Efficacy of contrast-enhanced ultrasound-guided percutaneous core needle biopsy in anterior mediastinal masses
Peili Fan, Jiaying Cao, Yunjie Jin, Hong Han, Wenping Wang,Huixiong Xu, Zhengbiao Ji,*
a Department of Ultrasound, Zhongshan Hospital, Fudan University, No.180 Fenglin Road, Xuhui District, 200032, Shanghai, China
b Shanghai Institute of Medical Imaging, No.180 Fenglin Road, Xuhui District, 200032, Shanghai, China
c Institute of Ultrasound Medicine and Engineering, Fudan University, No.180 Fenglin Road, Xuhui District, Shanghai, 200032, China
Keywords:Biopsy Ultrasound Contrast agent Core needle Mediastinal neoplasm
ABSTRACT
1. Introduction
The mediastinum is the general name for the organs,structures,and connective tissue between the two pleural sacs, while the anterior mediastinum is defined as the narrow gap between the sternal body and the anterior wall of the pericardium. Anterior mediastinal masses(AMMs)are the most common group of mediastinal tumours(54%),and 25–50%of AMMs are malignant.1AMMs have a low incidence and high heterogeneity. Early accurate diagnosis is important for the selection of treatment strategies.2Although non-invasive imaging techniques,such as computed tomography (CT), magnetic resonance imaging (MRI), and contrast-enhanced ultrasonography (CEUS), are widely used to distinguish AMMs and determine tumour extension,3,4such imaging is still not sufficient to make an accurate differential diagnosis.Treatment strategies are largely based on pathological results.
As early as 1992, ultrasound (US)-guided percutaneous core needle biopsy(PCNB)of AMMs had been reported to be a safe,cost-effective and reliable method and a good alternative to the traditional biopsy techniques via mediastinoscopy or thoracotomy.5The use of US-guided PCNB has several advantages, such as real-time imaging and dispensability of radiation exposure.First,continuous monitoring of the needle confirmed that the biopsy specimen was representative.Second,vascular structures(especially the internal thoracic artery) can be avoided more easily through the biopsy route. Furthermore, the rate of occurrence of lung injuries is very low. However, US-guided PCNB of AMMs also has disadvantages. Gas is a major barrier interfering with the ultrasonic imaging.Indeed,it is impossible to view the lesion when lung tissue is located between the US transducer and lesion. This limits US-guided PCNB of AMMs as only suitable for lesions directly contacting the thoracic wall or protruding into the thoracic outlet.
CT-guided PCNB is another common approach that is not restricted to lung tissues.The diagnostic yield of the US-guided approach is similar to that of the CT-guided approach,but it has fewer complications,including pneumomediastinum, pneumothorax, hemoptysis, hemothorax, and hematoma formation. In prior studies, the complication rate of the CTguided approach ranged from 2% to 17%, while that of the US-guided approach was consistently close to zero.6–9
The main reason for the diagnostic failure of US-guided PCNB is insufficient sampling due to necrotic or fibrotic tissue,especially in large lesions. CEUS has obvious potential in US-guided interventional procedures.CEUS can accurately delineate tissue vascularization.Biopsy of necrotic tissue can be avoided by directing the biopsy needle toward the contrast-enhanced areas within the target lesion.10
Herein,we conducted a retrospective study to compare the diagnostic yield,diagnostic accuracy,and complication rate of CEUS-guided vs.USguided PCNB for AMMs.
2. Material and methods
2.1. Patients
This retrospective study was approved by the ethics committee of our institution. Written informed consent was obtained from each patient before the CEUS and biopsy procedures. We reviewed the data from an institutional biopsy database and patients’ medical records between January 2015 and December 2021 in a single center.Three hundred and one consecutive patients with AMMs detected on chest CT were admitted to our department for a pre-biopsy US examination. Sixteen patients cancelled their original biopsy plans because the lesions were not visualized via the intercostal space by conventional US,or were smaller than 2.5 cm. One patient was cancelled because the lesion was completely cystic, as examined by CEUS. Finally, 284 patients (166 men, 118 women; mean age, 43.0 ± 18.4 years; age range, 13–82 years) were included in our study. According to the actual situation of the retrospective data, all the patients were divided into two groups: US-guided and CEUS-guided groups.
