide receptor CD14 (CD14) were analyzed by RT-qPCR with specific primers, as described in the previous study[14]. Briefly, the total RNA was isolated from the tissue samples and reversely transcribed into cDNA. The RT-qPCR was performed with SYBR®Premix Ex TaqTMqPCR SuperMix (Takara, China) on the ABI7500 real-time polymerase chain reaction (RT-PCR) machine with the two-step amplification protocol: 95 ℃ for 30 s, followed by 40 cycles of 95 ℃ for 5 s and 60 ℃for 60 s. The relative gene expression was normalized to internal control as β-actin.
Primers for TNF-α mRNA, IL-1β mRNA, IL-6 mRNA,iNOS mRNA, TLR2 mRNA, CD14 mRNA, and β-actin mRNA detection are shown in Table 2.
1.6 Enzyme-linked immunosorbent assay (ELISA)
The serum levels of inflammatory cytokines (TNF-α,iNOS, IL-1β, and IL-6) were detected using a commercial ELISA kit (Shanghai Elisa Biotech Inc., China). All experimental procedures were performed as recommended by the manufacturer.
1.7 Immunohistochemical and histopathological analyses
The brain sections were fixed using paraformaldehyde. After incubation with the primary antibody overnight at 4 ℃ (anti-tyrosine hydroxylase,Abcam, 1:1 000; anti-α-synuclein, Abcam, 1:1 000), the sections were incubated with goat anti-rabbit immunoglobulin G (IgG) secondary antibody (Boster Biological Technology Co., Ltd., China) for 30 min at 37 ℃. All procedures were performed according to the protocol of the immunoassay kit. The brain slices were stained concurrently with H&E prior to the histopathological assessment. Images were captured using a light microscope (Eclipse 80i, Nikon, Japan).
1.8 Western blotting (WB)
Standard WB procedures were carried out with the following antibodies: rabbit anti-myeloid differentiation factor (MyD) 88 (1:1 000, ab2064, Abcam, UK); rabbit anti-NF-κB p65 (1:1 000, ab32536, Abcam, UK); rabbit anti-Akt1 (phosphor S473, 1:1 000, ab215873, Abcam,UK); anti-β-actin (1:1 500, Sigma, USA), as previously described[28].
1.9 Amplification, sequencing of gut microbiota, and data analysis
Fecal microbial genomic DNA was extracted from 200 mg of each fecal sample using a FastDNA spin kit(Mobio, USA) according to the manufacturer’s recommendation. The V4 region of 16S rRNA gene was amplified with the forward primer 520F(5’-AYTGGGYDTAAAGNG-3’) and the reverse primer 802R (5’-TACNVGGGTATCTAATCC-3’) as described previously[29]. The V4 region of 16S rRNA gene amplicons followed the PCR condition: 95 ℃ for 3 min,then 30 cycles of 95 ℃ for 30 s, 55 ℃ for 30 s, 72 ℃for 30 s, and a final extension of 72 ℃ for 5 min. After being purified with an AxyPrepDNA kit (AXYGEN, Cat No.AP-GX-50) and quantified by QuantiFlour ST (Promega,USA), the PCR products were sequenced by the paired-end method on the Illumina Miseq platform according to manufacturer’s instructions. The 16S rRNA amplification and sequencing services were provided by TSINGKE Biological Technology Co., Ltd., China.
The raw sequencing data were filtered and trimmed according to the Phred score (the average Phred score<20 points) and then assembled by Flash software. The QIIME was employed to process the sequencing data and cluster the high-quality sequences with a 97%similarity to obtain operational taxonomic units (OTUs),and only those high-quality sequences without ambiguities were used for further analysis. The representative sequences of OTUs were annotated by comparing them to the Greegenes database (13.5). The biodiversity indexes of ACE, Chao, Shannon, and Simpson were computed from the number of OTUs to study the bacterial α-diversity. The principal coordinate analysis (PCoA) with unweighted UniFrac distance was performed to represent the β-diversity and similarity among the microbial communities.

