Functional explanation of extreme hatching asynchrony: Male Manipulation Hypothesis
Manuel Soler, Francisco Ruiz-Raya, Lucía Sánchez-Pérez, Juan Diego Ibáñez-Álamo, Juan José Soler
1 Departamento de Zoología, Facultad de Ciencias, Universidad de Granada, Granada E-18071, Spain
2 Unidad Asociada Coevolución: Cucos, Hospedadores y Bacterias Simbiontes, Universidad de Granada, Granada E-18071, Spain
3 Centro de Investigación Mariña, Universidade de Vigo, Grupo de Ecoloxía Animal, Vigo 36310, Spain
4 Departamento de Ecología Funcional y Evolutiva, Estación Experimental de Zonas Áridas (CSIC), Almería E-4120, Spain
ABSTRACT Hatching asynchrony in birds is considered an adaptation to facilitate brood reduction because under conditions of food scarcity, the smallest nestling usually dies soon after hatching, thereby minimizing parental effort. However, in species with extreme hatching asynchrony, the last hatchlings paradoxically experience a very low probability of survival and death can take so long that it can hardly be considered an adaptation. Here, we propose and experimentally tested a new adaptive hypothesis explaining the brood reduction paradox, namely the“Male Manipulation Hypothesis”. Our hypothesis suggests that by inducing asynchronous hatching,females increase the feeding requirements of the brood, which will induce males to increase provisioning effort. In addition, females may extend the period of male manipulation by feeding the smallest nestling just enough to sustain life. Our study showed that male common blackbirds (Turdus merula) increased their effort (i.e., number of food items per hour) in experimental asynchronous broods compared to synchronous broods, while females reduced their contribution, as predicted by the hypothesis.
Keywords: Brood reduction; Food allocation;Hatching asynchrony; Male manipulation hypothesis; Sexual differences in food allocation;Turdus merula
INTRODUCTION
In species with biparental care, parents are expected to optimize provisioning rate and food allocation among offspring to maximize their own fitness (Royle et al., 2012). In altricial birds, food provisioning and allocation are mediated by complex parent-offspring interactions (Mock et al., 2011;Wright & Leonard, 2002) and environmental conditions (Caro et al., 2016; Grodzinski & Johnstone, 2012). Under favorable conditions, or when food availability during the nestling period is predictable, females delay the onset of incubation until clutch completion, resulting in synchronously hatched nestlings of similar size and survival prospects (i.e., fed according to their begging signals of need (Caro et al., 2016;Stoleson & Beissinger, 1995)). However, under non-favorable conditions, or when food scarcity is predicted during the nestling period, females may begin egg incubation before clutch completion, leading to asynchronously hatched nestlings of different age and size. In these asynchronous broods, last-hatched nestlings are typically at a disadvantage relative to their older siblings, as preferential feeding of older and larger nestlings increases parental fitness (Caro et al.,2016; Jeon, 2008; Magrath, 1990; Mock & Parker, 1997;Stoleson & Beissinger, 1995). Thus, when resources are insufficient for the entire brood, hatching asynchrony is considered an adaptation to facilitate brood reduction (Lack,1947, 1954; Magrath, 1990; Stoleson & Beissinger, 1995).
In species with extreme hatching asynchrony, last-hatched nestlings experience a very low probability of survival. This raises the question of why females adopt this apparently maladaptive strategy of incubation, which often results in increased offspring mortality (i.e., “paradox of hatching asynchrony”; Stoleson & Beissinger, 1995). Brood reduction is not only the result of hatching asynchrony, but also a consequence of preferential parental feeding of larger nestlings at the expense of smaller ones (Mock et al., 2009;Price et al., 1996; Smiseth & Amundsen, 2002; Soler, 2001).The key question is why parents waste resources and expend effort in later hatched young if those young are convicted to die (i.e., “paradox of brood reduction”; Soler, 2001). Although the smallest nestlings sometimes die soon after hatching,which could be seen as an adaptation to minimize parental effort, death often takes such a long time that it can hardly be considered a form of adaptation (Clark & Wilson, 1981; Mock& Ploger, 1987; Soler, 1989). For example, Eurasian jackdaws(Corvus monedula) in southern Spain lay clutches of five to seven eggs in most (77.5%) nests (Soler & Soler, 1991).However, breeding success (defined as the mean percentage of eggs that produce fledglings) is only about 25% (Soler &Soler, 1996). Furthermore, although 43% of dead nestlings starved within the first five days of the nestling period,approximately 25% of chicks starved after 10 days, with some surviving until their siblings left the nest (Soler, 1989).Jackdaws never fledged more than four nestlings in this population, even under experimental conditions of food provisioning (Soler & Soler, 1996), as reported in other bird species (Amundsen & Stokland, 1988; Arcese & Smith, 1988;Slagsvold, 1982).
