Vocal repertoire of the critically endangered whiteheaded langur (Trachypithecus leucocephalus): Call types, acoustic structures, and related social-ecological contexts
DEAR EDITOR,
We examined the vocal repertoire of the critically endangered white-headed langur (Trachypithecus leucocephalus)inhabiting karst limestone forests in China. We identified 17 call types across all age/sex classes based on human auditory perception, spectrogram assessment, and quantitative analysis of 25 acoustic parameters extracted from 409 highquality calls. In total, 11 types of calls produced by adults corresponded to the simple one-male multi-female social groups of white-headed langurs. This supports the social complexity hypothesis of vocal communication, i.e., highly social species require more diverse call types to convey information and maintain inter/intragroup social relationships.We identified unique age/sex-dependent call types, with five call types specific to adult males, two to adult females, three to subadults and juveniles, and three to infants. Vocal differentiation among the age/sex classes reflected differences in social roles. Temporal and spectral parameters of vocalizations were associated with environmental characteristics. The acoustic signals presented here,especially male long-range loud calls, could be used for passive monitoring to explore population dynamics and habitat use of this critically endangered species in the wild.
The acoustic signals of a given species are influenced by multiple factors, including social system (Freeberg et al.,2012), phylogenetic relationships (Thinh et al., 2011), habitat characteristics (Zhao et al., 2021), and sexual selection(Delgado, 2006). Understanding the adaptative characteristics of call types and acoustic structures is critical for exploring the spatiotemporal mechanisms, functions, and evolution of vocalizations. The social complexity hypothesis of vocal communication suggests that highly social species living in large groups and featuring complex social organization and interactions require more diverse call types to convey information and maintain inter/intragroup relationships(Freeberg et al., 2012). However, phylogenetic relationships appear to constrain the extent and flexibility of primate vocalizations (Thinh et al., 2011). Notably, call type and structure of vocalizations are highly conserved among closely related taxa, despite differences in social systems(Hammerschmidt & Fischer, 2019).
Habitat characteristics and predation pressure can also affect species call types and acoustic structures (Ey & Fischer,2009; Zhao et al., 2021; Zuberbühler, 2001). The acoustic adaptation hypothesis suggests that specific environmental properties of habitats can exert selection pressure on the acoustic structure of vocal signals (Ey & Fischer, 2009; Zhao et al., 2021). For example, in forested habitat where visual cues are not always possible and sound attenuation is considerable, golden snub-nosed monkeys (Rhinopithecus roxellana) produce contact calls with adaptative acoustic traits,including several harmonics at low fundamental frequency, to transfer information and maintain group cohesion (Fan et al.,2018). In addition, selection pressures associated with predator threats can lead to predator-specific calls(Zuberbühler, 2001), thereby enriching vocal repertoires.
Interacting internal factors of callers, such as social role,sex, and age, also appear to influence call type and acoustic structure within a vocal repertoire (Bouchet et al., 2012; Fan et al., 2018). First, group living leads to differentiation in social functions between the sexes, for which sex-specific acoustic signals have evolved (Bouchet et al., 2012; Fan et al., 2018).For example, loud calls are usually emitted by adult males in association with male-male competition (Delgado, 2006).Second, age/sex-related differences in vocalizations,associated with development, are found in many primate species (Bouchet et al., 2012). For example, weeping calls are only produced by juvenile Tibetan macaques (Macaca thibetana) (Bernstein et al., 2016).
Our understanding of vocal communication in the critically endangered white-headed langur (T. leucocephalus) remains poor. This colobine primate species is restricted to the karst mountains (200 km2) of Guangxi Province (China), and typically resides in one-male-multi-female matrilineal groups(average group size of 12), with the resident male responsible for territorial defense (Jin et al., 2009). Habitat consists of seasonal karst limestone forest, which currently faces intense habitat fragmentation and human disturbance pressure. These forests are characterized by stunted trees, underbrush, and lianas, which allow a relatively broad field of vision (Zhang et al., 2021). Thus, the openness of the karst forest environment and the polygynous nature of white-headed langurs are useful for studying vocal signal adaptation and differentiation of social roles between the sexes.
Here, we investigated the following predictions concerning the vocal repertoire of white-headed langurs. First, we predicted that their social arrangement (small one-male-multifemale groups) would obviate the need for an extensive vocal repertoire (Freeberg et al., 2012). Second, we predicted that adult males would produce long-range calls to warn other males under fierce intergroup male-male competition(Delgado, 2006). Third, we predicted that alarm signals would be emitted by all age/sex classes due to strong predation pressure (Zuberbühler, 2001). Fourth, we predicted that ageand sex-specific call types would exist due to differences in social roles and body development (Bouchet et al., 2012).
