Nationwide retrospective study of hepatitis B virological response and liver stiffness improvement in 465 patients on nucleos(t)ide analogue
Alnoor Ramji, Karen Doucette, Curtis Cooper, Gerald Yosel Minuk,Mang Ma, Alexander Wong, David Wong,Edward Tam, Brian Conway, David Truong, Philip Wong, Lisa Barrett, Hin Hin Ko, Sarah Haylock-Jacobs,Nishi Patel, Gilaad G Kaplan,Scott Fung, Carla S Coffin
Abstract
Key Words: Nucleos(t)ide analog therapy; Functional cure; Hepatitis B virus surface antigen loss; Fibrosis regression; Liver stiffness measurement; Transient elastography
lNTRODUCTlON
Hepatitis B virus (HBV) nucleos(t)ide analog (NA) therapy is associated with fibrosis regression,reduced risk of hepatocellular carcinoma (HCC) and improved clinical outcomes[1]. The ultimate goal of HBV therapy is hepatitis B surface antigen (HBsAg) loss and considered a functional cure enabling treatment cessation, but rarely achieved with current NA. Without HBsAg clearance, stopping treatment may lead to severe viral and biochemical flares. Studies of European patients reported HBsAg loss in 20% patients after stopping long-term NA therapy. HBsAg loss among Asian virally suppressed patients was very low (< 5%) suggesting there are important differences among different groups of patients in terms of off-treatment response[2,3]. The approved 2ndgeneration NA,i.e., entecavir (ETV),tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) potently reduce serum viral load(HBV DNA) but they rarely achieve a functional cure despite prolonged therapy[4]. Studies in large Asian cohorts have compared the effect of NA with a low genetic barrier to drug resistance (i.e.,lamivudine, LAM) to higher potency (i.e., ETV, TDF/TAF) NA on liver fibrosis regression and viral response[5]. A single centre Asian study of 124 patients treated with anti-HBV NA documented HBV DNA suppression but all had persistently detectable HBsAg, and none achieved intrahepatic viral marker reduction (i.e., HBV covalently closed circular (ccc) DNA)[5]. A large retrospective Korean study showed lower risk of death or transplantation with ETV compared to LAM but no difference in HCC risk[6]. A recent global study involving 299 centers in 24 countries investigated outcomes after 10 years of follow-up of clinical trial patients treated with ETV or alternative NA[2]. The data revealed that 5305 Chinese ETV recipients maintained virological response with lower risk of HBV-related serious adverse events including HCC. However, approximately 17% of the cohort, and similar rate in other cohorts,continued to identify HBV-related liver disease progression[5,7]. Liver disease complications have also been reported in a Korean study of 440 cirrhotic patients (mostly genotype C) after 5 years of ETV therapy, with a 15% rate of liver disease decompensation in those with > 90% treatment adherence and 41% decompensation in patients with < 90% adherence[8].
There is limited real-world data evaluating the clinical impact of 1stvs2ndgeneration long-term NA therapy in North American chronic hepatitis B (CHB) patients. These patients maybe characterised by greater diversity and genotype heterogeneity based on a diverse regions of origin. Disparities in access to HBV NA is a global issue. Similarly, in Canada, NA reimbursement criteria vary by provincial or territorial jurisdiction[9]. Until recently, antivirals with a high barrier to resistance such as TDF and ETV were not funded as first line therapies for CHB in two large population provincial health jurisdictions [i.e., British Columbia (BC) and Ontario (ON)][9]. In particular, there were historic differences in reimbursement criteria with LAM utilized as first line in BC and ON, and TDF historically (before 2018)only reimbursed for persons with advanced fibrosis and/or decompensated cirrhosis. In BC and ON,HBV patients could access TDF if they failed LAM, developed resistance or very rarely, had intolerance to LAM. In this retrospective, multi-centre cohort study of 465 CHB patients followed in 8 provinces across Canada, we aim to assess HBV viral response, biochemical remission, liver fibrosis regression and HBsAg sero-clearance after treatment with median 4 years of either a 1stor 2ndline NA therapy.