In the current study, we present a new adaptive hypothesis explaining the brood reduction paradox and provide the first experimental test of this hypothesis (see below).
Male Manipulation Hypothesis
Sexual conflict over parental investment is an almost inevitable consequence of sexual reproduction and can occur in virtually any aspect of reproductive attempts (Lessells,2012). Sexual conflict theory predicts that one parent will attempt to minimize its investment in reproduction at the expense of its mate (Trivers, 1972). This prediction suggests that one member of a pair will motivate the other to work harder in reproduction, thus saving energy and time that can be devoted to other fitness-related activities. For instance,saving energy to maintain a better physical condition, thereby increasing the possibility of re-nesting during the same breeding season or surviving to the next breeding season(Harrison et al., 2009; Houston et al., 2005). In addition, those parents (especially males) impacted by shifting additional care are less likely to remate (Lessells, 2012; Olson et al., 2008).
Our “Male Manipulation Hypothesis (MMH)” proposes that,in some species, females may use asynchronous hatching to induce males to increase their parental investment in asynchronously hatched offspring. In other words, by using hatching asynchrony and allowing the youngest chick to survive for a period of time, females increase the feeding requirements of the brood, thus inducing males to work harder to provide additional food during the nestling period while the smallest nestling is alive.
More than 30 years ago, Slagsvold & Lifjeld (1989a)presented and tested their “Sexual Conflict over Parental Investment Hypothesis”, which was also related to sexual conflict theory. They suggested that by starting incubation before clutch completion, females will extend the nestling period, thereby forcing their male partners to care for nestlings for a few extra days (i.e., in comparison to synchronously hatched broods). The underlying mechanism of this hypothesis differs from that of MMH, where female strategy is linked to producing asynchronously hatched nestlings with a lower probability of survival, but which beg more intensely for food than their nest mates. Thus, while Slagsvold & Lijfeld’s hypothesis suggests that the female “manipulates” the male to contribute one or two extra days of paternal effort (i.e., the difference in duration of incubation between synchronous and asynchronous broods), MMH posits that the female strategy works during the nestling period while the smallest nestling is alive. Thus, the hypothetical benefits associated with female manipulation of males would be extended as long as the smallest nestling remains alive. MMH not only predicts the beneficial effects of females laying asynchronously hatched nestlings, but also that females are the sex more interested in keeping the smaller nestling alive. Therefore, the hypothesis can be divided in two independent components based on four strongly supported assumptions in the literature:
First component: the existence of a smaller nestling in a brood originates from female incubation behavior inducing males to increase their feeding effort. This first component can be adaptive and may explain cases of moderate and extreme hatching asynchrony. It relies on the following three assumptions:
1. Females can vary the degree of hatching asynchrony by deciding the onset of incubation during the laying sequence(Kontiainen et al., 2010; Magrath, 1990; Wiebe et al., 1998).
2. Given that undernourished nestlings typically signal their poor status by increasing their begging rates, the presence of runts in a brood will markedly increase the level of begging within the nest (Bengtsson & Rydén, 1981; Mock et al., 2011;Price et al., 1996).
3. Males will increase their food provisioning rates in response to increased begging intensity of the brood (e.g.,Bengtsson & Rydén, 1981; García-Navas & Sanz, 2010;Koenig & Walters, 2012; MacGregor & Cockburn, 2002).
