Isolation and Identification of Pathogenic Fungi in Kiwifruit during the Storage Period
Baolin SHAO, Luxi LIU, Baiyu SU, Yifen YANG, Ying ZHANG, Fang LIAO, Aiping ZHENG
1. Technical Center of Chengdu Customs, Chengdu 610041, China; 2. Animal & Plant and Food Inspection Center of Tianjin Customs, Tianjin 300461, China; 3. Sichuan Agricultural University, Chengdu 611130, China
Abstract [Objectives] The paper was to identify the pathogenic species of postharvest diseases of kiwifruit. [Methods] The pathogenic fungi were isolated from diseased kiwifruits during the storage period, and cultured to observe their culture traits and morphological characteristics. Molecular biological identification and pathogenicity test were carried out to confirm the main pathogens. [Results] There were 7 species of pathogenic fungi causing kiwifruit diseases during the storage period, including Alternaria spp., Botryosphaeria dothidea, Phomopsis/Diaporthe spp., Pestalotiopsis sp., Pseudocercospora sp., Colletotrichum sp., and Botrytis cinerea. [Conclusions] The research will provide a scientific basis for the prevention and control of postharvest diseases of kiwifruit.
Key words Kiwifruit; Storage period; Pathogenic fungi; Identification
1 Introduction
Kiwifruit is a favorite fruit with soft texture and sweet and sour taste. Kiwifruit contains 18 kinds of amino acids necessary for the human body, as well as calcium, iron, zinc, selenium and other elements, which is honored as "the kings of fruits" due to high content of vitamin C and rich nutrition. China is the origin and a big producer of kiwifruit. According to the statistical data of2017ChinaKiwifruitIndustryDevelopmentReport[1], China’s kiwifruit production reached 2.37 million t in 2016, mainly concentrated in Shaanxi, Henan and Sichuan, and the output of the three provinces accounts for 85.2% of the total output of kiwifruit in China. Because kiwifruit belongs to respiratory climacteric fruit, it will easily become soften and rot after harvesting, and its flavor and edible value will rapidly reduce. Besides, the lag of diseases and pests control, mechanical damage of storage and transportation, and imperfect preservation technology eventually lead to a large amount of decay and cause huge economic losses every year, seriously restricting the healthy development of the kiwifruit industry. The occurrence of postharvest diseases, on the one hand, is the natural latent infection of pathogens, or the invasion of pathogens caused by artificial and mechanical damage in the early storage period, resulting in rot and deterioration of fruits during the storage period. On the other hand, the pathogens will grow and diffuse on kiwifruit in the late storage period, especially in the case of poor preservation and storage conditions, the occurrence of diseases will lead to a sharp decline in the quality of kiwifruit and a sharp increase in the rate of rotten fruits, causing serious economic losses.
Kiwifruit diseases during the storage period have not been systematically studied, and there are many species of diseases during the storage period, which are inconsistently reported. Dingetal.[2]isolated 8 species of fungi from kiwifruit, 7 of which were pathogenic, and the dominant fungi wereBotrytiscinerea,Penicilliumsp., andPhomopsissp. Jiangetal.[3]pointed out that the pathogen of yellow rot that caused kiwifruit fruit decay wasPhomopsissp., and the "species" of the pathogen needed to be further identified. Wangetal.[4]identifiedBotryosphaeriadothideaas the pathogen causing kiwifruit soft rot based on the morphological characteristics of the isolates and the results of rDNA-ITS, β-tubulin and EF-1α gene sequence analysis. Huang[5]foundPseudocercosporaactinidiaeon the branches, leaves and fruits of kiwifruit, which was different from the reportedP.actinidiaeDeighton and considered as a new species, namedP.acdinidiaevar.fujianaT. Z. Huang. Duanetal.[6]suggested thatPenicilliumspp. was the dominant pathogen causing fruit mildew and rot of Huayou, Hayward and Qinmei kiwifruit in Zhouzhi County, Shaanxi Province during the storage period. With kiwifruit as the research object, the pathogenic fungi carried by kiwifruit during the storage period were isolated and identified, and the main pathogens were confirmed by pathogenicity test. The results can provide the scientific basis for the effective prevention and control of kiwifruit diseases during the storage period.
2 Materials and methods
2.1 MaterialsKiwifruits tested were from the online e-commerce sales in southwest China, and the fruits with disease spots or soft rot symptoms were selected as the test material for pathogen isolation and culture. Healthy fruits were kept fresh and stored for later use.