The study was conducted in the Banli area of the Guangxi Chongzuo White-headed Langur National Nature Reserve(N22°24′-22°46′, E107°22′-107°42′) in southwestern Guangxi,China. We studied two well-habituated groups of whiteheaded langurs from July 2020 to September 2021. Further details on study site and group information are available in the Supplementary Materials. We classified individuals into ageclass categories based on weaning age and behavioral development: i.e., adults (≥5 years old), subadults or juveniles(1.5-4 years old), and infants (≤1.5 years old). We collected data using focal andad libitumsampling over two time periods of the day (i.e., 0600-1200h and 1500-1930h). Vocalizations were recorded using a TASCAM DR-100 MK III (Tascam,Japan) and Zoom F6 digital recorder (Zoom, Japan) (44.1 kHz and 16 bits sampling rate) with a Sennheiser ME66 directional microphone (Sennheiser, Germany).
Avisoft-SASLab Pro v5.3.01 (R. Sprecht, Germany) was used to generate spectrograms with a fast Fourier transform(FFT) size of 1 024 points and an overlap of 87.5%, with a Hamming window (frequency resolution: 22 Hz, temporal resolution: 5.805 ms) and frame size of 100%. In addition to visual review of spectrograms, we assessed vocalizations by the human ear, and classified call types according to previously identified call types of other colobine species (Fan et al., 2018; Poirier-Poulin & Teichroeb, 2020). For each selected vocalization, we used Avisoft to measure one temporal and six spectral-based parameters from the entire vocalization, and 18 spectral-based parameters from three different locations within the vocalization (Supplementary Table S1). If calls were emitted in bouts, we considered each call separately for acoustic parameter extraction.Spectrograms were not generated for low-intensity call types that overlapped with background noise and calls of other individuals.
Whenever possible, we simultaneously filmed the socialecological context of each recorded call using a Sony FDRAX700 digital camera (Sony, Japan). For each call, the caller,date, time, location, potential receiver, and behavioral/vocal response of the potential receiver were recorded. A behavioral and vocal response was considered if it occurred within 5 s of initiation of the vocalization. The social-ecological context of each call was classified as vigilance, traveling, foraging,resting, agonistic interactions, natural disturbance, and human-induced disturbance (Supplementary Table S2).
Based on 1 884 h of direct observations over 284 days, we recorded 28 697 call samples (mean± standard deviation(SD)=101±62, range=41-235), yielding 409 high-quality calls for acoustic parameter extraction. We identified 17 call types based on auditory perception, spectrogram review, and statistical analysis of acoustic parameters (Figure 1). Details on social-ecological contexts, acoustic parameters, and statistical analyses of each identified call type are provided in Supplementary Tables S3, S4, and S5, respectively. In total,11 call types were emitted by adults, within the range reported for other small-group living colobines (Poirier-Poulin &Teichroeb, 2020). However, call diversity was lower than that of golden snub-nosed monkeys (18 call types), which reside in larger groups with a multi-level society (Fan et al., 2018).Overall, the small vocal repertoire of adult white-headed langurs in association with their small one-male-multi-female groups supports the social complexity hypothesis of vocal communication.
Three male-emitted long-distance loud calls, i.e., snorts,roars, and wahoos, were relatively commonly produced by the white-headed langurs, as reported in other primate species(Poirier-Poulin & Teichroeb, 2020). These energetically expensive calls may be produced as signals of male ability in male-male competition to establish intergroup spacing, attract mates, maintain group cohesion, and defend territory(Delgado, 2006; Poirier-Poulin & Teichroeb, 2020). Here, the three male-emitted loud calls were all short in duration, low in frequency, and syllabically repetitive. The acoustic characteristics of the loud calls produced by the white-headed langurs are consistent with the acoustic adaptation hypothesis, which proposes that low-frequency calls of short duration without complex harmonics carry well in open environments (Ey & Fischer, 2009), such as the steep karst limestone forests. Calls that are uttered repeatedly within a short period can achieve meaningful phonological syntax,while lexical syntax can be achieved by producing different call types in a combinational sequence. The three loud call types provide phonological syntax, reinforcing the information transmitted by a single signal and enabling the location of these vocalizations to be identified more easily by the receiver(Poirier-Poulin & Teichroeb, 2020). Complex vocal sequences produced by male adult white-headed langurs consisting of these three call types, with variations in order and number in different sequences, follow the characteristics of lexical syntax.