Second component: by feeding the smallest nestling just enough to maintain life, females will increase the time males spend working harder, which may explain extreme hatching asynchrony in which the death of the runt takes a very long time. This is based in the following assumption:
4. The time when brood reduction occurs is variable,depending on resources delivered to the smallest nestlings.Thus, since the hypothesis predicts benefits for females,females will be much more interested than males in keeping the smallest chick alive. This implies that the smallest nestling of the brood will be primarily fed by the female, as supported by many asynchronously hatching species (e.g., Bengtsson &Rydén, 1981; Budden & Beissinger, 2009; Gottlander, 1987;Lahaye et al., 2015; Ploger & Medeiros, 2004; Ryser et al.,2016). By providing some food to the smallest nestling in the brood (but not enough for overall survival), females delay brood reduction while prolonging the presence of alwaysbegging undernourished chicks in the nest with a very low probability of survival. For instance, in the Eurasian hoopoe(Upupa epops), a species with marked hatching asynchrony,males feed nestlings standing at the entrance of the cavity(i.e., stronger and more competitive ones), whereas females,by entering the nest cavity, allocate food more evenly among nestlings, showing a tendency to preferentially feed the smallest (Ryser et al., 2016).
Here, we performed an experiment to test the MMH in Eurasian blackbirds (Turdus merula), a passerine species with clear sexual dichromatism, moderate hatching asynchrony,and facultative brood reduction (Cramp, 1988; Del Hoyo et al.,2005). We established asynchronous and synchronous broods while controlling for the effects of nestling competition within the nest. Parental resource allocation can be influenced by scramble competition among nestlings (i.e., feeding winner nestlings) rather than selection among those begging for food(Macnair & Parker, 1979; Mock & Parker, 1997). Therefore,given that the potential effects of scramble competition on food allocation may mask the expected effects of experimental asynchronous broods, we prevented physical interactions among nestlings by including (experiment) or not (control) an artificial barrier that forced each nestling to stay in its position within the nest.
The main prediction derived from the MMH (first component) is that males should increase feeding effort when confronted with asynchronous broods, implying the presence of one smaller and hungrier nestling, while the opposite effect is expected for females (Prediction 1). The second component of the hypothesis predicts that the smallest hungry nestling should be preferentially fed by the female while the male will concentrate its feeding effort on larger nestlings (Prediction 2).
MATERIALS AND METHODS
Study area and species
Fieldwork was carried out in a Eurasian blackbird (hereafter blackbird) population, located in the Valley of Lecrín (southern Spain; N36°56′, W3°33′; 580 m a.s.l.) between mid-March and June in 2013 and 2015. As a medium-sized passerine with clear sexual dichromatism (adult males are black with a distinctive yellow-orange beak and eye-ring, while adult females are dull dark with lighter brown streaks on their breast; Cramp, 1988), the sex involved in each feeding event can be easily determined. In our study area, blackbirds can produce three broods in a spring breeding season, with clutch size ranging from two to five eggs (Ibáñez-Álamo & Soler,2010). Females start incubation before the last egg is laid,thereby inducing moderate hatching asynchrony (i.e., last egg hatches later than the rest), which sometimes results in brood reduction (Magrath, 1989), especially at the end of the breeding season or under poor environmental conditions(Pers. Obs.).
General field procedures and experimental design
Throughout the breeding season, we actively searched for blackbird nests in the study area. Once located, the nests were initially visited every two days and then daily when close to hatching to detect newly-hatched chicks. Nestlings were marked on their tarsus using colored permanent marker pens(Lumocolor), then later (days 6-7 post-hatching) tagged with numbered leg rings for individual recognition.
We performed cross-fostering manipulation in nests with similar hatching dates (±0-3 days) to create two different types of four-nestling broods: (i) asynchronously hatched broods, where a one-day-old nestling from a donor nest (small focal nestling; mean weight=7.4±0.3 g,n=14) was introduced into a recipient nest containing three three-day-old nestlings(±1 day, mean sibling weight=14.5±0.3 g,n=42). (ii)Synchronously hatched broods, where a three-day-old nestling from a donor nest (medium-sized focal nestling; mean weight=11.8±0.5 g,n=14) was introduced into a recipient nest containing three nestlings of similar hatching date (±1 day,mean sibling weight=12.4±0.5 g,n=42). The day after crossfostering manipulation (experimental day 1; 0900h-1300h),parental feeding activity (see below) was assessed in two consecutive and non-overlapping trials (1.5 h each). In one trial, siblings were allowed to physically compete for food,while, in the other trial, physical interaction among nestlings was prevented by placing an artificial (wooden) barrier in the nest (attached to the base with twine to prevent removal by parents) to ensure siblings remained separated. Artificial barriers have been shown to impede physical interaction among siblings within the nest (Ploger & Medeiros, 2004). The order of experimental trials was alternated in successive nests to avoid potential bias associated with barrier treatment order.