2.2. Pre-biopsy US examination
Before biopsies, patients were examined in the supine position.Conventional US scanning (Philips HD15, USA; Medison Acuvix V10,Korea;Mindary DC-8,China)or CEUS(Philips HD15,USA;Mindary DC-8,China)was performed with a low-frequency convex array probe in all lesions.
Conventional US examination was performed to observe the lesion's location,size,internal echo,and adjacent relationship with the heart and the large vessels.Color Doppler flow imaging was performed to observe the intralesional and surrounding vascularity of the lesions. The parenchyma of the lesion with relatively abundant blood supply was selected for biopsy.In this procedure,it is important to pay attention to the large vessels on the biopsy route,such as the internal mammary and intercostal arteries. “Internal necrosis” on conventional US was defined as a hypoechoic or anechoic area with a clear boundary from the surrounding tissue without a blood flow signal.
CEUS was performed after a bolus injection of 2.0 mL SonoVue®via a 20-gauge intravenous catheter in the cubital vein. Enhanced areas and necrosis(areas without enhancement)of the lesion were identified,and the enhanced areas were selected for biopsy.
2.3. Biopsy technique
PCNB was performed or supervised by a trained interventional ultrasound doctor with 3–20 years of experience. The biopsy route and device were selected at the discretion of the doctor. After the skin was sterilized,local anesthesia in the form of 2%lidocaine was administered.PCNB was performed with a 16-gauge/18-gauge fully automatic (Bard Medical, USA) or semi-automatic (CareFusion, USA) core needle device(Max cutter length 22 mm). The complete biopsy procedure was monitored under real-time US guidance to adjust the route. In the CEUSguided group, the target area for biopsy was the enhanced area selected from the previous CEUS examination.Two core specimens were obtained for each patient. If the parenchyma of the core specimens was too small,an additional one or two core specimens were obtained.Biopsy specimens were placed on a small piece of filter paper and stored in a specimen bottle containing 10% formalin for further histological diagnosis. Post-biopsy, the patients were observed for a minimum of 2 h in bed.
2.4. Diagnosis yield and accuracy
Pathological classification of the anterior mediastinal lesions was performed based on the World Health Organization tumour classification(2015 version).11Each biopsy was classified according to the following diagnostic results: malignancy, benignity, indeterminate result, or non-diagnostic result,based on the diagnosis of two trained pathologists with 5–20 years of experience.Non-diagnostic results were defined when an insufficient tissue sample was obtained via PCNB for a specific diagnosis.For example,the pathological examination revealed only blood or coagulative necrosis. Precise diagnosis of malignancy or benignity was considered the histologic result of PCNB in accordance with surgical pathology or clinicoradiologic diagnosis. Indeterminate results were defined as cases where the mediastinal lesion was not clear, whether benign or malignant, despite sufficient tissue sample.12

2.5. Statistical analysis
All data are expressed as mean ± standard deviation (SD). The difference between the two groups was analyzed using the independent samples t-test and the chi-square test. All statistical analyses were performed using the SPSS software (version 20.0; SPSS Inc.). Statistical significance was set at P <0.05.
3. Results
Ultimately,284 patients who underwent 285 biopsy procedures were enrolled, comprising 133 in the US-guided group and 151 in the CEUSguided group. In the US-guided group, one patient underwent two biopsy procedures (one US-guided and one CEUS-guided). Two hundred and sixty-three cases had a definite pathological diagnosis (114 cases confirmed by surgical pathological diagnosis and 149 cases confirmed by clinicoradiological diagnosis) (Fig. 1). The predominant final diagnoses in this study were thymoma (29.7%, 78/263), lymphoma (20.5%, 54/263), thymic carcinoma (13.3%, 35/263), and germ cell tumours(13.3%,35/263)(Table 1).
