Diagnosis of hepatic inflammatory pseudotumor by fine-needle biopsy
Min Lin, Lan Cao, Jianwei Wang, Jianhua Zhou
Department of Ultrasound,Sun Yat-Sen University Cancer Center,State Key Laboratory of Oncology in South China,Collaborative Innovation Center for Cancer Medicine,Guangzhou, China
Keywords:Hepatic inflammatory pseudotumor Fine-needle biopsy Hepatic perfusion disorder
ABSTRACT Hepatic inflammatory pseudotumor(IPT)is a benign lesion characterized by chronic infiltration of inflammatory cells and fibrosis that clinically, radiologically, and pathologically mimics malignancy. However, the epidemiology of IPTs remains unclear. IPTs are often misdiagnosed as malignant lesions because of the lack of characteristic features. We present the case of a 32-year-old man that was misdiagnosed as intrahepatic cholangiocarcinoma by CECT,CEMRI,and CEUS,which was finally confirmed as IPT by fine-needle liver biopsy.In this report, the key factor in the diagnosis of liver inflammatory masses was the presence of hepatic perfusion disorder.
1. Introduction
Hepatic inflammatory pseudotumor(IPT)is a rare,benign lesion that mimics malignancy clinically,radiologically,and pathologically.Thus,it is often misdiagnosed by imaging examinations.1We present a case of IPT that was misdiagnosed based on CECT, CEMRI, and contrast-enhanced ultrasonography (CEUS) findings. The masses were finally diagnosed correctly by fine-needle biopsy evaluation, and they gradually reduced in size after antibiotic treatment.
2. Case report
This study was approved by our institutional review board. The requirement for patient informed consent was waived for this retrospective study. A 32-year-old man was admitted to our hospital with a diagnosis of “liver cancer with intrahepatic and pulmonary metastases,pulmonary inflammation”after a health check-up at another hospital.He had a history of chronic hepatitis B infection.On CT, these liver lesions showed focal arterial phase hyperenhancement (APHE) in the arterial phase(AP)and“washout”in the portal venous phase(PVP)and delayed phase. Intrahepatic cholangiocarcinoma and multiple pulmonary metastases were suspected based on the CT findings(Fig.1 a–i).Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging provided a similar suggestion(Fig.1 j–o).
CEUS was performed using the SonoVue®.Grayscale US revealed two heterogeneous hypoechoic lesions with blurred edges (Fig. 2); their diameters were 71 mm (I) and 32 mm (VI). During the enhanced scan,these lesions showed heterogeneous APHE, early washout, and marked washout in the delayed phase (features that meet the criteria for CEUS LR-M) (Fig. 3). Based on these results, the masses were suspected to be malignant.
Laboratory tests revealed an increased WBC count (13.3 × 109/L;normal range, 4–10 × 109/L). Liver function tests revealed multi-fold increases in ALT, AST, and GGT levels. Serum AFP, CEA, and CA19-9 levels were within the normal ranges, but the PIVKA-II level was elevated(131.92 mAU/mL;normal range,5–40 mAU/mL).
US-guided fine-needle biopsy revealed chronic inflammation with infiltrating lymphocytes and eosinophils,fibrous tissue hyperplasia,and hepatocyte atrophy.These findings suggested that the lesions should be diagnosed as inflammatory pseudotumor(IPT).When asked whether he had a history of infection,the patient recalled a history of fever without obvious inducement about 20 days before attending our hospital. After intravenous administration of antibiotics, his WBC count and levels of serum transaminases and PIVKA-II gradually decreased to normalcy.Two CEUS scans were performed after 10 and 40 days,which revealed gradual shrinkage of the liver lesions(Fig.4).CT revealed a marked reduction in the size of the lung lesions.
After reviewing the CEUS video of the AP again, an interesting phenomenon was observed.The final area of hyperenhancement was larger than the lesion range on the grayscale scan at the early stage of AP,showing perilesional enhancement,and the washout area was also larger in the PVP and late phase.This observation suggested hepatic perfusion disorder(HPD)around the lesions.
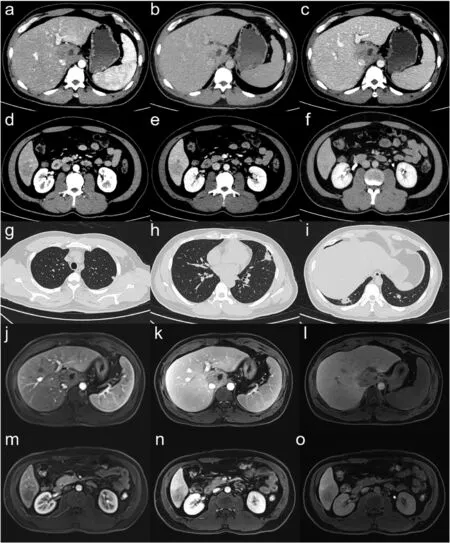
Fig.1. CT and MRI scan of the liver lesions and lung lesions.(a–c)CT images of the lesion in section I in the arterial phase(a),portal venous phase(b),and delayed phase(c). (d–e) CT images of the lesion in section VI. (g–i) CT images of lung lesions. (j–l) MRI T1W images of the lesion in section I in the arterial phase (j), portal venous phase (k), and hepatobiliary phase(l). (m–o) MRI T1W images of the lesion in section VI.