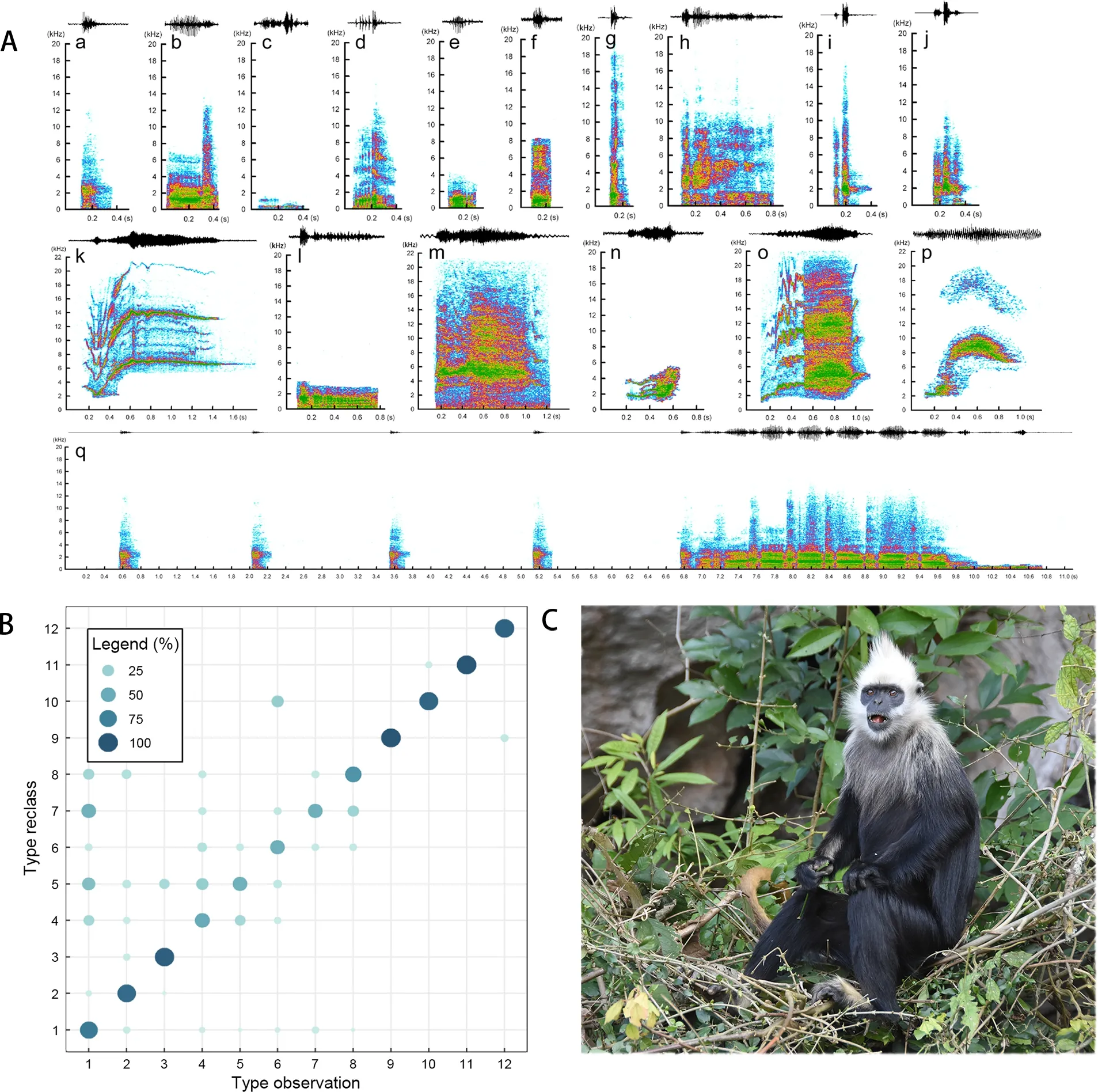
Figure 1 Representative spectrograms and correct classification rates based on discriminant function analysis (DFA) of vocalizations in repertoire of white-headed langurs
Four kinds of alarm calls (snort, roar, two-syllabled chuck,and three-syllabled chuck) were identified as part of the vocal repertoire of white-headed langurs. Sex-specific alarm signals,such as the snorts and roars produced by adult males and the two- and three-syllabled chucks produced by adult females,subadults, and juveniles in response to disturbances in the immediate environment, imply different sex roles in identifying and responding to perceived risk. However, the exact function of these alarm signals needs further confirmation.
