Benefit-Risk Assessment for PD-1/PD-L1 lnhibitors in the Treatment of Non-Small Cell Lung Cancer
Li Zhuangqi,Yang Yue,
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China;2.School of Pharmaceutical Sciences,Tsinghua University,Beijing 100085,China)
Abstract Objective To explore the benefits and risks of PD-1/PD-L1 inhibitors Atezolizumab and Nivolumab in the treatment of non-squamous non-small cell lung cancer and provide some references for clinicians.Methods Based on the data results of relevant studies published by ClinicalTrical.gov in the US clinical trial database and foreign peer-reviewed journals,the internationally recognized multi-criteria decision analysis (MCDA) model was used to assess the benefit and risk of PD-1/PD-L1 inhibitors for non-squamous non-small lung cancer comprehensively.Finally,a sensitivity analysis was performed to test the sensitivity of the weight to the evaluation.Results and Conclusion The benefit-risk evaluation result of Atezolizumab for the treatment of non-squamous non-small cell lung cancer is better than that of Nivolumab.Specifically,Atezolizumab has more benefits than Nivolumab with a lower risk.The results of MCDA model in drug benefit and risk evaluation are easy to understand.However,the selection of indicators in the model and the degree of data acquisition are limited.The evaluation results of the MCDA model should be comprehensively viewed with other evaluations to make decisions objectively.
Keywords: PD-1/PD-L1 inhibitor;non-small cell lung cancer;multi-criteria decision analysis (MCDA);benefit;risk
Lung cancer is the most leading cause of cancer death in the world.Approximately 1.6 million people die from lung cancer each year,of which 85% are histologic subtypes of non-small cell lung cancer,and nearly half are non-squamous non-small cell lung cancer[1,2].
PD-1/PD-L1 refers to the programmed death ligand,a kind of protein on T cells,which can weaken the immunity of T cells in tumors.Inhibiting the activity of PD-1/PD-L1 can significantly improve the self-immunity of T cells,thus achieving the purpose of anti-cancer[3,4].Therefore,PD-1/PD-L1 inhibitors have been developed and approved for the treatment of various cancers,significantly improving the survival and recovery rate of patients[5].However,the risk of PD-1/PD-L1 inhibitors in the treatment of cancer has received little attention.Taking lung cancer as an example,this study evaluated and analyzed the safety and risk of PD-1/PD-L1 inhibitors used in previously treated non-squamous non-small cell lung cancer based on a multi-criteria decision analysis (MCDA) model.
1 Materials and methods
1.1 Source of information
As of October 2020,the US Food and Drug Administration (FDA) has approved 5 PD-1/PD-L1 inhibitors for the treatment of non-small cell lung cancer.Among them,Atezolizumab and Nivolumab can be used to treat metastatic non-squamous nonsmall cell lung cancer that have been treated in the past.This study is based on the phase III clinical trial of the two drugs in ClinicalTrical.gov for the treatment of non-squamous non-small cell lung cancer,and the data results in related peer-reviewed articles[6,7].The clinical trials of the two drugs are open-label,multi-center randomized controlled trials,and their indication is for previously treated metastatic nonsmall cell lung cancer.
All studies were evaluated and graded according to the fifth edition of the “Common Terminology Criteria for Adverse Events (CTCAE)” of the National Cancer Institute of the National Institutes of Health for adverse event (AE)[8](Table 1).

Table 1 AE classification
1.2 Theoretical basis and research methods
The benefit-risk assessment of drugs,which is guided by risk management theory and modern decision theory,runs through the entire life cycle of drugs.Evaluation results are not only used for regulatory decision-making,but also an important support for post-market safety monitoring[9].Therefore,the status of quantitative evaluation has become increasingly important.The quantitative evaluation of benefits and risks is based on sufficient data,including clinical trial data and real-world data.The application of methodology in this process is crucial.