There was no significant difference in patient age, sex, number of percutaneous biopsies, or display rate of internal necrosis on conventional US between the US-guided and CEUS-guided groups.In the CEUSguided group,internal necrosis was observed in 72.2%(109/151)of the lesions after contrast agent injection,which was significantly higher than that before injection(41.7%,63/151;P <0.001)(Table 2).In the CEUSguided group,the display rate of internal necrosis was significantly lower in thymomas (40.5%, 17/42) than in lymphomas (63.9%, 23/36; P =0.039)and thymic carcinoma(95.2%,20/21;P <0.001)(Fig.2).The use ratio of the 18-gauge core needle was significantly lower in the CEUSguided group than in the US-guided group (3.3%, 5/151 vs. 21.8%,29/133;P <0.001)(Table 2).All tissue samples obtained using 18-gauge core needles provided a precise diagnosis. The diagnostic yield of the CEUS-guided group was significantly higher than that of the US-guided group (100% [151/151] vs. 89.5% [119/133]; P <0.001). There was no significant difference in diagnostic accuracy between the CEUSguided and US-guided groups (97.3%, 145/149 vs. 97.4%, 111/114; P=1.000).In the CEUS-guided group,one case was initially diagnosed as hyperplasia of the thymus gland by biopsy pathology, but was finally diagnosed as a seminoma with a large necrotic area by subsequent surgical pathology. In the US-guided group, one case underwent re-PCNB with CEUS guidance 5 days later. The result of the specimen first obtained by US-guided PCNB was non-diagnostic because of only coagulative necrosis in the pathological examination.The second result of the specimen obtained by CEUS-guided PCNB was diagnosed as a germ cell tumor confirmed by surgical pathology(Fig.3).
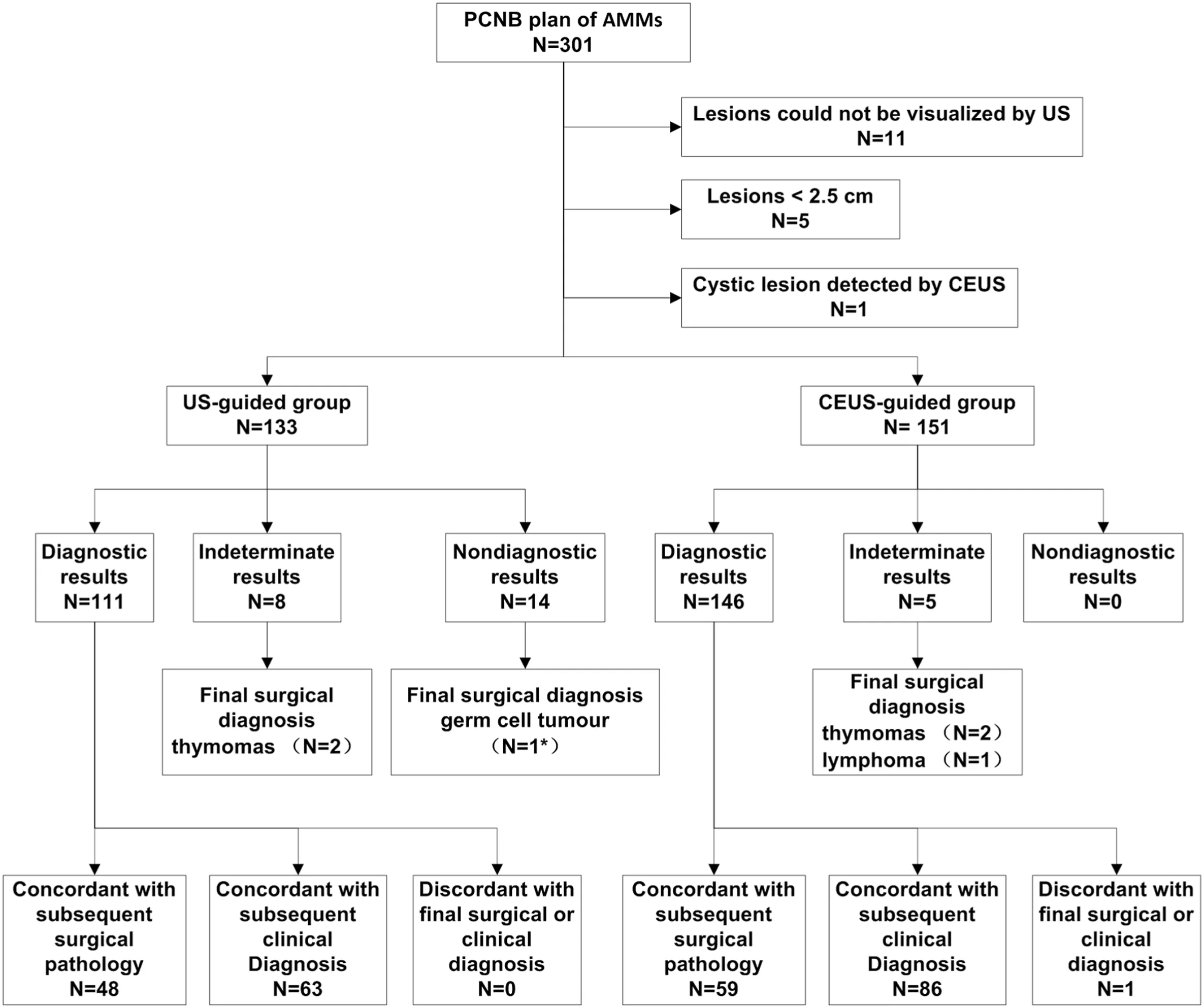
Fig. 1. Flow diagram shows the algorithm for the final diagnostic results of 284 patients with US-/CEUS- guided percutaneous core needle biopsy of AMMs. PCNB:percutaneous core needle biopsy. US: ultrasound. CEUS: contrast-enhanced ultrasound. *One case underwent re-PCNB by CEUS-guided.
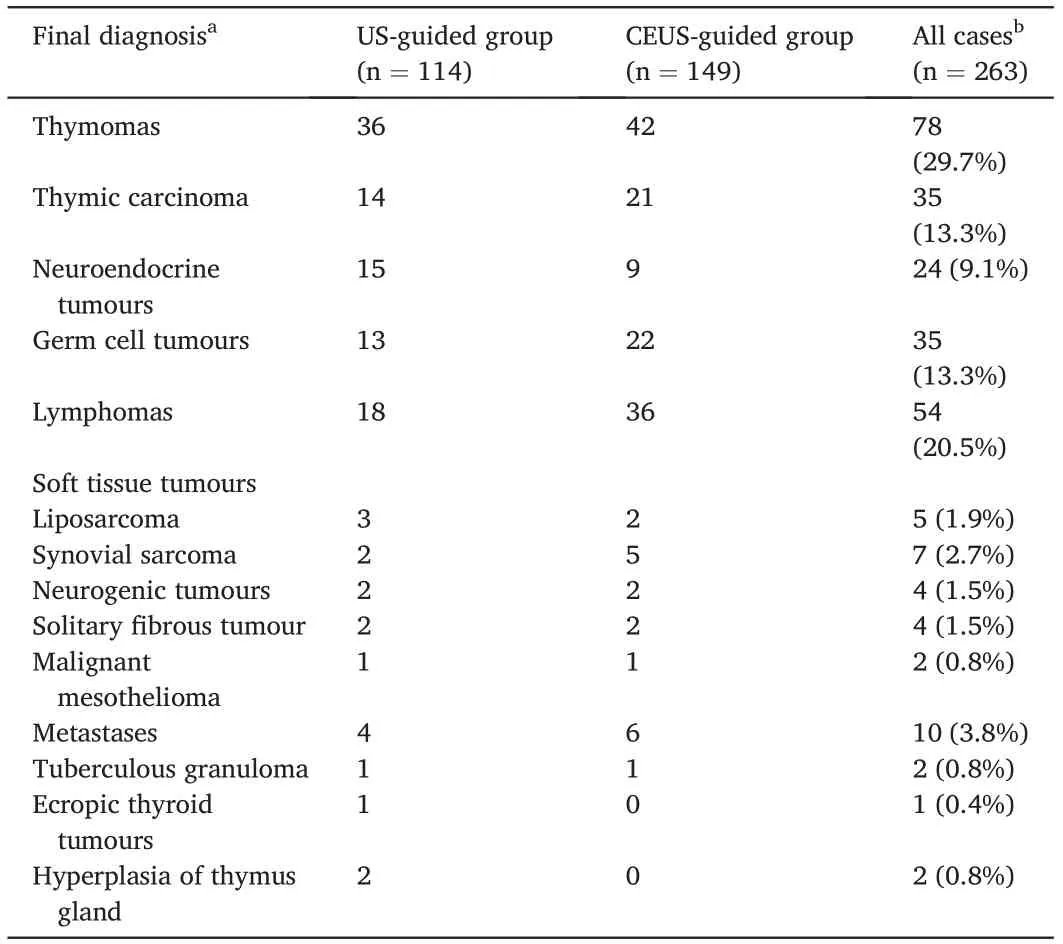
Table 1 Final diagnosis confirmed by surgical pathology or clinic-radiologic diagnosis of the US-guided and the CEUS-guided groups.
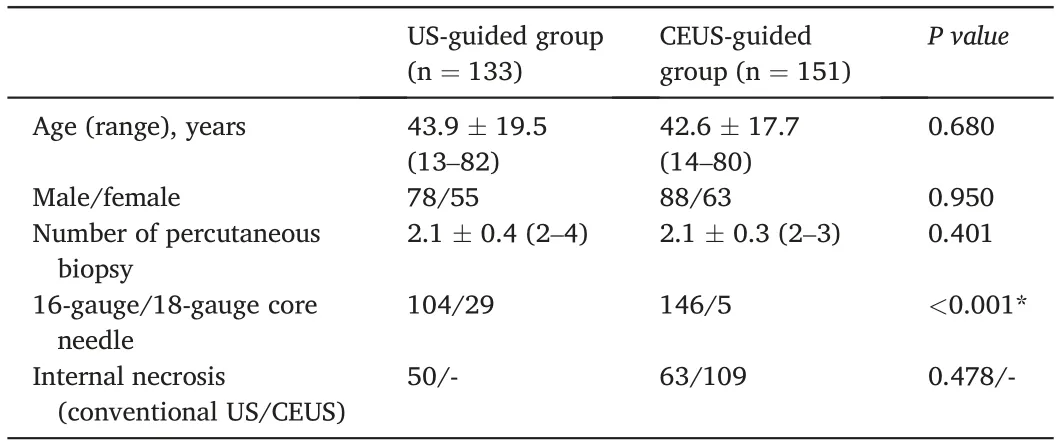