The MMH is based on the main characteristic of asynchronous broods: i.e., the presence of a smaller undernourished nestling, which begs at a higher intensity and rate (Bengtsson & Rydén, 1981; Mock et al., 2011; Price et al.,1996). Accordingly, before each trial, the experimental chick was removed from the asynchronous nest for 30 min and placed in an artificial nest (covered with cotton to reduce heat loss) to prevent parents from feeding it and increase its level of need. This procedure establishes a true asynchronous brood that includes a smaller nestling that will presumably beg at a higher rate and intensity than its nestmates.
Before each trial, a video camera (Panasonic HDC-SD40)was placed near the nest (3-5 m) to record parental feeding activity. The video camera was installed 1 h before the start of the first trial to allow adults to become accustomed to its presence. Recordings were used to obtain the following information: (i) number of visits to the nest made by each sex to feed the chicks, used as a proxy of parental feeding effort(i.e., feeding visits per hour); (ii) amount of food carried to the nest by parents at each visit (i.e., food items per visit). A food item was defined as prey representing a similar volume to the adult bill size. Thus, size categories of food items were estimated from the recordings by comparing the volume of each food item relative to the parent’s bill volume within 50%bins (e.g., 50%, 100%, and 150% of bill volume) (Hauber &Moskát, 2008). Food items smaller than 50% of the bill volume were quantified as 10% of bill volume as they were mostly small insects of similar size; (iii) overall food load (i.e., food items per hour) received by the focal nestling (Experimental),first-hatched nestling (A-nestling), second-hatched nestling (Bnestling), and third-hatched nestling (C-nestling). Thus, we quantified the number of food items delivered to each nestling by the parents in 1 h. The volumes (i.e., size categories) of all items delivered to a single nestling during the filming period were summed to generate the variable “food items per hour”.To distinguish different nestlings in recordings, we marked their bills with individual colors using permanent marker pens.When hatching order was unknown, position in the brood hierarchy was established according to body mass.
Blackbirds rarely carry more than one item per feeding visit to the nest, which was not affected by our experimental manipulation (Supplementary Table S1 and Figure S1). In fact, in our study, outcomes for feeding visits per hour(Supplementary Table S2 and Figure S2) were similar to food items per hour. Consequently, we only reported results related to food items per hour in the main text. Using food items carried to the nest as a proxy for parental feeding effort has the additional advantage of estimating the effects of experimental treatment on the feeding effort of males and females and on their food allocation patterns (i.e., food items per hour that nestlings of different size receive) in the same statistical model (see Table 1).
This study was conducted following all relevant Spanish national (Decreto 105/2011, 19 de Abril) and regional(permissions provided yearly by la Consejería de Medio Ambiente de la Junta de Andalucía) guidelines.
Statistical analysis
Food allocation among nestlings was recorded in 28 blackbird nests containing four nestlings. In four nests, it was not possible to unambiguously quantify the amount of food received by each nestling during the recording. Thus, 24 nests(14 asynchronous and 10 synchronous nests) were used to assess the effects of treatment on food allocation patterns. All nests were recorded under conditions that did (experimental)or did not (control) prevent scramble competition among nestlings in nests containing four 3-day-old blackbird nestlings(synchronous broods) and nests containing three 3-day-old nestlings plus one recently hatched nestling (asynchronous broods). Given the balanced design, we used a repeated measures approach to explore the effect of sex, nestling size hierarchy, and experimental treatment on three response variables (i.e., feeding visits per hour, food items per visit, and food items per hour). Briefly, repeated measures analysis of variance (ANOVA) included experiments with synchrony as the between factor (i.e., each nest was under a unique experimental treatment) and experiments with scramble competition, adult sex, and nestling size hierarchy as the repeated measures (i.e., within factors; all possible treatments and values occurring within each nest).
We used the Cox proportional hazards model (CPHM) to test the effects of synchronous vs. asynchronous brood manipulation on the survival prospects of the focal(experimentally cross-fostered) nestling from experimental days 1 to 12. Survival analyses were performed using thesurvivalpackage (Therneau, 2021) in R v4.1.0 (R Core Team,2021). Repeated measures ANOVA was performed using Statistica software v13 (Dell Inc, 2015). Values are presented as mean±standard error (SE).