The benefit-risk ratio is usually mentioned in the process of drug evaluation.But in fact,the benefit-risk ratio is not a good indicator to measure the relative importance of benefits and risks,because the two are not directly comparable.When measuring whether a drug can be approved for marketing,two aspects need to be considered.One is the comparability of benefits and risks and the other is the scientific nature of the quantitative methods and the interpretability of the results.Comparability is mainly the control group design and patient recruitment criteria in clinical trials.The scientific nature of evaluation methods and the interpretability of results depend on practice.According to previous research literature,there are currently three dimensions for comparing drug benefits and risks.The first is the evaluation based on preference and utility.MCDA model and Markov model solve the decision-making problems through this principle.The second is the incremental benefit and risk comparison.Since this method compares the absolute benefits and risks and does not take the clinical relevance into account,it is not often used.The third is to evaluate the benefits and risks through expert opinions and patient preferences.
The MCDA model is currently recognized as a good quantitative method for evaluating drug benefits and risks.In this study,MCDA model was used to quantitatively evaluate the benefits and risks of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer.MCDA model was first proposed in 1976,which was widely used in engineering,environmental,economic,and military fields.This model was introduced into the field of drug evaluation in 2007.In 2016,the International Society for Pharmacoeconomics and Outcome Research(ISPOR) proposed an implementation framework based on MCDA model in health decision-making,and explained operational precautions[10,11].After evaluation,the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency states that MCDA model is a systematic method with practicality and reliable operation among the existing quantitative evaluation methods for the benef its and risks of drugs.
The method for determining the weight of the subdivision indicators under risks and benefits adopts swing weighting,risk indicator and benefit indicator to assign weights independently.Swing weighting was proposed by Von Winterfeldt and Edwards in 1986.Its essence is to score according to the importance of the indicators in the evaluation system,and then determine the degree of importance of the indicators,thus obtaining standardized weights.The scoring range is 0-100,the more important the index,the higher the score.The swing weighting operation is simple and easy to understand.It is a way of combining quantitative and qualitative methods to determine weights.
2 Data processing
The evaluation of MCDA model includes 8 steps[12-14](Fig.1).First,it is necessary to clarify the background of the research problem and the practical significance of the research.This is the basis for carrying out the evaluation and a prerequisite for better evaluation.Then the benefit-risk value tree of the research object should be constructed to collect the corresponding data,assign weights,and calculate the benefit-risk value.Finally,a sensitivity analysis is performed on the weights of the indicators to verify the accuracy of the weight assignment and the research results[15],which can reduce the uncertainty in the value evaluation procedure and further improve the reliability of the evaluation results.

Fig.1 The evaluation steps of the MCDA model
The evaluation steps of the MCDA model include the core stages that regulatory agencies need to consider when evaluating the benefits and risks of drugs,namely the data collection and evaluation of benefits and risks.The final regulatory decision is based on the above evaluation results,which also comprehensively considers the actual medication needs.
After collecting data according to the indicators,the extreme value standardization method is used to process the data non-dimensionally.The extreme value standardization method distinguishes the positive and negative indicators.The positive indicator is the benef it indicator,and the reverse indicator is the risk indicator.


The steps for swing weighting are as follows[16]:
Determine the worst result of the index,that is,B=(X1,X2,...,Xn),and set its relative importance as 0 as a benchmark.
Construct the relative importance of each index asGj=(X1,X2,...,Xn),j∈{1,...,n},and the indexesGjandBare compared one by one,then the diff erence is determined to beDj.n Djcan be obtained from multiple indexes.
NormalizeDjand calculate the weight:

The overall benefit risk evaluation score is composed of two parts: The benefit index score and the risk index score.

3 Benefit-risk evaluation
First,the decision-making environment is determined.At present,PD-1/PD-L1 inhibitors for the treatment of cancer have attracted the attention of the public,but there are few studies on the benefitrisk evaluation of PD-1/PD-L1 inhibitors.This study took Atezolizumab and Nivolumab in the treatment of non-squamous non-small cell lung cancer as an example to explore the benefit-risk level of PD-1/PD-L1 inhibitors.Based on data from clinical trial database in the US,the trial drug Atezolizumab was injected intravenously at 2 100 mg every 21 days,and Nivolumab was injected at 3 mg/kg every 2 weeks.The test population was ≥ 18 years old and had received chemotherapy or drug treatment in the past.