MATERlALS AND METHODS
This was a retrospective observational cohort study, utilizing the Canadian Hepatitis B Network cohort data from January 1st, 2012-December 1st, 2019. Participating clinics provided data from electronic and/or paper charts. De-identified information was entered into a registry REDCap®database housed at the University of Calgary (U of C) under an approved U of C Conjoint Ethics Research Board (CHREB)protocol (Ethics ID # REB16-0041), and appropriate legal data sharing agreements[10]. Study subjects provided written informed consent to participate, or were included with a waiver of consent, according to local REB approval. Data extracted included demographics (age, sex, ethnicity), antiviral regimens,HBV DNA, hepatitis B e antigen (HBeAg), alanine aminotransferase (ALT) and liver stiffness measurement (LSM) prior to and during therapy. Inclusion: Adult patients >18 years, with known chronic HBV (i.e., HBsAg persistence > 6 mo duration), HBV monoinfected, treatment naïve at baseline,and were on only a single antiviral agent for the study duration. Exclusion: Participants were excluded if they had received previous HBV therapy, had a liver transplant or were co-infected with other hepatotropic viruses (e.g., HCV and HDV) or with HIV. ETV treated patients were also excluded for direct comparison of only two antiviral drugs. Persons who had their antiviral regiment switched(includes LAM-resistant patients switching to TDF) were also excluded. All patients had a minimum of 12 mo follow-up from treatment initiation, with at least annual HBV DNA evaluation using commercial assays (i.e., sensitivity 20 IU/mL or 50 copies/mL, Abbott and/or < 20 IU/mL, Roche) and serial LSM performed using FibroScan®(Echosens, Paris, Fr). Liver fibrosis, based on Metavir staging, was classified as F0-F1 (< 7.3 kPa), F2-F3 (7.3-9.5 kPa) and F4 or cirrhosis (> 9.5 kPa)[11]. CHB management and treatment monitoring was directed by the center physician(s) and based on the Canadian consensus guidelines[12].
Statistical analyses
For all analyses, patients with missing data were excluded. Continuous data were summarized with the mean, 95% confidence interval (CI) and count (n). For comparisons between treatment groups (LAMvsTDF) at a given time point, two-tailedttest was used. Repeated measures analysis of variance(ANOVA) with post hoc test was used to compare continuous variables at different time points for each treatment group. Categorical data were summarized as proportion using mean % (n/n known). Fisher’s exact test was used for comparison between dichotomous data. A linear mixed model was used to identify change over time in a multivariable linear mixed regression analysis. APvalue of less than 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS/STAT®software, IBM SPSS Statistics 27.0.1.0 and/or GraphPad Prism 9.0.
RESULTS
Characteristics of the study population
The study population included 465 patients who were treatment naïve at baseline. The mean age was 49(SD 12.9) years, 37% were female, and 35% were HBeAg (+) at baseline. Most were Asian (84%,n= 345)(albeit from different East and/or South-East Asian) countries, 9% Black (n= 37), and 6% White (n= 25).Patients either received TDF (n= 299, 65%) or LAM (n= 166, 35%) therapies with similar median treatment duration of 3.9 and 3.7 years, respectively. There was no difference between treatment groups in sex or ethnicity. However, TDF treated individuals were more likely to be HBeAg positive (Table 1).Historically, patients from one provincial health jurisdiction (i.e., British Columbia), only had access to TDF as first line therapy if they had cirrhosis. Thus, a greater proportion of persons in the TDF group also had advanced fibrosis (> stage F3/4) at baseline, compared to LAM (Table 1).
Summary of follow-up evaluation and outcomes
The mean baseline LSM prior to treatment was greater in TDF treated recipients (11.2 kPa, 95%CI: 9.9-12.4) than in those receiving LAM (8.3 kPa, 95%CI: 7.2-9.5) (Table 1). At baseline, serum HBV DNA and ALT levels were similar in both groups (Table 1). Compared to baseline, only TDF treated group achieved significant HBV DNA decline at 5 years follow up (p=0.0011) compared to LAM treated group(P= 0.37) (Figure 1A).