Table 2 Patient characteristics, biopsy technique, lesion characteristics: comparison of US-guided group versus CEUS-guided group.
CEUS examination was performed in 151 patients, and no adverse reactions to SonoVue®were observed.None of the patients experienced adverse reactions or complications after US-guided or CEUS-guided PCNBs.
4. Discussion
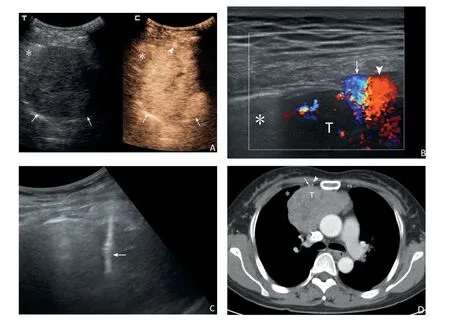
Fig. 2. The case in a 46-year female by CEUS-guided PCNBs. The anterior mediastinum lesion was confirmed as thymoma by subsequent surgical pathology. Before the biopsies,CEUS showed the lesion was heterogeneous hyper-enhancement without obvious necrosis(A,arrows;*lung),and the internal thoracic artery in front of the lesion was visible (A, arrowhead). High-frequency ultrasound could see the internal thoracic artery (B, arrow) and vein (B, arrowhead; T: lesion; * lung) more clearly. Biopsy route was adjusted to avoid blood vessel damage, and real-time US showed the transpleural pathway of the biopsy needle (C, arrow). Contrastenhanced chest computed tomography showed the heterogeneous enhanced lesion and the internal thoracic artery (D, arrow) and vein (D, arrowhead; T: lesion; *lung) in the soft tissue window.
