Comparative Study and Enlightenment on lnnovation Achievements of Pharmaceutical lndustry in Liaoning Province
Tao Na,Tian Lijuan
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To promote the innovation and development of pharmaceutical industry in Liaoning Province.Methods Literature study and comparative study were used to investigate the current situation and problems of innovationdriven development of pharmaceutical industry in Liaoning Province by comparing data of Heilongjiang,Jilin,Shandong,and Jiangsu from four aspects of priority review and approval,consistency evaluation of generic drugs,new drugs on the market,and scientific and technological innovation achievements.Results and Conclusion In terms of innovation-driven development,pharmaceutical industry in Liaoning Province has the following problems,such as small scale,weak transformation of scientific and technological achievements,tough business environment,and single platform module of scientific and technological achievements.Combined with the actual development of pharmaceutical industry in Liaoning province,the government should give full play to its leading role and guide “Benxi Pharmaceutical Capital” to establish characteristic industrial cluster and incubation park.After high quality enterprises and high-level innovative talents get together,enterprises can analyze their actual situation to plan R&D layout,focusing on the input-output ratio.Meanwhile,the benign development of the enterprises,universities and research institutes should be promoted to integrate technical innovation and product supply.This can enhance the cooperation among the government,enterprise,university,and research institute,and provide reference for further development of pharmaceutical industry in Liaoning province.
Keywords: Liaoning pharmaceutical industry;innovation-driven;innovative product
Innovation-driven development is the core strategy of the “National 13th Five-Year Plan”.As one of the pillar industries of innovation and development,pharmaceutical industry is a sunrise industry with strong growth,relevance,and initiative.The implementation of the innovation-driven development strategy is of great significance for deepening the reform of pharmaceutical industry,promoting the construction of a healthy China,and supporting the upgrading of pharmaceutical industry chain.
Liaoning Province has a good foundation for pharmaceutical industry.It has leading enterprises such as Northeast Pharmaceutical and Neusoft Medical,as well as some pharmaceutical colleges and research institutes,providing an opportunity for innovation-driven development.In recent years,the state and the province have issued a series of policies to promote the development of Liaoning pharmaceutical industry and encourage technological innovation,including the establishment of Benxi National Biomedical Industry Base,Liaoning Sishui Medical Device Technology Industry Base,and other first-class national pharmaceutical industry bases with local characteristics[1].
However,the overall level of pharmaceutical industry in Liaoning Province is lower than the national average,and the gap between Liaoning Province and other provinces with developed pharmaceutical economy (Jiangsu,Shandong) is widening gradually.Even the neighboring provinces (Heilongjiang,Jilin)also have a large gap with Liaoning.The innovationdriven development of pharmaceutical industry in Liaoning is still faced with many problems.For example,the lack of innovation ability of enterprises leads to the low technical content of products.Therefore,they do not have better competing products to occupy the market.Besides,their R&D capacity is low,and the output of innovative drugs is insufficient.The small scale and weak strength of enterprises make it difficult for them to develop healthily.Therefore,the reform and innovation of pharmaceutical industry in Liaoning Province is imperative.
1 Evaluation of the innovation of pharmaceutical industry in Liaoning Province
The main criterion for evaluating the innovation ability of pharmaceutical industry is the output of achievements.Based on the latest data and the actual situation of innovation of pharmaceutical industry in Liaoning province,four indicators such as priority review of the number of drug approval listed,the number of drugs by generic consistency evaluation,quantity listed drugs,and transformation of scientific and technological achievements were selected through to make the comparison with the developed provinces of Shandong,Jiangsu,Heilongjiang,and Jilin.Furthermore,the problems of innovation-driven development of pharmaceutical industry in Liaoning Province were found out.
1.1 Priority for review and approval
Following the special approval and special approval system,China has established the drug priority review system to further encourage drug innovation research and development,accelerate the efficiency of clinically urgent drugs on the market,and improve the enthusiasm of drug innovation[2].The priority review and approval system mainly aims at new drugs in urgent need,such as children’s drugs,and drugs for rare diseases,etc.