Then,a value tree is built.Based on the primary and secondary clinical endpoints of PD-1/PD-L1 for non-squamous non-small cell lung cancer previously treated in the US clinical trial database,as well as the occurrence of adverse events,the value tree of benefitrisk indicators was determined[17,18],including 4 benefit indicators and 3 risk indicators (Fig.2).
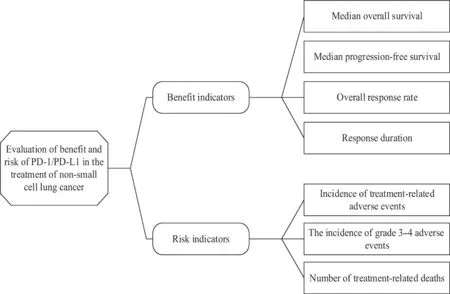
Fig.2 The value tree of benefit-risk PD-1/PD-L1 inhibitors for the treatment of non-small cell lung cancer
Based on the results of two clinical trials and peer-reviewed articles,data items of benefit indicators and risk indicators were extracted,and the data were all below the 95% confidence interval level.After that,weights were assigned to each indicator based on the swing weighting method (Table 2).According to the theory of risk management,the safety of drugs is a relative concept,which is a balance between benefits and risks.Rather than blindly seeking benefits or focusing only on risks,both are equally important.Therefore,the weights of the benefit index and the risk index are determined to be 0.5.According to modern decision-making theory,the importance of specific benefit indicators and risk indicators to overall benefits and risks is scored,and the weight data is finally determined according to the proportion of indicator scores in the overall benefit or risk system.

Table 2 Benefit-risk indicators of PD-1/PD-L1
Based on the value tree and the value and weight of each indicator,the benefit-risk score of PD-1/PDL1 inhibitors for previously treated non-squamous non-small cell lung cancer is calculated according to the following formula.The result is between 0-1,the higher the score,the better the effectiveness of the treatment and the lower the risk (Table 3).The results show that the benefit-risk evaluation results of Atezolizumab for the treatment of non-squamous non-small cell lung cancer are better than Nivolumab.Specifically,the benefits of Atezolizumab are better than Nivolumab with lower risk since there is one treatment-related death of Nivolumab.
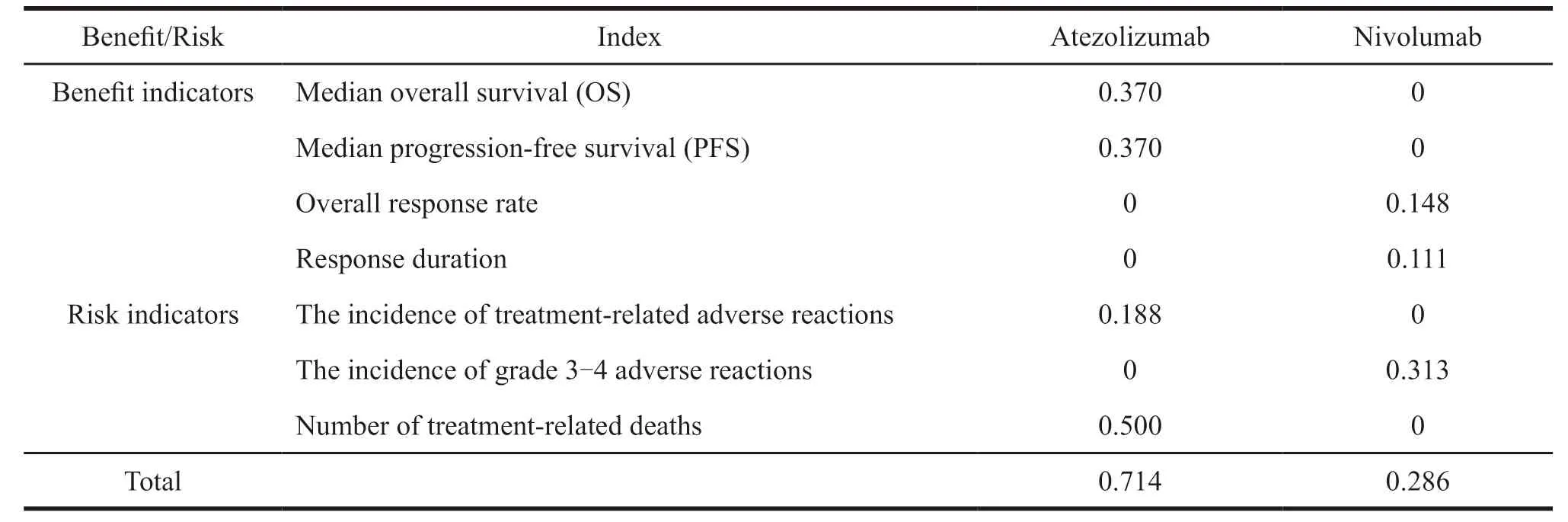
Table 3 Benefit-risk situation of PD-1/PD-L1
As to the sensitivity analysis,there is some subjectivity in swing weighting,so it will result in bias to a certain extent.If the relative importance of each index remains unchanged,the perturbation method will be used for the sensitivity analysis of the weight.The perturbation method refers to the influence on the output result after certain conditions change[19].The changing factor in this study is the weight.At present,it is not clear how much the weight changes when the model is considered stable.According to the research of other scholars,it can be considered that the model is stable if the degree of weight change is greater than 20%,which will affect the results[20].In this study,the weight changes by 20% or less,and it has no influence on the results.Therefore,it is believed that the weight setting is reasonable,and the model is relatively stable.