By year 5, 194/299 (64.9%) TDF patients had undetectable HBV DNA (< 10 IU/mL) than 88/166(53.0%) LAM patients (P= 0.012, Chi-square test, Supplementary Table 1). Furthermore, a higher proportion of TDF treated patients had suppressed HBV DNA (< 10 IU/mL) (n= 170/190, 89%)vsLAM-treated (n= 35/58, 60%) (P <0.05), but none achieved functional cure (HBsAg loss) at the end of the study period.
Both treatment groups had decline in ALT from baseline to 5-year follow-up (P= 0.014 for LAM andP <0.0001 for TDF) (Figure 1B). As well, ALT normalization was achieved for both treatment groups by 1-year follow-up. More specifically, by year 5, 167/299 (55.9%) TDF patients achieved ALT normalization compared to 74/166 (44.6%) LAM patients (P= 0.020, Chi square test, Supplementary Table 2).
In patients with available serial long-term LSM data, NA treatment also led to improvement in liver inflammation and fibrosis regression with lower mean LSM at 5-year follow-up (7.0 kPa, 95%CI:5.8-8.2 for TDF and 6.7 kPa, 95%CI: 4.9-8.6 for LAM treated patients). Multivariable linear mixed regression with a linear mixed model showed a significant difference in fibrosis regression between antiviral treatment groups. Mean fibrosis regression was greater in TDF treated patients with -4.2 kPa change compared to -1.6 kPa in LAM recipients from baseline,P <0.05 (Figure 2).
As well, by 4-year follow-up, LSM improved by ≥ 1 stage in 92/299 (20.8%) TDF patients compared to 29/166 (17.5%) LAM patients (P= 0.002, Chi square test).
Of the 26 HBeAg positive patients that received LAM treatment, 6 patients became HBeAg negative(23.1%). Of the 99 HBeAg positive patients that received TDF treatment, 27 patients (27.3%) achieved HBeAg loss. No data was available on LAM resistance however all patients enrolled in the study were on a single NA agent. It is standard clinical practice to switch to TDF in individuals who developed LAM resistance (and criteria for public formulary drug reimbursement in some provinces). Genotype data was not available in most patients.
DlSCUSSlON
In this multi-centre, retrospective real-world Canadian study, we assessed clinical outcomes in CHB patients receiving long-term NA therapy. Both 1stgeneration (LAM) and higher potency antivirals (TDF)reduces HBV DNA and improves liver inflammation and fibrosis based on serial LSM evaluation even after ALT normalization. This study highlights the effectiveness of both antiviral drugs in inducing fibrosis regression in a diverse HBV patient cohort. However, up to 5-year follow-up period no CHB patients achieved HBsAg loss or functional cure. A clinically relevant proportion of patients continued to show low-level viremia, especially if treated with LAM. Persons with LAM- resistance switched to TDF were not included to avoid bias of 2 different regimens. Data on antiviral resistance testing is not available, and it is likely these patients would have been switched to TDF in both BC and ON pharmacare reimbursement criteria. The clinical implications of low-level viremia, especially association with residual HCC risk is unclear and requires further study[13,14].
为解系统面临的安全威胁,提升生态系统的安全可信,当前的IoT技术面临着诸多安全挑战,但归根结底是以下2方面的特性所致:
Many studies including randomized clinical trials, observational cohorts and meta-analyses demonstrate that NA treatment improves clinical outcomes for persons living with chronic hepatitis B[1]. Observational studies from clinical databases also reflect real-world practice and complement clinical trials[15,16]. Single centre real-world Canadian studies have observed reduced HCC risk[17].The majority of observational studies conducted on long-term NA therapy outcomes were done in HBVendemic countries with a particular focus on Asia. Canada is non-endemic for HBV but due to high rates of immigration from many endemic regions, CHB remains an important health issue and affects a diverse, multi-ethnic population as persons have from multiple regions and encompasses 40 countriesas based on Canadian HBV Network Data. Compared to other published real-world studies, the data is also relevant/applicable in a single-payer universal health care system with equal access care albeit differences in medication re-imbursement providing for the unique aspect of this study. The Canadian health care system does provide some financial assistance to cover the cost of antiviral therapy, and other associated costs (i.e., physician visits, hospitalization, laboratory monitoring, diagnostic imagingetc.) is reimbursed by provincial health care plans.