Fig.2. B-mode and CDFI scan of the liver lesions.The greyscale images demonstrate the presence of lesions in both I and VI,which were heterogeneous hypoechoic lesions with blur edges. CDFI shows no significant color signal. (a–b) Images of the lesion in I. (c–d) Images of the lesion in VI.
3. Discussion
Inflammatory pseudotumor(IPT)is a benign lesion characterized by chronic infiltration of inflammatory cells and fibrosis.1The epidemiology of IPTs remains unclear, but possible etiologies include congenital diseases, trauma, infection, biliary inflammation, vascular conditions, and autoimmune disorders.2IPTs are often misdiagnosed as metastatic or primary liver tumors because of their diverse imaging findings and lack of characteristic features.3The definitive diagnosis of IPTs depends on needle biopsy findings. The most common symptoms of IPTs are abdominal pain and fever, but approximately 15.6% of patients are asymptomatic.4The most common CT and MRI findings can be summarized as an obscure boundary and a delayed hyperattenuating mass with an internal hypoattenuating component.5Some lesions regressed spontaneously,and some regressed after antibiotic treatment.In our case,CEUS findings indicated LI-RADS M,but differentiation from malignancy was difficult,which led to a tumor biopsy.
The liver has a unique dual blood supply from both the hepatic artery(25%)and portal vein(75%).6The arterial and portal venous supplies to the liver are not independent systems.There are several communication routes between the vessels,including the transsinusoidal,transvasal,and transplexal routes. When vascular compromise occurs, there are often changes in the volume and direction of blood flow in the individual vessels.7When the ratio of the dual blood supply changes,the area may show different enhancements.These conditions are known as HPDs.On MRI, HPD areas are undetectable on T1WI and T2WI according to the isointensity on diffusion-weighted images,but enhanced scanning shows hyperintense signals on AP images and isointense or hyperintense signals on PVP and delayed phase images.8On CEUS, the affected area around the lesion appears as an area of hyperenhancement on AP images and returns to normal on PVP images. This reflects an enhanced redistribution of arterial flow to the surrounding liver parenchyma,usually due to decreased portal or hepatic venous flow, resulting in an arterioportal shunt.The causes of such perfusion disorders are portal vein obstruction,liver cirrhosis, hepatic neoplasms, hepatic trauma, hereditary hemorrhagic telangiectasia, hepatic vein obstruction, steal phenomenon by hypervascular tumors, inflammatory changes, aberrant blood supply,hepatic parenchymal compression, etc.9The hepatic perfusion index,which is defined as the ratio of the gradient of hepatic arterial to total hepatic blood flow,has been proven to be sensitive to differences in liver perfusion associated with established metastatic liver disease on imaging and can be used as a histopathological indicator of prognosis in some cases.10,11HPD can be divided into vessel abnormalities, hepatic focal lesions,hepatic perfusion disorders,and other causes.HPD also occurs in acute inflammatory diseases of surrounding organs, such as acute pancreatitis and cholecystitis.12–14A possible mechanism of HPD is the reduction in portal blood flow,which causes hepatic arterial hyperemia and increased venous drainage because of the inflammatory process. In hepatic focal inflammatory lesions, such as liver abscesses, characterization of lesions solely by grayscale US or noncontrast CT may be challenging due to the mass-like appearance. CEUS often reveals irregular rim enhancement of the inflammatory lesion in the AP and PVP,with the possibility of hyperenhancement in the surrounding liver parenchyma (thought to be due to perilesional hyperemia), which was also seen in our case.15
4. Conclusion
It is difficult to distinguish IPTs from other hepatic malignancies.HPD of the lesion surrounding the area is helpful in identifying inflammatory lesions.Fine-needle biopsy should be performed in cases that are difficult to identify.

Fig. 3. CEUS images of I lesion at 14 s (a), 30 s (b), 2 min (c), and 3 min 53 s (d) from the SonoVue® injection. The lesion appeared heterogeneous arterial phase hyperenhancement (APHE) quickly after the injection and started to show a lower enhancement compared with the surrounding liver parenchyma at 30 s after the injection. It showed a marked washout in the late phase.
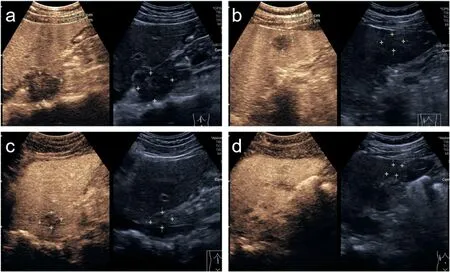
Fig.4. CEUS images of the lesions at 10 days(a,b)and 40 days(c,d)after the first scan.(a,c)The lesion in segment I.(b,d)The lesion in segment VI.The patient was treated with antibiotics and the lesions gradually receded.
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Interventional Medicine2022年3期
Journal of Interventional Medicine2022年3期
- Journal of Interventional Medicine的其它文章
- A review of high-intensity focused ultrasound as a novel and non-invasive interventional radiology technique
- Ultrasound-guided microwave ablation for symptomatic adenomyosis: More areas of concern for more uniform and promising outcomes
- Balloon-occluded retrograde transvenous obliteration with lauromacrogol sclerosant foam for gastric varices
- A canine model of aortic arch aneurysm created with autologous pericardium
- Recent advances of multimoda ultrasound in image-guided prostate-targeted biopsy
- Efficacy of contrast-enhanced ultrasound-guided percutaneous core needle biopsy in anterior mediastinal masses