2.2 Isolation and culture of fungiIf there was mildew in the affected area of fruits, the mildew was directly picked for cultivation. If there was no mildew in the affected area, the surface of the diseased fruit was disinfected with 70% ethanol, and the pulp tissue at the junction of diseased and healthy part was taken for isolation and culture of fungi. The isolates were transferred to PDA plates containing chloramphenicol and placed in a constant temperature incubator at 25 ℃. The colonies were observed regularly, and isolated and purified on PDA medium in time.
2.3 Morphological observationThe culture traits of colonies were observed and recorded, including the color and shape of colonies, and the shape and size of conidia were measured and recorded under a microscope.
2.4 Molecular biological identification
2.4.1Liquid culture of pathogens. A proper amount of mycelia were picked from the isolated and purified colony plate and transferred to the triangle flask containing PDB medium for liquid culture. The mycelia were cultured at 28 ℃, 130 r/min for 2-3 d. The incubation could be prolonged if the amount of mycelia were few.
2.4.2DNA extraction and detection. The mycelia obtained from liquid culture were collected, dried with sterilized filter paper, transferred into a 1.5 mL centrifuge tube, frozen with liquid nitrogen, and ground with a plastic grinding pestle for later use. DNA was extracted by CTAB extraction method or commercial genome extraction kit. The quality of DNA extraction was detected by ultramicro nucleic acid protein assay and agarose gel electrophoresis.
2.4.3PCR assay. The samples were amplified by ITS4/ITS5 Primers: ITS4:5′- TCCTCCGCTTATTGATATGC-3′; ITS5: 5′-GGAAGTAAAAGTCGTAACAAGG-3′. PCR was performed in a 30 μL system containing 3.0 μL of 10×PCR buffer (containing Mg2+), 2.0 μL of dNTPs (2.5 mmol/L), 1.0 μL of each upstream and downstream primer (10 μmol/L), 0.4 μL ofTaqDNA polymerase, 1.0 μL of DNA template (30 ng/μL), and 21.6 μL of ddH2O. The PCR reaction procedures were as follows: predenaturating at 94 ℃ for 3 min; 94 ℃ denaturating for 30 s, 55 ℃ renaturating for 30 s, 72 ℃ extension for 1 min, 35 cycles; extension at 72 ℃ for 7 min and storage at 4 ℃. The amplified products were detected by electrophoresis in 2.0% agarose gel 1×TAE buffer, and gel imaging was performed after EB staining. If the band of target fragment size (500-700 bp) appeared, the amplified products were sequenced.
2.4.4rDNA-ITS sequence analysis. The sequences obtained by sequencing were aligned with related sequences in GenBank on NCBI website for homology analysis, and the classification status was analyzed.
2.5 Pathogenicity testThe healthy kiwifruits were inoculated, and the surface of fruits was wiped and disinfected with 70% ethanol. Mycelium blocks were inoculated by trauma method and sterile water was set as blank control. There were 3 treatments in the test, 3 replicates each treatment. The fruits were moisturized at 20-22 ℃ for 2 d, and continued to culture and observe at room temperature. After the onset of symptoms, the tissues at the junction of diseased and healthy part were collected for re-isolation of fungi, and Koch’s rule verification was completed to confirm whether the isolated fungi were pathogenic fungi.
3 Results and analysis
3.1 Isolation, culture and identification of fungiA total of 45 fungal strains were isolated from fruits with diseased spots or soft rot symptoms. The culture traits (Fig.1) and morphological characteristics (Fig.2) of the isolated cultures were observed, and a total of 12 strains were obtained by merging the same species.
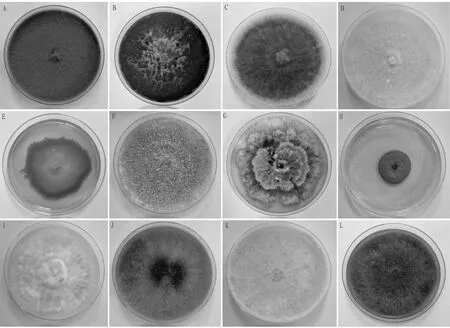
Note: A-L. number of isolated strains.
