Research on Problems and Countermeasures of Patent Evaluation System in Pharmaceutical Enterprises
Zeng Yingying,Shen Manzhu,Yuan Hongmei,Han Xu
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To provide some suggestions and countermeasures for improving the patent evaluation system in pharmaceutical enterprises.Methods Literature research method was used to collect the relevant documents of pharmaceutical enterprises and patent evaluation system,and then the problems of traditional patent evaluation methods for pharmaceutical patents were studied.Results and Conclusion The evaluation of pharmaceutical patents plays an important role for pharmaceutical enterprises.However,it is urgent to improve the pharmaceutical patent evaluation system because there are many difficulties in evaluating pharmaceutical patents,such as large fluctuation of patent value,strong subjective interference,the incomplete pharmaceutical information,unapplicable traditional evaluation method,and the insufficient role of evaluation agencies.Therefore,some suggestions are put forward: (1) The application of the real option method;(2) Developing evaluation institutions and training evaluation personnel;(3) Improving the laws and policies related to the evaluation of pharmaceutical patents;(4) Building a pharmaceutical patent evaluation database.
Keywords: pharmaceutical enterprise;patent;evaluation system
Under the background of in-depth development of knowledge economy,intellectual property has become the focus of international competition.China attaches great importance to intellectual property in improving international competitiveness.The pharmaceutical industry is an intellectual propertyintensive industry.For pharmaceutical enterprises,intellectual property is an important indicator to measure their comprehensive strength.In the business transactions such as patent transfer of pharmaceutical enterprises,patent evaluation is necessary.However,the existing patent evaluation system is not fully applicable to pharmaceutical patents in China.Therefore,this paper reviews the current patent evaluation method,listing its problems in the evaluation of pharmaceutical patents,and putting forward some suggestions to improve the evaluation system.The perfect evaluation system can effectively help pharmaceutical enterprises to market their R&D achievements and enhance their innovation ability.
1 Introduction
1.1 Patent valuation
Patent evaluation is the process of making analysis,evaluation and offering suggestions by professional evaluation organizations and their staff according to certain principles,considering the factors affecting the patent value in accordance with the legal procedures.It is a special intangible asset evaluation that focuses on the future economic benefits of patents.Through the evaluation,the value of intellectual labor can be understood more specifically,which is in line with the general background of the era of knowledge economy and promotes the commercialization of patented technologies[1].
1.2 Patent evaluation system
The existing patent evaluation system consists of quantitative method and qualitative method.For quantitative evaluation methods,the cost approach,market approach and income approach in economic methods are generally accepted[2].As for the qualitative evaluation method,the patent value analysis index system is relatively mature in China,which is jointly developed by the China National Intellectual Property Administration and China Technology Exchange.
1.2.1 Quantitative evaluation method
The cost approach refers to the method of calculating the value of the asset by deducting the depreciation from the complete replacement cost of repurchasing assets that have the same purpose and function as the asset to be assessed.
The market approach refers to a method that selects several similar assets to be evaluated in the market as a reference,compares the trading conditions and prices of these similar assets,adjusts the characteristics of the assets,and determines the value after comprehensive analysis.
The income approach is a method to determine the value of assets by estimating the expected income of assets in the future and converting it into present value according to a certain discount rate.
The calculation formula is:,the meaning of each parameter in the formula is shown in Table 1.