The histological types of mediastinal lesions are complex, and their clinical and imaging manifestations of mediastinal lesions usually lack specificity. More than 50% of mediastinal lesions seen in adults are observed in the anterior mediastinum.2An accurate pathological diagnosis is crucial to decide on the most effective therapeutic strategy.In the past, the diagnosis was mainly based on thoracotomy or mediastinoscopy, both of which were associated with invasive damage and even morbidity.13Currently,image-guided PCNB of AMMs,including US, CT and MRI,has been verified to be a minimally invasive and relatively safe procedure.9,14–18
AMMs directly contacting the thoracic wall or with the soft tissues of the jugular fossa can be visualized by US scan through the parasternal intercostal space. CDFI can distinguish the relationship between lesions and the surrounding large vessels and the heart. Therefore, US-guided PCNB has become a cost-effective, time-saving, and feasible method to obtain pathological specimens of AMMs.8The diagnostic yield of US-guided PCNB of AMMs ranged from 79% to 100%.5,8,15,19,20The pathological tissue obtained by PCNB represents only a small part of the lesion,and the tissue composition in different parts of the same lesion may vary greatly. Obtaining adequate tissue specimens representing the essential characteristics of the lesion is essential to improve the diagnostic yield and accuracy of PCNB. Conventional US-guided percutaneous biopsies can usually be used to obtain adequate tissue specimens.Nevertheless,necrosis or liquefaction caused by insufficient blood supply often occurs during tumour growth. Conventional US cannot accurately distinguish the active area from necrosis;therefore,the diagnostic yield of US-guided percutaneous biopsy may be unsatisfactory in these circumstances. Combined with CDFI, necrosis of the lesion may be avoided by experienced operators.However,AMMs are adjacent to the heart and large blood vessels, and their pulsation may affect the presentation of low-velocity blood flow on CDFI. CEUS can accurately display the blood flow perfusion of different tissue structures in the lesion and is not disturbed by the pulsation of large cardiac vessels. In our study, the CEUS-guided group showed a higher rate of internal necrosis of the lesions.CEUS can accurately distinguish the active area and necrosis of the lesion to allow an optimal route to guide PCNB and improve diagnostic yield.Our study showed that the diagnostic yield of CEUS-guided PCNB for AMMs was significantly higher than that of US-guided PCNB(100%vs.89.5%).
There was no significant difference between the diagnostic accuracies of the CEUS-guided and US-guided groups. Although the 18-gauge core needle was used more frequently in the US-guided group than in the CEUS-guided group, all tissue samples obtained using 18-gauge core needles had a precise diagnosis in our study. For safety purposes, 18-gauge core needles were used for small AMMs in our study. Some researchers have reported that using a thinner needle (i.e., a 20-gauge needle) is the sole significant risk factor for diagnostic failure. As such,both 16-gauge and 18-gauge core needles are safe and effective for PCNB of AMMs, and tissue specimens obtained by PCNB were adequate for pathological examination, although care should be taken regarding necrosis.