According to the statistics of the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA),from February 28,2016 to May 27,2020,a total of 1 109 drugs were given priority for review and approval,covering 533 varieties.Among them,a total of 240 drugs from these five provinces,covering 140 varieties,were included in the priority review and approval.The number of drugs included in priority review and approval in Jiangsu and Shandong was 171 and 51,respectively.While the number of drugs included in Liaoning was only 10,accounting for 4.2% of the total number of drugs in the five provinces,as shown in Fig.1.The number of drugs included in the priority review and approval from the three provinces in Northeast China is small.Although Liaoning has certain advantages compared with Heilongjiang and Jilin,there is still a big gap between Liaoning and other developed provinces.
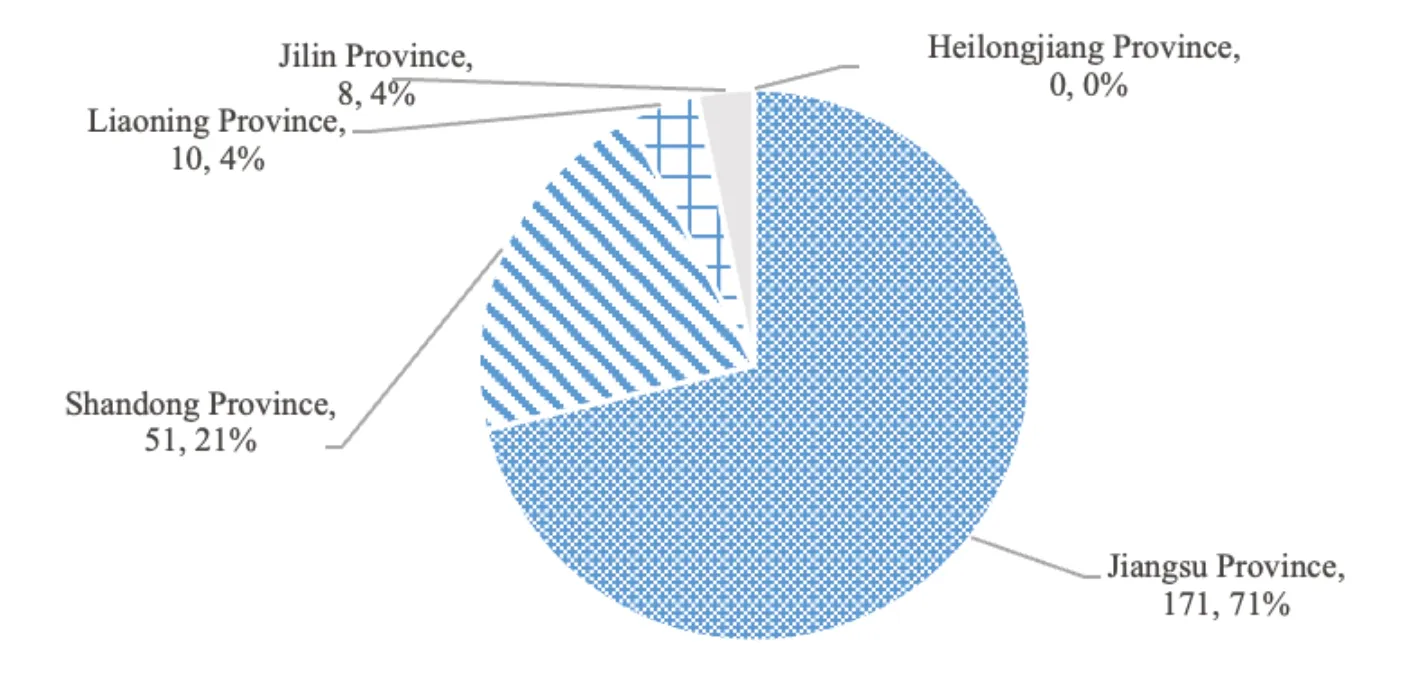
Fig.1 The number and proportion of drugs included in priority review and approval from the five provinces
The number of drugs that have passed the priority evaluation and approval shows the scientific research and innovation ability of an enterprise.By 2019,179 drugs had been approved to market faster due to the preferential review and approval system,which eased market demand.In terms of the number of drug varieties,21 drug varieties are in Jiangsu Province,accounting for 12%,and 3 drug varieties in Shandong Province,accounting for 2%.In Liaoning Province,only carrimycin tablets (specifications:0.2 g) from Shenyang Tonglian Pharmaceutical Co.,Ltd.was included in the priority review and approval as a special new drug,which was approved to be marketed on June 24,2019.In terms of the scope of priority review and approval,76 new drugs have obvious clinical value,accounting for 57%,followed by 17 new drugs for children,accounting for 13%.As shown in Fig.2,new drugs with obvious clinical value account for the largest proportion (57%) among the drugs included in the priority review and approval.It further indicates that this system accelerates the research and development and marketing of innovative drugs,adding new impetus to the research and development of innovative drugs for enterprises,which also points out the direction for pharmaceutical enterprises in Liaoning Province.