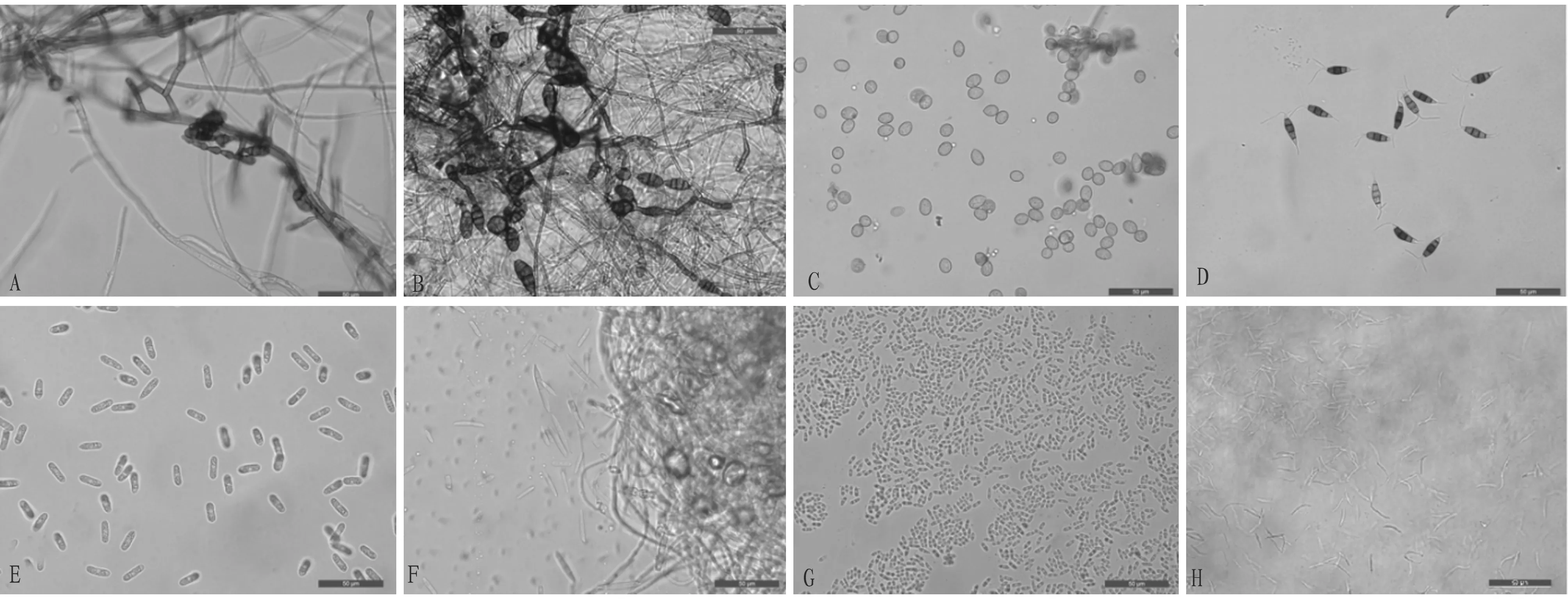
Note: A-H. number of isolated strains.
3.2 PCR assay results and rDNA-ITS sequence analysis
The DNA of the above fungi was extracted by CTAB extraction method, and PCR assay was performed with ITS4/ITS5 primers. The amplification products were detected by electrophoresis in 2.0% agarose gel 1×TAE buffer, and the gel imaging was performed after EB staining. The target fragment size was 500-700 bp, and the products were sequenced in the gene company. The sequence obtained by sequencing was aligned with the relevant sequences in GenBank on NCBI website for homology analysis (Table 1). Combined with culture traits, morphological characteristics and the phylogenetic tree constructed (Fig.3), the identification results of strains were as follows: A and J,Alternariasp.; B,B.dothidea; C,Fusariumsp.; D.Pestalotiopsissp.; E.B.cinerea; F and G, Diaporthe/Phomopsissp.; H.Pseudocercosporasp.; I.Xylariabambusicola; K.Coprinellusradians; L.Colletotrichumsp.