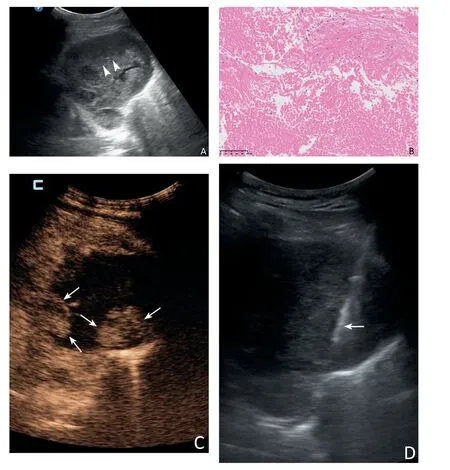
Fig.3. A 52-year male underwent two PCNBs.The first time,US showed a heterogeneous hypoechoic anterior mediastinum lesion and selected a common target area for biopsy (A, arrowheads). The result of the specimen by US-guided PCNB was nondiagnostic due to only coagulative necrosis seen microscopically (B). Five days later,CEUS showed the deep area of the lesion was obviously enhanced(C,arrows),and the area of the first biopsy was unenhanced massively.PCNB was performed successfully in the active area of the lesion subsequently,and real-time US showed the transpleural pathway of the biopsy needle(D,arrow).Microscopically,atypical epithelioid cells could be seen (E), and the specimen of CEUS-guided PCNB was diagnosed as a germ cell tumour confirmed by surgical pathology.
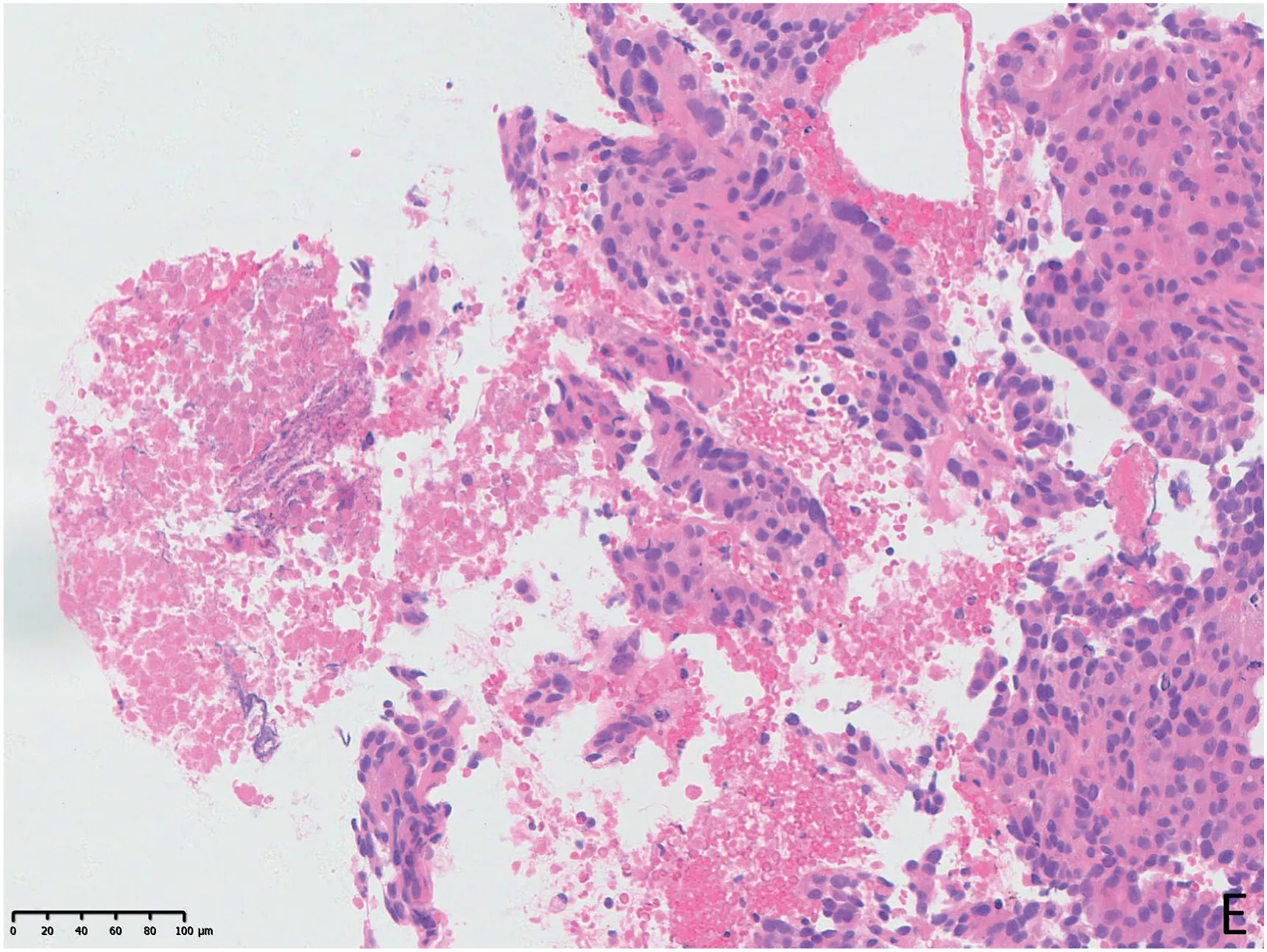
Fig. 3. (continued).