Fig.2 Distribution of the scope of inclusion priority review and approval
1.2 Consistency evaluation of generic drugs
A series of technical requirements,such as reference preparation,prescription process,quality research and control,and stability research become the main obstacles in the consistency evaluation of enterprises.Therefore,if enterprises want to pass the consistency evaluation,they need to comprehensively improve their technological research and development ability,which can only be realized by continuous improvement and innovation of enterprises.In other words,consistency evaluation forces enterprises to carry out technological innovation,achieving the consistency of quality and efficacy of drugs.This method can encourage and promote pharmaceutical enterprises to research and develop innovative drugs.
By May 10,2020,a total of 9 drugs in Liaoning Province had passed the consistency evaluation of generic drugs,covering 7 drug varieties from 4 companies,as shown in Table 1.Among them,pyrazinamide tablets (specifications: 0.25,0.5 g)developed and produced by Shenyang Hongqi Pharmaceutical Co.,Ltd.,as the first application in Liaoning Province,passed the evaluation of generic drug quality and efficacy consistency on January 9,2019.It is the first variety of this type to obtain the approval of the NMPA.Pyrazinamide tablets successfully approved by the consistency evaluation of generic drugs in Liaoning province is of great significance.It both made a major breakthrough in new drug research and development,and set up a good model of technological innovation to other enterprises in the province.Therefore,it has a positive radiationdriven effect[3].

Table 1 Drugs of pharmaceutical enterprises in Liaoning Province through consistency evaluation
Since the implementation of generic drug consistency evaluation,according to CDE data statistics,from September 29,2017 to May 10,2020,a total of 845 drugs applied for the evaluation,including 267 drug varieties.They all passed or were deemed to have passed the consistency evaluation of generic drugs,involving 304 enterprises.160 drugs in Jiangsu and 97 drugs in Shandong passed the consistency evaluation and obtained the approval document number.The number of drugs approved in the two provinces accounted for 30.41% of the total.The number of approved document numbers in Liaoning,Jilin and Heilongjiang is far less than that in developed provinces.Both Liaoning and Jinlin have 9 drugs that passed the consistency evaluation and obtained the approval document numbers.Heilongjiang had 10 that obtained the document number.Liaoning only accounts for 3% of the total number of drugs in the five provinces,as shown in Fig.3.

Fig.3 Number and proportion of approved document numbers passed the consistency evaluation in the five provinces
On May 14,2020,the CDE of the NMPA issued three documents,including “Technical Requirements for Consistency Evaluation of Quality and Efficacy of Generic Drug Injections for Chemical Drugs”(NMPA [2020] No.9),etc.,which provided detailed provisions on the specific techniques and application contents for consistency evaluation of injections.By May 18,2020,the total number of acceptance numbers for consistency evaluation of listed generic drugs had reached 2 052,among which 721 were accepted for consistency evaluation of injections.At present,26 provinces and cities have applied for the consistency evaluation of injection.Jiangsu Province has the largest number of drug acceptance,with 172,accounting for 24%.The drug varieties accepted for consistency evaluation of injections in Liaoning Province only accounted for 5% of the total number in the five provinces,as shown in Fig.4.Although it is ahead of the neighboring provinces of Jilin and Heilongjiang,but falls far behind Shandong,Jiangsu and other provinces with developed pharmaceutical industry.In addition,by the end of May 2020,33 varieties of injections had applied for the evaluation,among which only azithromycin for injection passed the consistency evaluation,and the rest were regarded as passed.A total of 15 injections in Liaoning Province were included in the generic drug consistency evaluation,but none of them passed the generic drug consistency evaluation.