4 Discussion
The MCDA model is applied to the benefit-risk evaluation of drugs,and the framework is clear and easy to operate and understand.The result obtained by using MCDA model is an exact value.The evaluation result is Atezolizumab is better than Nivolumab,with a gap of 0.428.Whether such a small gap can get an accurate evaluation of the efficacy of the two,or how much difference between the scores can be used to draw an accurate conclusion is worthy of in-depth discussion.
Although the results of using MCDA model to evaluate the benefits and risks of drugs quantitatively are intuitive,they cannot be taken as the only criteria for regulatory decision-making and clinical drug use.It is necessary to combine this evaluation with other evaluations to obtain objective results to the greatest extent.
5 Conclusion
In this study,the innovative use of MCDA model to quantitatively evaluate the benefits and risks of drugs is of practical significance.The MCDA method has clear steps,easy operation,intuitive results,and can be applied to the benefit-risk evaluation research of all drugs.It should be used as evidence support for regulatory decision-making.
People are the subject of regulatory decisionmaking.Modern decision-making theory holds that human reason is limited as well as their cognition.Drugs are extremely complex substances,and their properties and information on safety and effectiveness are constantly recognized.People’s research on drugs is endless.At present,since people’s knowledge and research on a certain drug are always limited,their decisions cannot be correct all the time.In addition,decision-making is based on data and information.The quantity and quality of data and information can directly affect the decision-making.The concept of“cumulative data” is deduced from this,which means the more data on the safety and effectiveness of drugs,the more comprehensive people’s understanding of drugs,and the more authentic and reliable the regulatory decisions will be.
In summary,based on the accumulated data on drug safety,MCDA model for drug benefit-risk assessment is a feasible method,and the results of the assessment are interpretable.In the context of regulatory science,it can be better used to help decision-making.
- 亚洲社会药学杂志的其它文章
- Analysis of Factors Affecting Online Drug Purchase -Based on Factor Analysis
- Research on the Price Level of Drugs in Short Supply in China
- Research on Problems and Countermeasures of Patent Evaluation System in Pharmaceutical Enterprises
- Comparative Study and Enlightenment on lnnovation Achievements of Pharmaceutical lndustry in Liaoning Province
- Research on the Relationship between Cooperation lnnovation Expenditure and Economic Output of China’s Pharmaceutical lndustry
- Development Strategy of Green Marketing of Pharmaceutical Enterprises against the Background of Building a Beautiful China