RESULTS
Parental feeding effort
The amount of food delivered did not differ between synchronous and asynchronous broods, but scramblecompetition among siblings did influence the amount of food delivered (Table 1). The number of food items delivered per hour by parents did not differ between asynchronous (logtransformed values,n=14; 1.24±0.14) and synchronous broods (log-transformed values,n=10, 1.03±0.11, Table 1). As predicted by our hypothesis, the effect of brood asynchrony manipulation on the number of food items per hour depended on parental sex (P=0.03) and largely on whether scramble competition was controlled for experimentally (P=0.008) (see interactions in Table 1; Figure 1). Interestingly, the effect of brood asynchrony on sex interactions was independent of scramble competition among siblings (P=0.095) (see threeway interaction among sex, scramble competition, and asynchrony in Table 1), suggesting that the detected differential effects of hatching asynchrony on feeding behavior of males and females were not dependent on scramble competition. As expected by the MMH, females provided less food to asynchronous broods, although the effect on males mainly occurred in those nests where nestlings could not interact (Figure 1). Therefore, because the number of food items per hour in asynchronous and synchronous broods was similar (see non-significant effect for this factor in Table 1), the reduced number of food items carried by females to asynchronous nests does not necessarily imply negative effects for the entire brood.
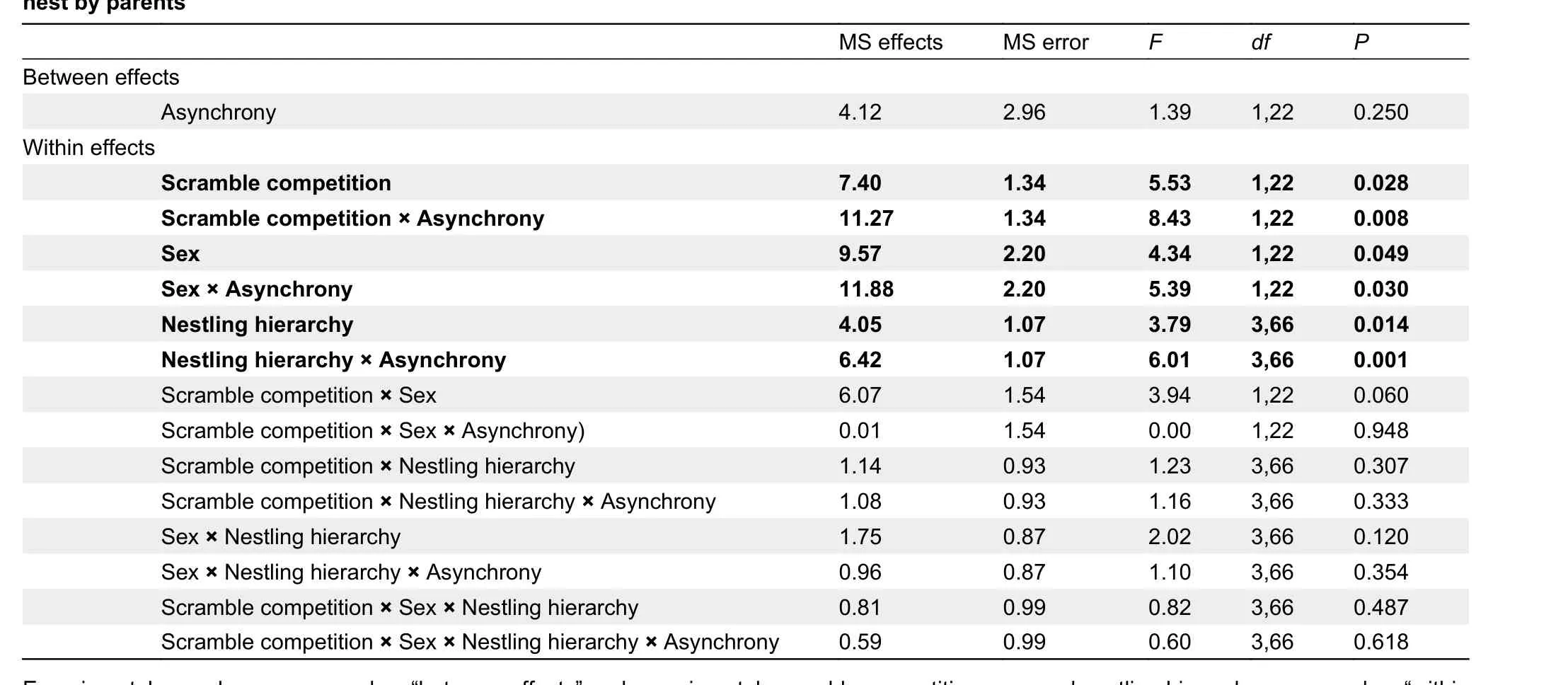
Table 1 Summary of repeated measures ANOVA of effects of experimental manipulation on number of food items per hour carried to the
Food allocation patterns

Figure 1 Mean (±95% CI) amount of food delivered to nests by male and female parents in synchronous (white bars) and asynchronous (gray bars) broods
Overall, parental food allocation among nestlings was dependent on hierarchy rank position, with larger nestlings receiving more food items per hour than smaller nestlings (logtransformed values,n=24, Size Experimental: 0.94±0.24, Size C: 0.96±0.28, Size B: 1.29±0.27, Size A: 1.33±0.23). The effects of nestling hierarchy did not depend on scramble competition among siblings, as evidenced by the nonsignificant interactions (Table 1). Although two-way interactions between parental sex and nestling hierarchy did not reach statistical significance (Table 1), food allocation by males was biased toward the two largest nestlings (least significant difference (LSD)post-hoc,P<0.003), while females fed all nestlings equally (Figure 2). Interestingly, while the described effects