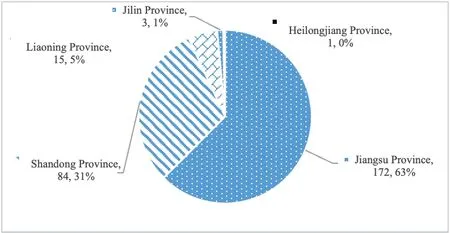
Fig.4 The proportion of accepted numbers in consistency evaluation of injections in the five provinces
1.3 Major project of drug innovation
“New Drug R&D Project” is a national scientific and technical program to improve the drug innovation system,enhance the innovation ability of pharmaceutical industry,accelerate the transformation of China’s pharmaceutical research and development from imitation to creation,and transform China from a country of pharmaceutical production to a pharmaceutical creation.The approval of the “New Drug R&D Project” for enterprises is conducive to promoting the leapfrog development of pharmaceutical enterprises from imitation to independent innovation[4].
Since the launch of the “New Drug R&D Project”,China has set up more than 1 900 projects,with a total investment of nearly 20 billion from the central government and about 200 billion from local governments,enterprises,and other sources.An innovation system with various technology platforms has been initially put in place,playing an important role in ensuring and improving people’s livelihood.By July 2019,a total of 139 drug varieties had obtained new drug certificates,including 44 first-class new drugs,which was 8 times as much as the number before the start of the special program[5].
With the support of the “New Drug R&D Project”,from 2018 to June 2020,China approved 29 varieties of independently researched innovative drugs (including chemical drugs and therapeutic biopharmaceuticals),among which 25 were firstclass new drugs.A total of 10 first-class new drugs have been approved for marketing in five provinces,among which 8 first-class new drugs were approved in Jiangsu,accounting for 80%,as shown in Fig.5.Shandong Province and Liaoning Province received 1 first-class new drug approval respectively,accounting for 10%.The only one in Liaoning Province is the first-class new drug carrimycin tablets from Shenyang Tonglian Pharmaceutical Co.,Ltd.,which was approved in July 2019.After retrieval and analysis,we found that “New Drug R&D Project” has made great progress under the national major scientific and technical project.Among the approved first-class new drugs,clicillamycin tablets from Liaoning Province,carrizumab for injection,cindilizumab injection,pyrrottinib maleate tablets,amrottinib hydrochloride capsules,abvetai for injection,and recombinant cytokine gene-derived protein injection from Shandong Province were all approved with special support.

Fig.5 The first-class new drugs approved by NMPA for the domestic marketing from June 2018 to 2020
1.4 Transformation of scientific and technological achievements
Transformation of scientific and technological achievements is an important basis to promote economic development.To implement the innovationdriven development strategy,the transformation of scientific and technological achievements is particularly critical[6].Therefore,the ability and level of transformation of scientific and technological achievements have become another factor to measure the innovation ability of pharmaceutical enterprises.
Based on the data from the National Scientific and Technical Achievements Database,the achievements of national scientific and technical plan,department/local science and technology plan,state science and technology award and department/local science and technology award are analyzed.The results are as follows.By May 28,2020,955 transformation projects of scientific and technological had been achieved in pharmaceutical industry.373 projects are in Liaoning,Jilin,Heilongjiang,Shandong,and Jiangsu provinces.Jiangsu has achieved the most with 168 transformation projects,accounting for 30%.Jilin and Heilongjiang have 26 and 31,respectively.There are 37 in Liaoning,accounting for 10%.Although the transformation of scientific and technological achievements in Liaoning has increased a little,the overall level of technological transformation is still low compared with developed provinces,as shown in Fig.6.