Our findings indicated that age- and sex-specific call types existed in the vocal repertoire of white-headed langurs.Specifically, five call types (i.e., snort, roar, wahoo, purr, and soft snort) were specific to adult males, two call types (i.e.,squeal and long squeal) were specific to adult females, three call types (modulated tonal scream, grunt, and squeak) were specific to subadults and juveniles, and three call types (coo,compound squeak, and scream) were specific to infants.Differentiation in social roles is the most plausible explanation for age- and sex-specific call types in primates (Bouchet et al.,2012; Fan et al., 2018). For white-headed langurs, malespecific calls may be linked with intergroup spacing, territory,or food resource defense. Resident males will defend their tenure in the group from extra-group males, thereby indirectly protecting the group (Jin et al., 2009). Female-specific call types may be associated with female-female competition for food items or spatial position within the group (Jin et al.,2009). The distinct distress signals emitted by subadults,juveniles, and infants appear to indicate anxiety or to seek contact reassurance.
In conclusion, this study provides the first evidence of the call types, acoustic structures, and related social-ecological contexts of white-headed langur vocalizations, which likely evolved in response to their polygynous society and open karst limestone forest habitats. Of the 17 call types documented, 11 were produced by adults, suggesting that small groups and simple one-male-multi-female social organization correspond to smaller vocal repertoires. Playback experiments could be used to determine whether long-range loud calls or chucks carry multiple messages, such as individual identity, predator type, strength of the motivational state of the caller, and distance to the caller. The acoustic signals identified in this study, especially male long-range loud calls, could be used to investigate the population dynamics and habitat use of these critically endangered langurs in the wild.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Permission for field surveys in Banli was granted by the Guangxi Chongzuo White-headed Langur National Nature Reserve. Non-invasive methods were used to collect all data.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
All authors declare that they have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
P.L.F., Q.H.Z., and M.L. conceived and designed the study.J.X.L., L.T.Y., and S.J.W. collected the data. P.L.F. and L.T.Y.analyzed the data. P.L.F. wrote the manuscript. P.L.F., T.S.,C.C.G., C.M.H., Q.H.Z., and M.L. revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We are grateful to the Guangxi Chongzuo White-headed Langur National Nature Reserve for allowing us to conduct this study. We thank reserve staff Jian-Bao Wu, Deng-Pan Nong, and Nong-Zhi Li for providing substantial support during the study period. We thank Dr. Li Yang from the School of Life Sciences, Sun Yat-Sen University, for advice on manuscript writing.
Peng-Lai Fan1,2,3, Jia-Xing Li2,3, Li-Ting Yang2,3,Tao Sun2,3, Shi-Jun Wu4, Cyril C. Grueter5,6,7, Cheng-Ming Huang1,2,3, Qi-Hai Zhou2,3,*, Ming Li1,8,*1CAS Key Lab Animal Ecology&Conservation Biology,Institute of Zoology,Chinese Academy of Sciences,Beijing100101,China2Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University),Ministry of Education,Guilin,Guangxi541006,China
3Guangxi Key Laboratory of Rare and Endangered Animal Ecology,College of Life Sciences,Guangxi Normal University,Guilin,Guangxi541006,China4Guangxi Chongzuo White-headed Langur National Nature Reserve,Chongzuo,Guangxi532200,China
5Department of Anatomy,Physiology,and Human Biology,School of Human Sciences,University of Western Australia,Perth WA6009,Australia6Centre for Evolutionary Biology,School of Biological Sciences,
University of Western Australia,Perth WA6009,Australia7International Center of Biodiversity and Primate Conservation,Dali University,Dali,Yunnan671003,China8Center for Excellence in Animal Evolution and Genetics,Chinese
Academy of Sciences,Kunming,Yunnan650223,China
*Corresponding authors, E-mail: zhouqh@mailbox.gxnu.edu.cn;lim@ioz.ac.cn
- Zoological Research的其它文章
- Diversity of reptile sex chromosome evolution revealed by cytogenetic and linked-read sequencing
- Coevolutionary insights between promoters and transcription factors in the plant and animal kingdoms
- Deficiency of transmembrane AMPA receptor regulatory protein γ-8 leads to attention-deficit hyperactivity disorder-like behavior in mice
- Global cold-chain related SARS-CoV-2 transmission identified by pandemic-scale phylogenomics
- The Hippo pathway and its correlation with acute kidney injury
- Genomics and morphometrics reveal the adaptive evolution of pikas