of sex on parental food allocation (i.e., in interaction with nestling hierarchy) did not vary between asynchronous and synchronous broods (see three-way interaction in Table 1), in asynchronous broods, females reduced the number of food items per hour provided to the first and last nestlings in rank hierarchy (LSDpost-hoc,P=0.003, Figure 2). Similarly, males reduced the number of food items provided to the smallest nestling (LSDpost-hoc,P=0.044, Figure 2), but increased food items provided to the larger nestlings (LSDpost-hoc, Nestling B:P<0.016, Nestling A:P=0.80, Figure 2). These results strongly suggest that the presence of a smaller nestling in a nest has differential effects on males and females, and that the reduced effort of females for larger nestlings is compensated by the enhanced feeding effort of males due to the presence of asynchronous hatchlings.
Nestling survival
Survival of focal (cross-fostered) nestlings to 12 days of age was lower in asynchronous than in synchronous broods(CPHM: asynchrony: 2.98; 95% confidence interval (CI): 1.65,5.36;z=3.63,P<0.001).
DISCUSSION
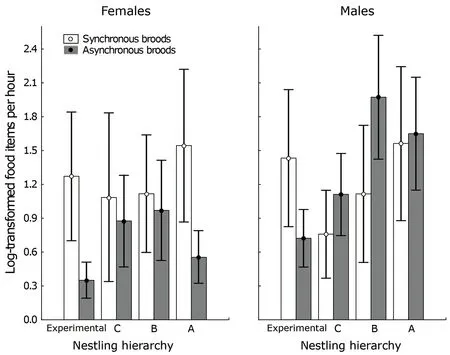
Figure 2 Food delivered by female (left) and male (right) parents to nestlings of different size hierarchy in asynchronous and synchronous broods
Since Lack (1947, 1954) proposed that hatching asynchrony adaptatively facilitates brood reduction in situations of reduced food availability in unpredictable environments (Brood Reduction Hypothesis), more than 20 different functional hypotheses have been suggested to explain the biological significance of hatching asynchrony (reviewed in Slagsvold et al., 1995; Stoleson & Beissinger, 1995; see Mock & Forbes,1995, for other original hypotheses). Most of these hypotheses have received experimental support in different species, and it is now broadly accepted that hatching asynchrony evolved as a consequence of a considerable variety of selection pressures (Caro et al., 2016; Jeon, 2008). Regardless of the functional explanation, in asynchronously hatching broods, the last smaller and hungrier nestling usually starves soon after hatching because parents preferentially feed larger nestlings(Mock et al., 2009; Price et al., 1996; Soler, 1989). This broadly assumed consequence of hatching asynchrony (i.e.,parental feeding preferences toward larger nestlings) is supported by our results given that both males and females significantly decreased feeding rates to the smallest nestling in the experimental asynchronous broods (Figure 2). Indeed, the smallest nestling exhibited a lower probability of survival than the focal nestling introduced in the experimental synchronous broods.