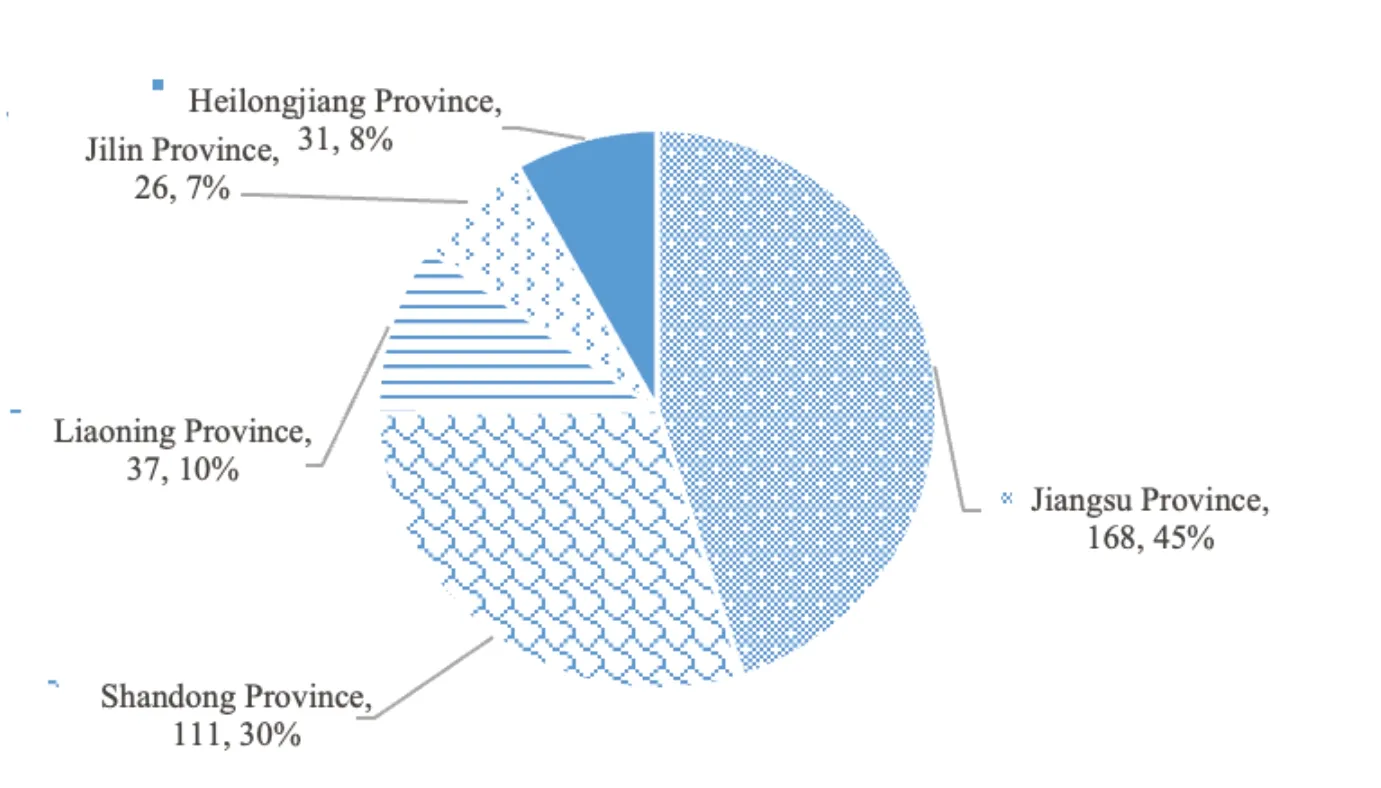
Fig.6 Number and proportion of transformation projects of national scientific and technical achievements in the five provinces
2 Results and discussion
2.1 Result analysis
Through the study of four innovation indicators,it can be seen that the innovation ability and output of innovation achievements of pharmaceutical industry in Liaoning Province are much lower than that in Jiangsu and Shandong Province,and close to Jilin and Heilongjiang.Compared with other provinces and cities,pharmaceutical industry in Liaoning Province has problems such as imperfect industrial structure,low innovation ability of enterprises,insufficient R&D investment,and low output value of new products.Pharmaceutical enterprises in Liaoning Province are generally small scale.In the list of the top 100 pharmaceutical enterprises in China in 2019,only one pharmaceutical enterprise is in Liaoning Province.There are few driving enterprises with competitiveness and influence,and small and medium-sized enterprises with outdated technology and low production rate further affect the innovation output of pharmaceutical industry in Liaoning Province.At the same time,the lack of high-tech talents limits enterprises’ innovative development,which hinders the development of pharmaceutical enterprises.Moreover,the lack of external support for the innovation and development of pharmaceutical industry in Liaoning Province leads to a poor business environment,which makes it more difficult to introduce domestic and foreign investment.Therefore,the good environment of industrial innovation needs to be strengthened urgently.Through looking up information on the National Scientific and Technical Achievements Database,Liaoning Pharmaceutical Industry Association and other platforms,we found that the platform module for scientific and technological achievements in pharmaceutical industry in Liaoning province is single,the degree of sharing of information resources is not high,and the government does not give policy support in the production and resource integration.Besides,the connection between the universities,research institutes,and enterprises is not close enough.Due to the insufficient channels of transformation,scientific and technological achievements are unable to be changed into practical products.Therefore,a good cycle of funds feeding back scientific research cannot be formed,thus affecting the output of innovation achievements.