Table 2. Primers for mRNA detection
1.10 Statistical analysis
Analyses were conducted with SPSS version 20.0 statistical software. Data were tested for normal distribution and homogeneity of variance. Data fitting normal distribution were presented as mean ± standard deviation (±s). One-way analysis of variance (ANOVA)was used to compare the differences among the groups,and the least significant difference (LSD) was used to compare the differences between two groups. Pairedt-test was used for self-comparison before and after the intervention. Rank-sum test was used if the data did not fit normal distribution.P<0.05 indicated that the difference was statistically significant.
2 Results
2.1 Effect of acupuncture on gastrointestinal and locomotor functions
To investigate the locomotor function after madopar or acupuncture intervention in MPTP-induced PD mice,we performed open-field text and pole descent test.Compared with the initial status of MPTP-induced PD mice, madopar- and acupuncture-intervened animals exhibited locomotor recovery in open-field and pole descent tests (Figure 1), indicating that madopar and acupuncture interventions can improve the locomotor defects of PD mice.
Compared with the control group, intestinal motility and fecal water content in the MPTP decreased. With respect to the MPTP-intervened mice, there was no significant difference in colon motility as assessed by one-hour stool weight or stool water content after madopar intervention. However, the intervention of acupuncture significantly enhanced the colon motility of MPTP-induced PD mice (Figure 2). Furthermore, the stool water content, another indicator of colon function,also increased after the acupuncture intervention(Figure 2). These results indicate that acupuncture intervention can recover the gastrointestinal function of PD mice caused by MPTP.
2.2 Acupuncture rescued tyrosine hydroxylase (TH)and α-synuclein pathologies induced by MPTP
MPTP-induced dopaminergic neuron injury is related to the loss of TH and aggregation of α-synuclein in the SN[30]. The expression of TH and α-synuclein in the SN is shown in the representative photomicrographs(Figure 3 and Figure 4). Compared with the control group, the expression of TH in the SN in the MPTP group decreased significantly, and the expression of α-synuclein in the MPTP group increased significantly.Although significantly decreased expression of α-synuclein was also detected in the SN of madoparand acupuncture-intervened mice (Figure 4), the intervention of acupuncture significantly increased the expression of TH in the SN (Figure 3). These data indicate that the MPTP-induced dopaminergic neuron injury is rescued by acupuncture but not madopar.
2.3 Effect of acupuncture on inflammation
The activated microglia and astrocytes can release pro-inflammatory mediators such as TNF-α, iNOS, IL-1β,and IL-6 to aggravate the neuroinflammation in MPTP-induced PD[6]. Madopar and acupuncture both reduced the infiltration of microglia and promoted the recovery of neurons (Figure 5). Compared with the MPTP-intervened mice, the anti-inflammatory reaction was observed in the madopar- and acupunctureintervened mice, with significantly decreased serum levels of inflammatory cytokines (TNF-α, iNOS, IL-1β,and IL-6) and significantly decreased expression levels of TNF-α, iNOS, IL-1β, and IL-6 in the SN. However, there was no difference between madopar and acupuncture interventions in anti-inflammatory effects on PD mice.Please see Figure 6 and Figure 7.
Since most TLR2 signaling pathways activate NF-κB through MyD88-dependent or MyD88-independent pathways, the expression of downstream TLR2 signaling was evaluated. The expression levels of TLR2 and CD14 in the SN were decreased after madopar and acupuncture interventions (Figure 8). Interestingly,there was no significant difference in the protein level of MyD88, but the expression of NF-κB p65 was significantly decreased after madopar or acupuncture interventions, suggesting that the effect of madopar or acupuncture on NF-κB is not dependent on MyD88(Figure 9 and Figure 10). Furthermore, the intervention of acupuncture significantly increased the expression of Akt1 in the SN, indicating that the anti-inflammation effect of acupuncture is related to activating the Akt-signal pathway.