It is worth emphasizing that increasing the level of need in smaller nestlings by preventing parents from feeding them for 30 min prior to the start of the feeding trials, which allowed us to simulate realistic asynchronous broods (Bengtsson &Rydén, 1981; Price et al., 1996; Mock et al., 2011), is unlikely to provoke a decrease in the parental feeding rate. Indeed, a decrease in feeding rate would imply the opposite (i.e.,increase in feeding rate) as smaller nestlings would be presumed to beg more intensively (Lotem, 1998; Price et al.,1996; Smiseth & Amundsen, 2002). Similarly, this manipulation is unlikely to impact the survival prospects of offspring, as blackbird nestlings frequently spend longer periods of time without being fed by their parents due to different reasons (e.g., presence of a predator, poor weather conditions, or presence of humans in the vicinity of the nest;Pers. Obs.).
Importantly, we found that preventing physical interaction among nestlings did not affect the feeding efforts of males and females for small-sized nestlings, confirming previous results suggesting that scramble competition has little or no influence on food allocation by parents in asynchronous blackbird broods (Soler et al., unpublished data).
The fact that, in some species, offspring do not die soon after hatching, but often after a few days in the nest, casts doubt on the adaptive role of the Brood Reduction Hypothesis(see examples and references above). Thus, the MMH is expected to apply to those species that frequently experience extreme hatching asynchrony. The MMH states that by starting incubation before clutch completion and inducing hatching asynchrony, females force males to increase paternal feeding effort, allowing her to decrease her own investment. Our results unambiguously support this first prediction: in asynchronous broods, males increased their feeding rates (significantly when scramble competition was avoided), while females reduced feeding rates independently of whether scramble competition was experimentally avoided(Figure 1). As the feeding rates in asynchronous and synchronous experimental broods did not differ significantly,the detected sexual differences in feeding behavior benefited females without influencing nestling development. The lower energetic costs associated with reduced feeding rates of females may be crucial for their survival prospects, as supported by several studies. For instance, Slagsvold & Lifjeld(1989b) reported that body mass in female pied flycatchers(Ficedula hypoleuca) (but not males) is heavier at the end of the nestling period when rearing asynchronous broods compared to synchronous broods, suggesting that females work less in asynchronous than in synchronous broods.Furthermore, Hõrak (1995) showed that female (but not male)survival increases with brood reduction rate in great tits (Parus major), also suggesting that hatching asynchrony-induced brood reduction mostly benefits females. In addition,Slagsvold & Lijfeld (1989a) found that body mass of females rearing asynchronous broods is heavier at the end of the nestling period than that of females rearing synchronous broods, providing important support for their Sexual Conflict over Parental Investment Hypothesis (see above). Here, we showed that laying additional eggs that hatch asynchronously can be advantageous for females because the presence of small and hungry nestlings in the nest enhances paternal but not maternal feeding effort. Thus, it is possible that the detected beneficial effects identified by Slagsvold & Lijfeld(1989a) may also be explained by the MMH. In fact, the Sexual Conflict over Parental Investment Hypothesis, as explained by Slagsvold & Lijfeld (1989a), and our MMH are not mutually exclusive, and both may work in species or situations where asynchronously hatched nestlings survive during the nestling period.
Regarding food allocation among nestlings, the Brood Reduction Hypothesis does not predict any sexual differences in food allocation. Thus, when food is limited, larger nestlings of asynchronous broods are expected to obtain more food than smaller nestlings regardless of their begging intensity(Mock et al., 2011; Smiseth & Amundsen, 2002), leading to nestling starvation when food is scarce. However,accumulating evidence suggests that male and female parents follow different food allocation rules among their nestlings, i.e.,males preferentially feed larger nestlings, while females distribute food more evenly among offspring, or even favor smaller nestlings (Gottlander, 1987; Lahaye et al., 2015;Ploger & Madeiros 2004; Ryser et al., 2016; Soler et al.,2022). Our experimental manipulation affected parental food allocation by interacting with sex, supporting the general statements regarding food allocation in asynchronous broods.Notably, while males increased feeding rates to the largest nestlings in the body-size hierarchy within asynchronous broods, females decreased feeding rates independent of nestling body-size hierarchy (Figure 2).