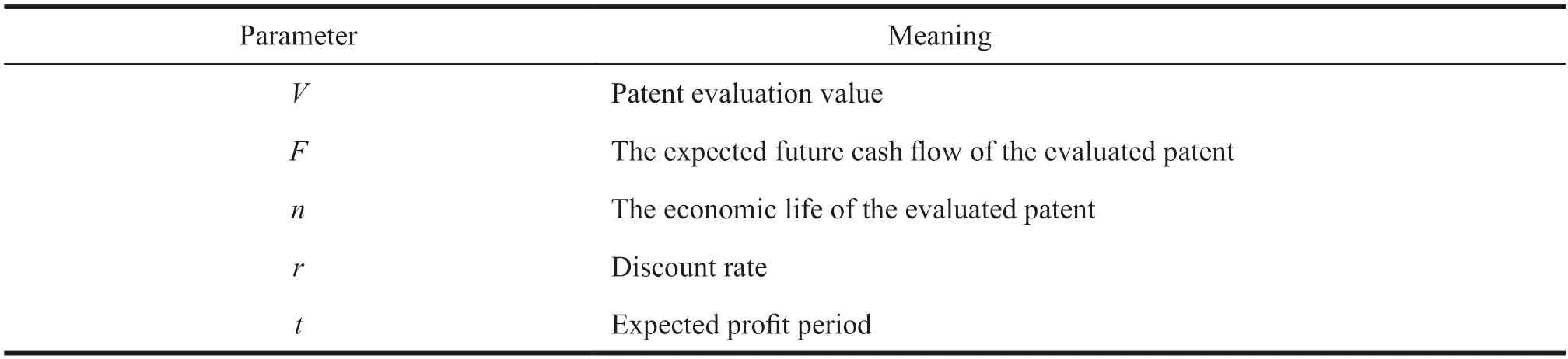
Table 1 Parameter meaning
1.2.2 Qualitative evaluation method
The patent value analysis index system is a system that can reflect the overall characteristics of the evaluated patent.Each index in the system is internally linked and complementary to each other.This system points out that patent value is composed of legal,technical,and market value.These three values have their own secondary and tertiary indicators.18 secondary indicators are factors that affect the value of patents,and the tertiary indicators are specific evaluation criteria.Patents are scored based on these indicators.Then the weight of each secondary index is artificially specified.Finally,the total value scores are obtained[3].Table 2 shows the primary and secondary indicators in the patent evaluation index system.

Table 2 Patent evaluation indicator
1.3 Characteristics of patent evaluation of pharmaceutical enterprises
The high risk and large investment in the pharmaceutical industry makes pharmaceutical patents have the following characteristics.
1.3.1 Difficulty in patent evaluation
It takes a lot of time from the discovery of lead compound,pre-clinical research,clinical phase I to III trials to the approval of marketing of pharmaceutical products[4].In different stages of the value of the product is not the same.For example,drugs in preclinical research and clinical trials have no economic value.The number of factors inpharmaceutical patent evaluation makes the work more difficult.
1.3.2 Large fluctuation of patent value
The value of pharmaceutical patents is easily affected by external factors such as national pharmaceutical approval policies and industrial policies[5].If the government holds a loose policy on the examination and approval of pharmaceutical products,and the marketing process of pharmaceutical products will be accelerated,then the patent value will rise.On the contrary,it goes down.Related technologies within the industry will also affect the value of patents.For example,as the technology in a certain field becomes better,the value of pharmaceutical patents in that field will decline.
1.3.3 Strong subjective interference
When a pharmaceutical enterprise conducts a patent transaction,due to the lack of a scientific evaluation system,the two parties usually negotiate the price multiple times.This transaction method is biased.The price of pharmaceutical patents of patentees with high social prestige is higher when they are traded.Sometimes even the price of the same patent transfer is not the same,depending on the outcome of the negotiation between the two parties.
2 Analysis on the problems and causes of patent evaluation in pharmaceutical enterprises
2.1 The problems of patent evaluation in pharmaceutical enterprises
2.1.1 Pharmaceutical information is not completely true
Medical information and related data are very important to the patent evaluation.The more comprehensive the data,the more it can help enterprises evaluate patents from different angles and make the evaluation results scientific.However,in actual pharmaceutical patent transactions,the disclosure of relevant patent information is limited,or the information disclosed is intended to confuse the company’s competitors,which is not completely true.Pharmaceutical enterprises need to spend more time and energy on market research and comparative analysis of relevant information in the application activities such as patent transfer to improve the accuracy of the evaluation,which greatly increases the cost of patent evaluation.The high expectations of both parties sometimes make them unable to finish the transaction.Therefore,the marketization of pharmaceutical products is blocked,which slows down the development of China’s pharmaceutical industry to a certain extent.