Table 1 Summary of baseline characteristics of 465 study patients who were treated with either lamivudine or tenofovir disoproxil fumurate enrolled in the Canadian hepatitis B virus network
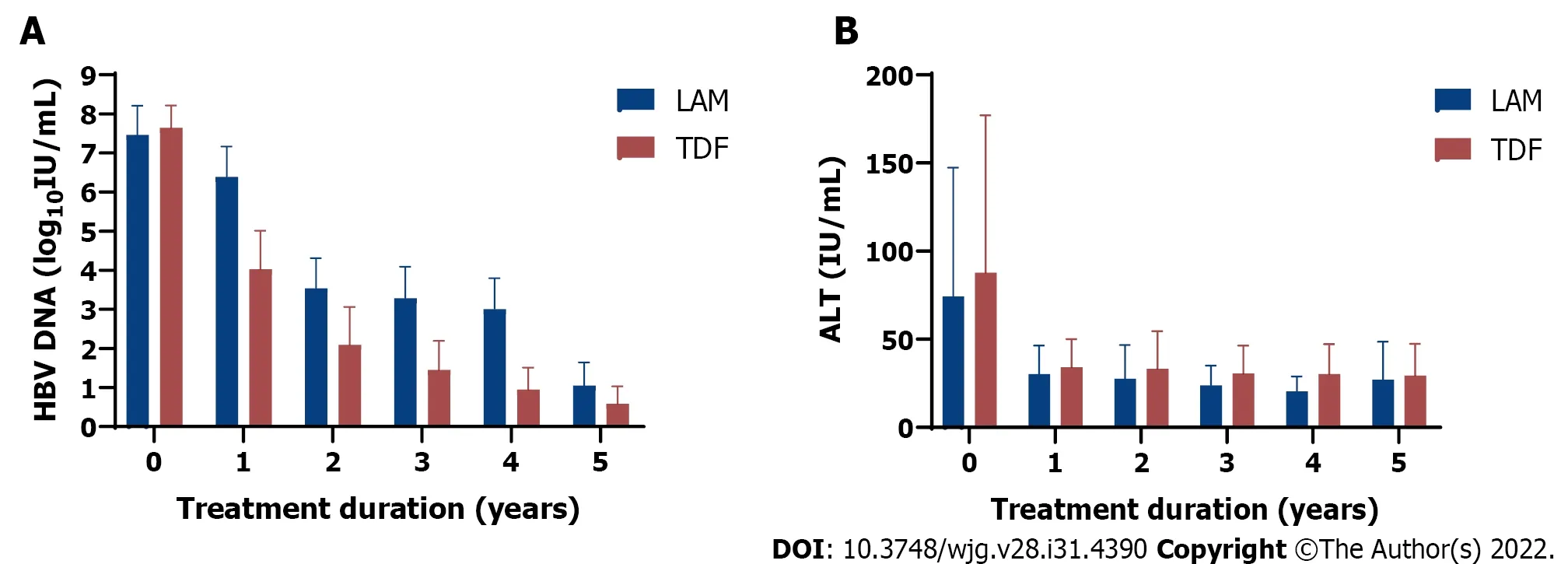
Figure 1 Comparison of clinical outcomes in 465 chronic hepatitis B patients receiving 1st generation (lamivudine or LAM, solid bar) vs 2 nd generation (tenofovir disoproxil fumarate or TDF, dotted bar) hepatitis B virus nucleos(t)ide analog therapy from baseline (pretreatment) followed for up to 5 years. A: Hepatitis B virus DNA decline (log10 IU/mL); B: Mean alanine aminotransferase (IU/mL) decline from baseline after starting lamivudine or tenofovir disoproxil fumarate. Mean with error bars representing standard deviation is plotted. LAM: Lamivudine; TDF: Tenofovir disoproxil fumarate; HBV: Hepatitis B virus; ALT: Alanine aminotransferase.