Figure 1. Effect of madopar and acupuncture on locomotor functions

Figure 2. Effect of madopar and acupuncture on gastrointestinal functions

Figure 3. The tyrosine hydroxylase protein expression in the substantia nigra (immunohistochemical staining, ×400)

Figure 4. The α-synuclein protein expression in the substantia nigra (immunohistochemical staining, ×400)

Figure 5. Effect of madopar and acupuncture on the pathology of the substantia nigra (hematoxylin-eosin, ×400)

Figure 6. Contents of inflammatory cytokines in each group

Figure 7. Expression levels of inflammatory cytokines in each group

Figure 8. Expression levels of TLR2 and CD14 in each group

Figure 9. Expression levels of MyD88, NF-κB, and Akt1 in the substantia nigra
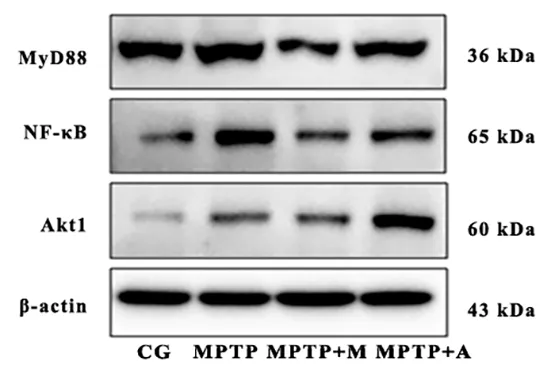
Figure 10. Expression levels of MyD88, NF-κB, and Akt1 in each group detected by Western blotting
2.4 Effect of acupuncture on the diversity and structure of fecal microbiota
Given the recent evidence that gut microbial dysbiosis occurs in MPTP-induced PD mice[15-16], and excessive stimulation of the innate immune system resulting from gut microbial dysbiosis may produce central nervous system (CNS) inflammation[31], the fecal microbiota analysis using Illumina HiSeq pyrosequencing was performed in this study. Multiple alpha diversity metrics of richness and diversity were used to reveal the distinctions among microbial populations. MPTP-induced PD mice showed a lower alpha diversity[15], but the alpha diversity metrics of richness (ACE and Chao 1) significantly increased in madopar- and acupuncture-treated mice. However,there was no significant difference in the alpha diversity metrics of diversity (Shannon and Simpson), (Figure 11 and Figure 12).
Beta diversity was further evaluated with PCoA based on the UniFrac metric to uncover the relationships of bacterial groups as well as their phylogenetic relatedness. In unweighted UniFrac PCoA, the composition of the bacterial population in each sample was shown in the multidimensional space using the explained variance of principal component analysis (PC)[PC1 (21.8%) and PC2 (16.5%)] to explain the similarity of microbial composition in each sample (Figure 11 and Figure 12). The MPTP-intervened mice had no significant difference compared with madoparintervened mice (P=0.055), but were significantly different from acupuncture-intervened mice at PC1-axis(P=0.033), indicating that there is a significant difference in the composition of fecal microbiota between the MPTP-intervened mice and acupunctureintervened mice (Figure 11 and Figure 12).

Figure 11. Effect of acupuncture on the diversity and structure of fecal microbiota

Figure 12. The diversities and structures of fecal microbiota in each group
2.5 Alterations in fecal microbiota associated with acupuncture and madopar
The composition of fecal microbiota from acupuncture-intervened mice clustering differently compared with MPTP-intervened mice was additionally confirmed by Hierarchical clustering of the samples based on the Bray-Curtis distance (Figure 13-Figure 16).Bacteroidetes,Firmicutes, andProteobacteriawere the three most dominant phyla detected in the feces of MPTP-induced PD mice. MPTP-induced fecal microbial dysbiosis was characterized by a lower relative abundance ofFirmicutesand a higher relative abundance ofProteobacteria[15-16], which is consistent with the observations of human subjects with PD[18,32].Compared with MPTP-induced PD mice, the intervention of acupuncture significantly increased the relative abundance ofBacteroidetesand decreased the relative abundance ofFirmicutesandCyanobacteria(Figure 13-Figure 16). However, there was no significant difference between the MPTP-intervened and madoparintervened mice at the phylum level.