2.1.2 Traditional evaluation methods are not applicable
There are some problems in the traditional method of evaluating the value of pharmaceutical patents.The value of a patent cannot be determined solely by cost.It is not that the larger the cost,the greater the value of the patent.There are some examples in the market where the final research or development of pharmaceutical products with large investment fails or the value is zero due to no market demand.For instance,in May 2018,Kanghong Pharmaceutical launched the global phase III clinical trial of conbercept for the treatment of neovascular age-related macular degeneration (nAMD).It invested a total of 1.397 billion yuan in this clinical trial over the past two years.However,on April 9,2021,the company announced that due to multiple factors,including the COVID-19 pandemic,it decided to terminate this clinical trial[6].Although the company invested huge costs,the value of this patent did not exist.In addition,the value of a patent is difficult to identify and measure since the value of an intangible asset depends more on the benefits of related projects or products,rather than the expense and cost of acquiring it.Therefore,domestic scholars believe that the cost method should not be applied to the evaluation of patented technology.
The premise of using the market approach is the existence of an active trading market.Fig.1 shows the number of transactions in the market of new biological and pharmaceutical product transfer each year.In 2016,the number of transactions was the largest,but there were only 375,and the number of transactions each year accounted for only about 0.1% of the total.This means the market of China’s pharmaceutical patent transaction is not mature at present,and it is not easy to obtain the reference and relevant data parameters for market approach.Therefore,it is not common to use market approach due to conditional restrictions.There are two problems when we use the income approach to evaluate patents.First,the future net income of patents is unpredictable.Second,the value of patents is the discount of expected income,but the discount rate is difficult to determine.

Fig.1 Number of contracts transacted in the transfer market of new biological and pharmaceutical products
The difficulty of applying traditional evaluation methods directly affects the transfer of innovative technologies and products of pharmaceutical enterprises.It is manifested in that high value of pharmaceutical patents will increase the transfer fee and license fee,which can reduce the enthusiasm of the patent assignee.It is not conducive to the marketization of pharmaceutical products.On the contrary,it will greatly reduce the expectation of income from the transfer of pharmaceutical enterprises and discourage their enthusiasm.
2.1.3 The role of evaluation agencies is insufficient
The development of evaluation agencies in China is not fully mature.Under the situation of increasing patent trading activities and problems in the evaluation system,the deficiency of evaluation agencies becomes more obvious.There are many factors that affect the value of pharmaceutical patents.For instance,the evaluation agency cannot make a complete and accurate evaluation result,and the evaluation personnel of the agency mainly possess the background of assets or financial accounting.They not only lack laws and regulations related to the patent knowledge,but also cannot fully understand medical products,which makes the patent evaluation more challenging.
2.2 The causes of patent evaluation in pharmaceutical enterprises
2.2.1 Having difficulty in selecting appropriate evaluation parameters
Traditional evaluation methods involve multiple parameters when evaluating patents.In practice,it is difficult to select appropriate evaluation parameters.At the same time,in the process of collecting patentrelated information,pharmaceutical enterprises do not extensively collect the relevant information of both parties in the patent transaction and strictly examine the authenticity of the data,which makes the parameter selection difficult and the evaluation results inaccurate.
2.2.2 Lack of perfect pharmaceutical patent evaluation system
There is not a complete evaluation system for pharmaceutical patents in China because the evaluation is still based on three traditional methods.There are some problems in the traditional evaluation methods,which affect the comprehensive and accurate evaluation of pharmaceutical patents.Therefore,it can lead to the sluggish market of pharmaceutical patents,reducing the speed of the development of the pharmaceutical industry.
2.2.3 Imperfect laws and policies for patent evaluation
The laws,regulations and policies related to patent evaluation are an important guarantee to ensure the legal evaluation agencies to carry out their work properly.Besides,they can keep the access of illegal agencies to disrupt the industry evaluation standards and destroy the evaluation market.At the same time,since there are a large number of evaluation agencies in China,the industry competition is fierce.Due to the lack of legal constraints,some of the evaluation personnel of the agencies violate professional ethics and make false evaluation.Their behaviors should be punished.The imperfect regulations and policies make the evaluation agencies unable to play a full role.