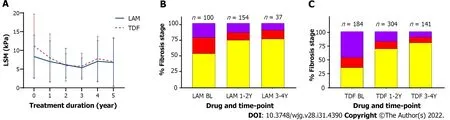
Figure 2 Comparison of lamivudine vs tenofovir disoproxil fumarate on liver stiffness measurement over time. A: Liver stiffness measurement change from baseline (before treatment) and while on treatment; mean with error bars representing standard deviation is plotted; B and C: Comparison of fibrosis severity at baseline, 1-2 years, and 3-4 years post-treatment for lamivudine (B) and for tenofovir disoproxil fumarate (C). F0-F1 (yellow), F2-F3 (red), and F4 (purple).LSM: Liver stiffness measurement; LAM: Lamivudine; TDF: Tenofovir disoproxil fumarate.
Our study is similar to previous published work that highlights the benefits of antiviral treatment in improving liver fibrosis. A systemic review and meta-analysis including 34 published studies of treated and untreated CHB patients also found low rates of NA-induced HBsAg seroclearance, highlighting the need for improved curative therapies[18]. In fact, HBsAg seroclearance generally occurred in untreated individuals with less active disease[18], and is consistent with retrospective data from the Canadian HBV Network of CHB patients comparing outcomes in individuals that remained HBsAg positivevsthose with HBsAg loss[19].
The current study is limited by retrospective data and missing data at all defined time-points,including serial lab and LSMs, and highlights the limitation of real-world studies (i.e., missed appointments, adherence and incomplete clinician documentation or reporting). We also excluded patients treated with the other approved 2ndgeneration NA (i.e., ETV) for purposes of the study analysis to compare a single 1stvs2ndgeneration NA therapy. Moreover, although our cohort was multi-ethnic most patients were Asian, albeit individuals were born in many different east and south-east Asian countries. Additionally, there was missing ethnicity data on 52 patients (11%). Overall, this data represents immigration from up to 40 countries, based on Canadian HBV Network Data. The 2016 Canadian census reported the top Asian countries of birth for recent immigrants are Philippines, India,China, Iran, Pakistan, Syria and South Korea and Syria (www.statcan.gc.ca). There is increasing data on LSM for assessment of liver inflammation and HBV-related fibrosis, although transient elastography is not widely available in resource limited countries compared to other non-invasive fibrosis markers such as aspartate aminotransferase to platelet ratio index (APRI) and Fibrosis4 (FIB4) calculator[20]. It is noteworthy that our analysis represents the largest North American study conducted to date on longterm follow-up with NA therapy utilizing this novel technology.
CONCLUSlON
In summary in this real-world diverse cohort study of chronic hepatitis B patients in Canada, long-term nucleo(s)ide analog (either 1stor 2ndgeneration) therapy suppresses HBV DNA and improves hepatic inflammation and liver fibrosis, as determined by non-invasive testing (i.e., transient elastography). In patients treated for up to 5 years, none achieved HBsAg loss (functional cure), highlighting the need for improved therapeutic strategies to reduce the life-long burden of antiviral therapy.
ARTlCLE HlGHLlGHTS


Research objectives
To assess HBV viral response, biochemical remission, liver fibrosis regression and hepatitis B surface antigen (HBsAg) sero-clearance after treatment with either a 1stor 2ndline NA therapy. These objectives were realized and the future impact would be the consideration of utilizing 1stvs2ndline NA’s. Further,other outcome measure to include HBsAg loss and development of hepatocellular carcinoma (HCC)may be considered.