Consistent with the second prediction, males in asynchronous broods concentrated their feeding efforts on larger nestlings, although the smallest nestling was not primarily fed by females. This is not surprising for two reasons:First, blackbirds typically live in food-abundant situations,allowing males to provide more food than required to larger nestlings, which may instead be delivered to the smallest nestling. In addition, the blackbird is a multi-brooded species with small clutch sizes in each breeding attempt (Cramp,1988; Del Hoyo et al., 2003; Ibáñez-Álamo & Soler, 2010),which is likely associated with the high predation rate suffered by this species (Ibáñez-Álamo & Soler, 2010). Notably, bird species living under high predation risk often produce smaller clutch sizes and more broods per year to reduce the costs of nest predation (Ferretti et al., 2005; Julliard et al., 1997;Slagsvold, 1984).
Second, although the blackbird is a good model species to test Prediction 1 given its facultative brood reduction, it is probably not the most appropriate model species to test Prediction 2, because although blackbirds suffer brood reduction, this situation is not very common (Magrath, 1989).The most appropriate model species to test Prediction 2 would probably be one with extreme hatching asynchrony and elevated brood reduction, such as the Eurasian jackdaw or Eurasian hoopoe.
Sexual conflict over parental investment occurs in almost all species with sexual reproduction, unless the evolutionary interests of the two sexes coincide entirely, which can be the case in bird species with obligate lifelong monogamy(Lessells, 2012). However, this is not the case for the blackbird due to its high within-season divorce rate(11%-14%; Wysocki, 2005) and potential for poly-territorial polygyny (Wysocki & Jankowiak, 2018). In addition, frequency of copulation is high and attempts at forced extra-pair copulation are common, indicating that frequent paircopulation behavior has evolved in response to both sperm competition and sexual conflict (Halupka, & Wysocki 2004).
In conclusion, our results support the first prediction of the MMH, i.e., in asynchronous broods, males increase their feeding rate, which directly benefits females who simultaneously reduce their parental effort. However, the effect of rearing synchronous or asynchronous broods on fitness outcomes for each sex remains to be demonstrated.Thus, further research is required to explore the link between brood rearing type and parental fitness outcomes and to test the novel MMH in other contexts and species.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
We performed the study following all relevant Spanish national(Decreto 105/2011, 19 de Abril) and regional (permissions provided yearly by la Consejería de Medio Ambiente de la Junta de Andalucía) guidelines. Ethics approval for this study was not required in that time. Collection of nestlings from their nests and transportation to other nests were made in accordance with CEEA (Comité de Ética de Experimentación Animal de la Universidad de Granada) recommendations,taking all necessary measures to minimize disturbance of both nestlings and breeding adults. Permission for field surveys in Lecrín, cross-fostering, and collection of nestlings was licensed by the Andalusian authority for wildlife protection(Dirección General de Gestión del Medio Natural de la Junta de Andalucía; Ref.: SGYB/FOA/AFR/CFS; 15/03/2012). The time spent at each nest was the minimum necessary. No females deserted their nests after experimental manipulation.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
M.S. conceived of the study, designed the study, coordinated the research, obtained funding, and wrote the first draft of the manuscript. F.R.-R. conducted field work and participated in statistical analysis and preparation of the first draft of the manuscript. L.S.-P. participated in field work, processed the recordings, and critically revised the manuscript. J.D.I.-A.contributed to experimental design, participated in field work,and critically revised the manuscript. J.J.S. carried out statistical analysis and participated in the preparation of the first draft of the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Teresa Abaurrea for assistance with field work and two anonymous reviewers for their constructive comments.
- Zoological Research的其它文章
- Diversity of reptile sex chromosome evolution revealed by cytogenetic and linked-read sequencing
- Coevolutionary insights between promoters and transcription factors in the plant and animal kingdoms
- Deficiency of transmembrane AMPA receptor regulatory protein γ-8 leads to attention-deficit hyperactivity disorder-like behavior in mice
- Global cold-chain related SARS-CoV-2 transmission identified by pandemic-scale phylogenomics
- The Hippo pathway and its correlation with acute kidney injury
- Genomics and morphometrics reveal the adaptive evolution of pikas