Figure 13. Relative abundance of fecal microbiota in each group

Figure 14. Alterations in the family level of fecal microbiota associated with acupuncture and madopar

Figure 15. Alterations in the genus level of fecal microbiota associated with acupuncture and madopar
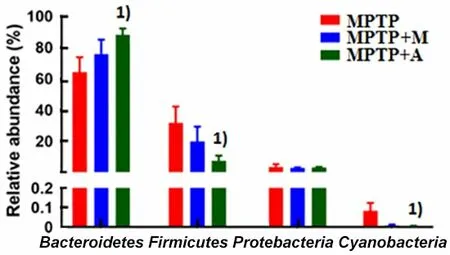
Figure 16. Alterations in the phylum level of fecal microbiota associated with acupuncture and madopar
Some bacteria sharply varied from the family and genus classification level, but the effect of acupuncture on fecal bacteria of MPTP-induced PD mice was distinct from that of madopar. Specifically, the intervention of acupuncture significantly decreased the relative abundance ofLachnospiraceae,Ruminococcaceae,Desulfovibrionaceae, andRhodospirillaceae, but increased the relative abundance ofBarnesiellaceaeandClostridiaceae1 at the family level (Figure 13-Figure 16). However, the intervention of madopar only significantly increased the relative abundance of
BarnesiellaceaeandEubacteriaceaecompared with MPTP-intervened mice. At the genus level (Figure 13-Figure 16), the intervention of acupuncture significantly decreased the relative abundance ofLachnospiraceae
unclassified,Flavonifractor,Oscillibacter,Oscillospira,Desulfovibrio,Anaerotruncus,ClostridiumIV,Butyricimonas,Insolitispirillum, andBlautia. However,the intervention of madopar significantly increased the relative abundance ofAnaerostipes,Erysipelotrichaceae-unclassified,Porphyromonas, andEubacterium. Furthermore, the relative abundance ofPrevotellaceae-unclassifiedwas increased after the intervention of either acupuncture or madopar.
3 Discussion
PD is a neurodegenerative disorder. Gastrointestinal dysfunction is the most common non-locomotor symptom of PD. The loss of dopaminergic neurons and the presence of Lewy bodies in the SN pars compacta are the main pathological features of PD[33]. The α-synuclein is the main component of Lewy corpuscles,and the abnormal aggregation of α-synuclein plays an important role in the development of PD[34].
In our study, the locomotor dysfunction and aggregation of α-synuclein were improved by madopar intervention, but there was still a significant reduction of dopamine neurons in the SN. Consistent with the result of OLANOW C W,et al[35], madopar replacement therapy can improve the symptoms of MPTP-induced PD mice, but cannot prevent the progression of PD.However, the symptoms of locomotor and gastrointestinal dysfunction were efficaciously improved after the acupuncture intervention,accompanied by the recovery of dopamine neurons,and a decrease of α-synuclein in the SN. There were no differences in the TLR2-downstream MyD88 or Akt1 after the madopar intervention, but madopar inhibited the expression of NF-κB and decreased the levels of inflammatory cytokines. So, we consider that the inhibition effect of madopar on microglia-mediated neuroinflammation may be related to the reduction of α-synuclein.
Interestingly, there was no significant difference in the MyD88 between MPTP-intervened and acupuncture-intervened mice, but acupuncture significantly increased the expression of Akt1. TLR2 regulates NF-κB through different mechanisms,including the TLR2-MyD88 pathway of activation and the TLR2-mediated inhibition of PI3K/Akt pathway[36].Therefore, acupuncture can inhibit NF-κB-mediated neuroinflammation by activating the Akt signal pathway.Defects in the Akt signaling are one part of the neurodegenerative process of both Alzheimer disease and PD[37-38]. The Akt signaling is important in the actions of dopaminergic receptors[39]and the homeostatic regulation of dopaminergic transporters[40].Furthermore, drugs targeting the dopaminergic system are neuroprotective via Akt activation in PD mice[41-42].Certain Akt1 haplotypes may act in a protective role against PD[43], which has been corroborated in autosomal recessive Parkinsonism caused by Akt gene mutations[44]. In concordance, the expression of constitutively active Akt protects against dopaminergic cell deaths[45]. Therefore, the Akt signal pathway may be a neuroprotective target of acupuncture on PD.