Research methods
We performed a retrospective observational cohort study utilizing a National network in HBV. Novel aspects that allowed this study is the utilization of a National Network which was able to capture differences in NA utilization based on regional differences of reimbursement. The strength of this study lies in the diversity that the Canadian HBV Network provides. The utilization of liver stiffness by transient elastography in a North American cohort for this objective is also unique.
Research results
As per differences in utilization, a larger proportion of patients with advanced fibrosis were initiated with tenofovir disoproxil fumarate (TDF) compared to LAM. At the end of the study period there were similar stages of fibrosis between the 2 groups. There was an increased fibrosis regression in those treated with a high potency compared to a low-potency NA. More patients in the TDF group also achieved virological suppression, though alanine amino transferase (ALT) normalization was similar.
Research conclusions
In this diverse cohort treatment with low and high potency NA’s achieves high rates of viral suppression, ALT normalization and a large proportion achieve fibrosis regression. The strength of national collaboration within a network is exemplified in this study in particular taking advantages of diversity within an overall similar medical system. These differences within a Network can be a powerful tool to answer research questions and can reduce a number of biases inherent when comparing populations/studies between countries or regions.
Research perspectives
The future direction would include potential for long-term outcomes with differential NA usage to include HBsAg loss or seroconversion and any differences in development of HCC albeit in a population with differences in stage of fibrosis at baseline. This study population and Network provides a unique perspective to answer this question.
FOOTNOTES
Author contributions:Ramji A contributed to study design, data contribution, and manuscript draft; Coffin CS contributed to manuscript draft, data contribution, data analysis, and resource support; Haylock-Jacobs S contributed to data analysis and manuscript draft; Patel N and Kaplan GG contributed to data analysis; all other authors contributed to data contribution, manuscript review and feedback.
lnstitutional review board statement:The study was reviewed and approved by the Conjoint Health Research Ethics Board, No. REB17-2321_REN4.
Conflict-of-interest statement:Dr. Alnoor Ramji and Dr. Carla S Coffin didn’t receive at any time payment from a third party for any aspect for the submitted work; there are no relevant conflict of interest; there are no patents related to this work; Dr. Alnoor Ramji and Dr. Carla S Coffin have nothing to disclosure.
Data sharing statement:No data sharing is approved by the institutional ethics board.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Canada
ORClD number:Alnoor Ramji 0000-0003-4059-8767; Karen Doucette 0000-0002-1660-9166; Gerald Yosel Minuk 0000-0002-2687-940X; Mang Ma 0000-0003-2587-1788; Alexander Wong 0000-0002-5984-3083; David Wong 0000-0002-1145-1611;Edward Tam 0000-0002-8306-3968; Brian Conway 0000-0001-6821-7829; David Truong 0000-0002-3190-784X; Philip Wong 0000-0002-3446-4116; Hin Hin Ko 0000-0002-2740-2486; Gilaad G Kaplan 0000-0003-2719-0556; Scott Fung 0000-0003-0501-8800; Carla S Coffin 0000-0002-1472-0901.
S-Editor:Chen YL
L-Editor:A
P-Editor:Chen YL
 World Journal of Gastroenterology2022年31期
World Journal of Gastroenterology2022年31期
- World Journal of Gastroenterology的其它文章
- Duodenal-jejunal bypass reduces serum ceramides via inhibiting intestinal bile acid-farnesoid X receptor pathway
- Preoperative contrast-enhanced computed tomography-based radiomics model for overall survival prediction in hepatocellular carcinoma
- Prevalence and clinical characteristics of autoimmune liver disease in hospitalized patients with cirrhosis and acute decompensation in China
- Application of computed tomography-based radiomics in differential diagnosis of adenocarcinoma and squamous cell carcinoma at the esophagogastric junction
- Radiomics and nomogram of magnetic resonance imaging for preoperative prediction of microvascular invasion in small hepatocellular carcinoma
- Insights into induction of the immune response by the hepatitis B vaccine