3 Suggestions on improving the patent evaluation system of pharmaceutical enterprises
3.1 Application of real option method
A real option is an option on a real asset.As for pharmaceutical patents,an enterprise can first invest part of the capital to start drug research and development,and then take actions according to its development and the changes of market.For example,if the market prospects are clear,the enterprise will continue to invest,otherwise,it will abandon the investment[7].
The applicability of the real option method to the evaluation of pharmaceutical patents is embodied in the following.Firstly,the real option method is suitable for evaluating the high-input,high-yield,and highgrowth enterprise,and pharmaceutical enterprises have these characteristics.Secondly,the real option method is suitable for some evaluations with uncertain factors.Pharmaceutical enterprises face the risk of failure in all links of research and development of pharmaceutical products,which are full of uncertainty.And this kind of uncertainty can be solved by the real option method.
3.2 Developing evaluation agencies and training professional personnel
With the development of the patent transaction,it is urgent to give full play to the role of evaluation agencies.In order to develop evaluation agencies and train professional personnel,laws and regulations must be used to restrict the market access of evaluation agencies and personnel.The government should increase supervision of evaluation agencies and personnel to form a standardized evaluation system.Meanwhile,it is necessary to pay attention to the professional quality of the evaluators,which is closely related to the evaluation results.A series of measures should be taken cultivate the evaluators who have the knowledge of pharmaceutical patents and related laws,so as to make professional and accurate evaluation.
3.3 Improving the laws and policies related to the evaluation of pharmaceutical patents
Accurate evaluation of pharmaceutical patent is inseparable from normative legal mechanism.The legal mechanism of patent evaluation in China has been improving for a long time.The current legal system related to patent evaluation includes the “Guidelines for the Evaluation of Intellectual Property Assets” and other provisions.However,there are still problems in the specific implementation of pharmaceutical patent transactions.We should continue to improve the evaluation rules according to the needs of pharmaceutical patent transaction market.They can become an important basis for scientific and reasonable evaluation of pharmaceutical patents.
3.4 Building a pharmaceutical patent evaluation database
With the continuous development and update of the Internet,big data has become a part of the future development of the pharmaceutical industry[8].Therefore,government departments can use network technology to build an information platform and evaluation database for pharmaceutical patents to solve the problem of inaccurate evaluations.Besides,it can provide a communication platform for market entities such as pharmaceutical companies and evaluation agencies,so that they can exchange information about pharmaceutical patents.The evaluation database can also be used to provide services related to pharmaceutical patents and to realize resource sharing,which will make the evaluation of patent more objective and accurate.
4 Conclusions
Under the background of knowledge economy,patent is an important index to measure the comprehensive strength of pharmaceutical enterprises,and the evaluation of pharmaceutical patents is an important link in the daily business activities of pharmaceutical enterprises.Due to the particularity of pharmaceutical patents,there are some difficulties in evaluating pharmaceutical patents.Therefore,the government should improve the related laws and regulations,strengthen the supervision and management,set up professional pharmaceutical patent evaluation agencies,train high-quality evaluation personnel,provide strong support for the patent evaluation of pharmaceutical enterprises,and promote the vigorous development of China’s pharmaceutical industry.
- 亚洲社会药学杂志的其它文章
- Analysis of Factors Affecting Online Drug Purchase -Based on Factor Analysis
- Benefit-Risk Assessment for PD-1/PD-L1 lnhibitors in the Treatment of Non-Small Cell Lung Cancer
- Research on the Price Level of Drugs in Short Supply in China
- Comparative Study and Enlightenment on lnnovation Achievements of Pharmaceutical lndustry in Liaoning Province
- Research on the Relationship between Cooperation lnnovation Expenditure and Economic Output of China’s Pharmaceutical lndustry
- Development Strategy of Green Marketing of Pharmaceutical Enterprises against the Background of Building a Beautiful China