LAI F,et al[46]have shown that neurotoxin MPTP causes gastrointestinal dysfunction and intestinal pathology prior to locomotor dysfunction, which supports the BRAAK staging theory that the pathogenesis of PD starts from the gut to the brain[9].Here, we showed that the gut microbiota from acupuncture-treated mice was different from MPTP-induced PD mice.
Therefore, acupuncture may improve locomotor function in PD by regulating gut microbiota.Acupuncture and madopar both can increase the relative abundance ofBarnesiellaceaeat the family level andPrevotellaceae-unclassifiedat the genus level,but the alteration in fecal microbiota associated with acupuncture was more significant than that associated with madopar. Especially, the acupuncture intervention decreased the relative abundance ofLachnospiraceaeandRuminococcaceae, which was significantly increased in MPTP-induced mice[16].
Gut microbial dysbiosis-activated intestinal immune system will release the inflammatory cytokines to regulate the CNS through the vagus nerve[47]. Moreover,metabolites from gut microbiota can activate TLR in the intestinal nervous system and CNS[48]. The nerve inflammation response of microglia is reliant upon CD14,which acts together with TLR4 and TLR2 to activate intracellular signaling and promote cytokines’secretion[49-50]. Therefore, acupuncture can regulate the gut microbiota to activate TLR2-Akt1 signaling,improving locomotor deficits and neuroinflammation in MPTP-induced PD mice.
Although this study did not directly confirm the sequence of onset of the changes of intestinal flora and motor symptoms in PD mice from the existing evidence,certain intestinal flora disorders are indeed related to neuroinflammation and certain signal pathways. As for acupuncture intervention, whether neuroinflammation affects the intestinal flora or whether it reduces neuroinflammation by adjusting the intestinal flora still needs in-depth research.
In conclusion, we found that the locomotor functions of MPTP-induced PD mice were improved by the intervention of acupuncture or madopar. The activation of Akt may be a neuroprotective target of acupuncture in treating PD.
Conflict of Interest
There is no potential conflict of interest in this article.
Acknowledgments
This work was supported by Natural Science Basic Research Program of Shaanxi Province (陕西省自然科学基础研究计划, No. 2020JM594, No. 2017JM8108).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria.
Received: 19 January 2021/Accepted: 29 September 2021
 Journal of Acupuncture and Tuina Science2022年5期
Journal of Acupuncture and Tuina Science2022年5期
- Journal of Acupuncture and Tuina Science的其它文章
- Efficacy of mild moxibustion combined with surgery for meniscal injury and its effect on TGF-β1 and PDGF levels in the fluid of knee joint
- Effects of acupuncture plus medication on hippocampus SIRT1 and FOXO3a expression, MDA content, and SOD activity of rats with Alzheimer disease
- Effects of Mo-Rubbing abdomen manipulation on glucose metabolism and inflammatory factors in rats with type 2 diabetes mellitus
- Observation on the therapeutic efficacy of Tuina plus “three-bridge” exercise for non-specific low back pain
- Influence of herbal cake-partitioned moxibustion on lumbar functions and inflammatory factors in patients with lumbar disc herniation due to kidney deficiency and blood stasis
- Effect of acupuncture-like transcutaneous electrical nerve stimulation on labor pain in nulliparous women: a randomized controlled trial