CT is the most commonly used modality for the localization of mediastinal lesions and to guide biopsy. The diagnostic yield of CTguided PCNB ranged from 74% to 97%.7,14,18,21–23The pooled proportions of the diagnostic yield and accuracy of CT-guided PCNB for mediastinal masses were 92%and 94%,respectively,in a meta-analysis from 18 eligible studies.12The US-guided approach had a similar diagnostic yield to the CT-guided approach.No complications were reported in prior studies assessing the US-guided approach Conventional US,CDFI, and CEUS can be used to visualize vessels and guide the percutaneous biopsy process in real time to avoid important structures.Complication rates in CT-guided procedures range from 4% to 17%.6A transpleural route was reported as the only variable associated with an increased complication rate by CT-guided approaches.6It is impossible to view the lesion when the lung tissue is between the US transducer and the lesion; therefore, US-guided procedures usually avoid this route. This may be another reason for the fewer complications associated with the US-guided procedures.
The current study had several limitations. First, the retrospective design will have resulted in the introduction of selection bias. Second,only patients deemed suitable to undergo US-guided or CEUS-guided PCNB underwent this procedure. Third, the technique (including the approach, route, and biopsy gauge) was chosen by a trained and experienced interventional ultrasound doctor based on the case study.
5. Conclusions
Our study shows that US-guided and CEUS-guided PCNBs for AMMs are safe methods with high diagnostic yield and diagnostic accuracy.CEUS-guided PCNB can improve the diagnostic yield by allowing optimization of the biopsy procedure.
Author contributions
Peili Fan:Data curation, Formal analysis, Writing - original draft,
Jiaying Cao:Writing-review&editing,Yunjie Jin:Investigation,Hong
Han:Investigation,Wenping Wang:Funding acquisition, Validation,Supervision,Huixiong Xu:Validation, Supervision,Zhengbiao Ji:Conceptualization,Investigation,Methodology,
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Natural Science Foundation Project of Shanghai “Science and Technology Innovation Action Plan” (Grant No.20ZR1452800), Clinical Research Plan of SHDC (Grant No.SHDC2020CR1031B),and Shanghai Municipal Key Clinical Specialty of China(Grant No.shslczdzk03501).
 Journal of Interventional Medicine2022年3期
Journal of Interventional Medicine2022年3期
- Journal of Interventional Medicine的其它文章
- Safety and efficacy of the SeparGateTM balloon-guiding catheter in neurointerventional surgery: A prospective, multicenter, single-arm clinical trial
- Uterine artery embolization combined with ultrasound-guided dilation and curettage for the treatment of cesarean scar pregnancy: Efficacy and 5–8-year follow-up study
- Microwave ablation of colorectal liver metastases adjacent to the cardiophrenic angle: Parallel versus non-parallel placement of the antenna relative to the diaphragm
- Diagnosis of hepatic inflammatory pseudotumor by fine-needle biopsy
- Recent advances of multimoda ultrasound in image-guided prostate-targeted biopsy
- A canine model of aortic arch aneurysm created with autologous pericardium