2.2 Suggestions
Firstly,the government should attach great importance to the innovation-driven development of pharmaceutical industry,and all the government departments should clarify their responsibilities and roles in providing enterprises with introduction of review experience,policy answers,innovation project awards and technical guidance.The government should also make relevant policies such as financing and investment support,building financing platforms,and investing funds in key projects for innovative development to alleviate the difficulties of financing for enterprises.At the same time,the government should also increase the investment support,attracting capital to carry out innovation activities in Liaoning Province.“Benxi Pharmaceutical Capital” should be actively guided to establish characteristic of industrial cluster and Liaoning biomedical R&D incubation park.High-quality enterprises and highlevel innovative talents should be gathered with good policy support.Besides,resources integration between enterprises should be encouraged to drive the development of small and medium-sized enterprises.Liaoning Pharmaceutical Enterprise Incubator is established to provide a good business environment for the innovation and development of pharmaceutical industry.A comprehensive platform for scientific and technological innovation of pharmaceutical industry should be set up to concentrate the transformation resources of scientific and technological achievements.Then,the related resources,technologies,and information of pharmaceutical innovation industry can be shared to enhance their innovation abilities.
Secondly,the distribution of pharmaceutical industry in Liaoning province is given priority to biological pharmaceutical and chemical medicine.Other fields are weak.Based on the present situation of pharmaceutical industry,at this stage enterprises should focus on layout of reference preparations with specifications,formulation optimization,and market competition.Meanwhile,the input-output ratio should be taken into consideration to promote the generics.The independent innovation can be gradually realized.Then,the introduction and training talents should be strengthened.For instance,an innovative incentive salary system can be established as well as welfare policies and infrastructure.Meanwhile,the autonomy of researchers should be encouraged and their material and spiritual needs must be met to create an atmosphere for technological innovation.
Under the guidance of the government,pharmaceutical colleges and universities can be fully relied on to establish medical platforms in Liaoning Province.In addition,enterprises should set up a special talent strategic fund with Shenyang Pharmaceutical University to train innovative talents,combine scientific research with practice,thus promoting the relationship between enterprises,universities,and research institutes.When the technological innovation and the product supply closely cooperate,the integration of government,industry,universities,and research institutes can be promoted for the innovative pharmaceutical industry chain,which will stimulate the scientific and technological innovation vitality of pharmaceutical industry in Liaoning Province.
- 亚洲社会药学杂志的其它文章
- Analysis of Factors Affecting Online Drug Purchase -Based on Factor Analysis
- Benefit-Risk Assessment for PD-1/PD-L1 lnhibitors in the Treatment of Non-Small Cell Lung Cancer
- Research on the Price Level of Drugs in Short Supply in China
- Research on Problems and Countermeasures of Patent Evaluation System in Pharmaceutical Enterprises
- Research on the Relationship between Cooperation lnnovation Expenditure and Economic Output of China’s Pharmaceutical lndustry
- Development Strategy of Green Marketing of Pharmaceutical Enterprises against the Background of Building a Beautiful China