Fig.3 Phylogenetic trees of 12 fungal strains from kiwifruit based on ITS gene sequences
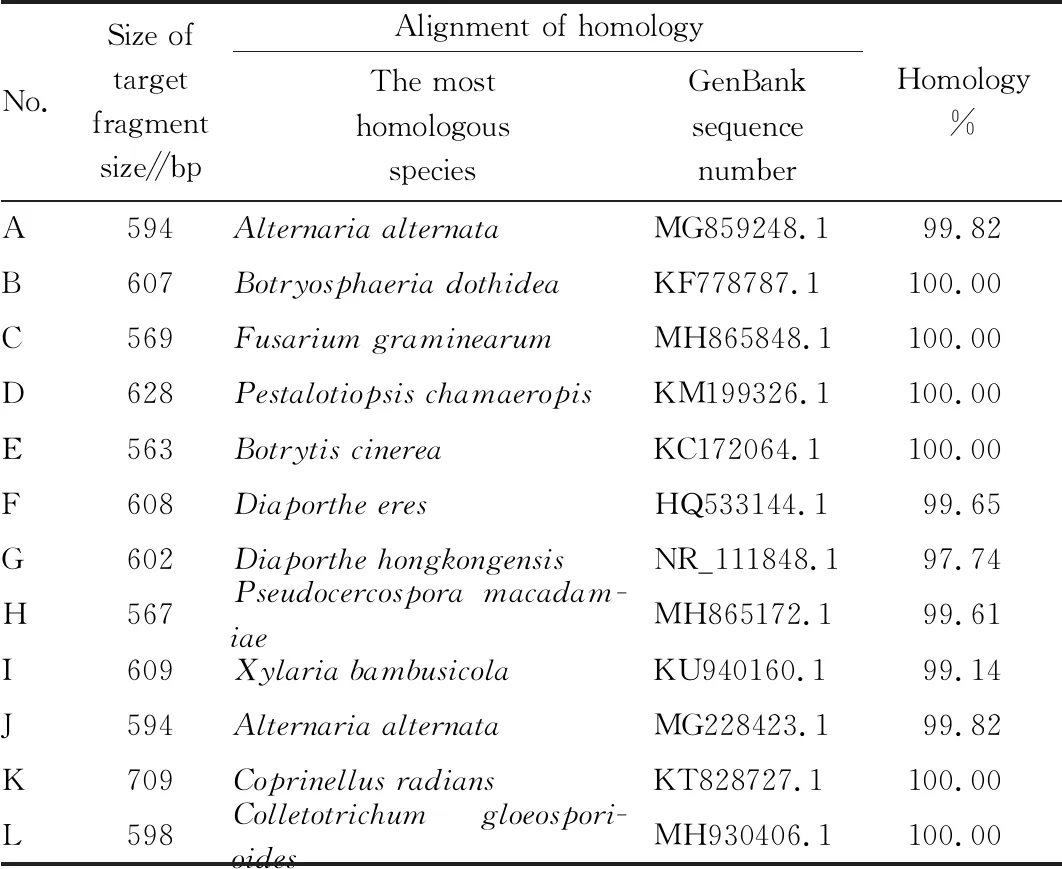
Table 1 ITS identification results of different strains
3.3 Pathogenicity testThe 12 fungal strains isolated were re-inoculated to healthy red heart kiwifruits under stabbing condition. Except for strains C, I and K, the other 9 fungal strains showed the same symptoms as the natural symptoms, and the fungi with the same traits as the original isolates were obtained. In terms of pathogenicity,B.cinereahad the strongest pathogenicity (Fig.4).
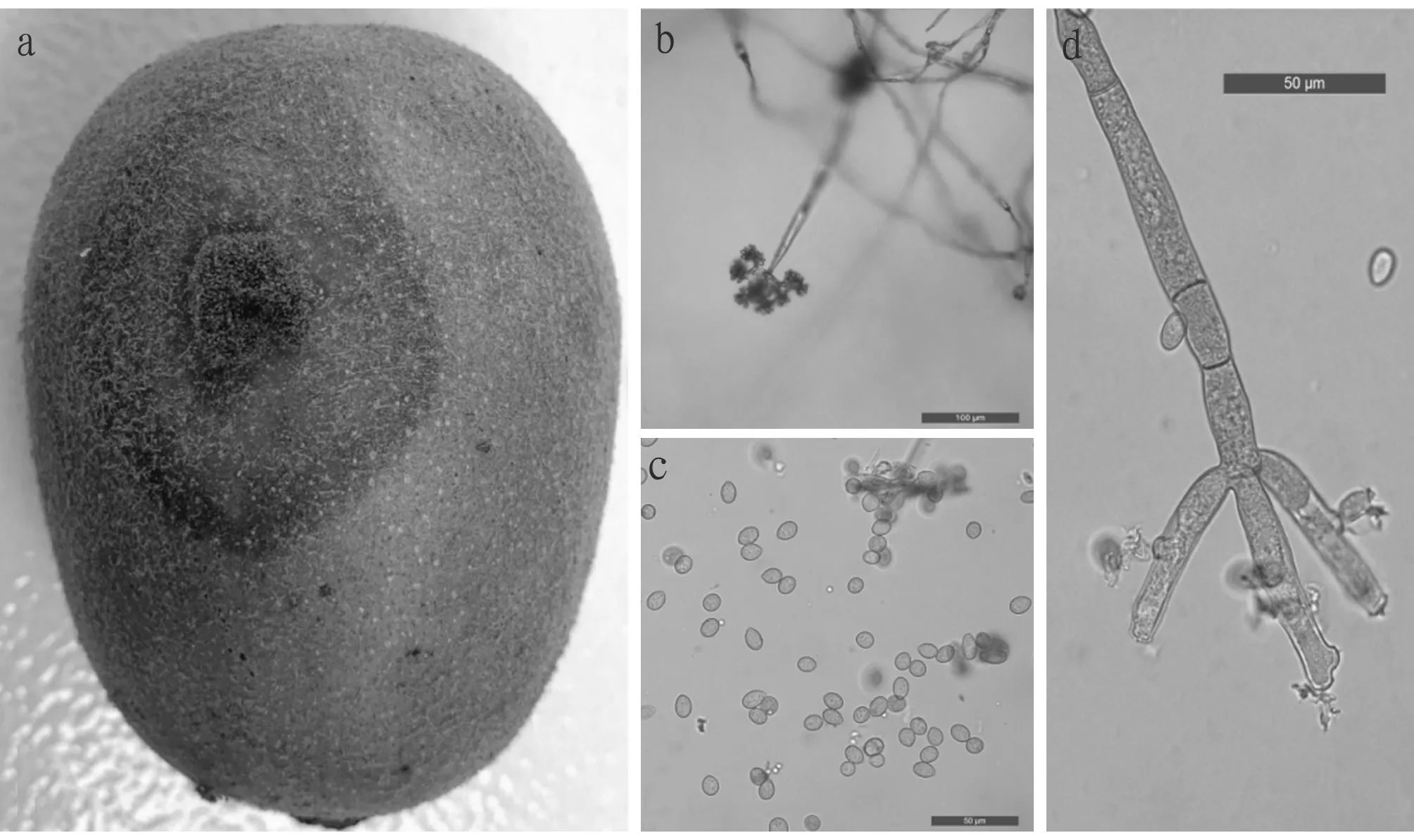
Note: a. Symptoms of inoculation; b. Grape-shaped conidia on conidiophore; c. Conidia; d. Conidiophore.
4 Discussion and conclusions
Kiwifruit is a kind of fruit hard to store, especially Hongyang kiwifruit is extremely resistant to storage.B.dothideacausing soft rot of kiwifruit was isolated in this test, with an isolation frequency up to 40.0%; followed byDiaporthe/Phomopsissp., with an isolation frequency of 22.2%. These two strains were the main pathogens leading to kiwifruit soft rot, and it is generally recognized at present[7]. In addition,B.cinereawas mainly isolated in the late
stage, with an isolation frequency of only 4.4%, but it had the strongest pathogenicity. Foreign studies have reported thatB.cinereawill produce ethylene when infecting kiwifruit[8-9], which will accelerate the premature ripening of fruits and shorten the storage period.
In terms of pathogen isolation and identification, some fungi isolated in this test were not easy to sporulate on PDA culture plates, which brought difficulties to identification. Based on the strictness, some fungi has not been identified to species and need to be further confirmed. Although molecular sequencing can yield twice the result with half the effort, accurate identification of pathogen species needs to be supported by a variety of methods.
Latent infection of pathogens in kiwifruit before harvest easily leads to the occurrence of postharvest diseases during the storage period, especially soft rot. At the early stage of postharvest, the appearance of fruits is intact without harmful symptoms, but at the later stage of storage the symptoms are prominent and can not be prevented. It is necessary to strengthen the research on preharvest control and rapid diagnosis technology of pathogens after harvest, and diseased fruits should be removed in time to avoid economic losses. All the fruits used in this study were spoiled and lost their edible value after the test. Combined with the actual production, the possible causes of kiwifruit rot are as follows: widespread application of swelling agent reduces the storage resistance; due to inappropriate harvest time, fruits are more likely to soften and rot when they enter the peak period of respiration; improper harvesting and storage of fruits cause scratches and extrusion, which creates favorable conditions for the invasion of pathogens; poor management after harvesting and storage, especially under the circumstances of incomplete disinfection, inaccurate temperature control, mixed storage of goods, over-dense stacking and no ventilation, seriously reduces the storage time and quality of fruits.
- 植物病虫害研究(英文版)的其它文章
- Preliminary Study on Acoustic Characteristics of Ips grandicollis Larvae
- Analysis and Study on Characteristics and Detection Methods of Cotton Diseases and Insect Pests
- Prevention and Control Test of Semiaphis heraclei in Spring
- Effects of Different Fertilization Measures on Chemical Composition and Quality of Flue-cured Tobacco

