Approaches to reconstruction of inferior vena cava by ex vivo liver resection and autotransplantation in 114 patients with hepatic alveolar echinococcosis
Yusufukadier Maimaitinijiati,Tuerganaili Ai, Tie-Min Jiang, Bo Ran, Ying-Mei Shao, Rui-Qing Zhang, QiangGuo,Mao-Lin Wang, Hao Wen
Abstract
Key Words: Ex vivo liver resection; Alveolar echinococcosis; Inferior vena cava; Vascular reconstruction;Liver transplantation; Artificial vessel
lNTRODUCTlON
Echinococcosis is an infectious zoonotic parasitic disease[1,2]. Notably, 90% of the global burden of this disease occurs in China, and echinococcosis has been a major public health problem in the Northwestern area of China[3,4]. Hepatic alveolar echinococcosis (AE) has been considered invasive growth and systematic metastasis[5,6], but its insidious onset and slow progression usually contribute to the delayed diagnosis. The mortality rate within 10 years after diagnosis is over 90% if the lesion is inadequately or not treated[7-9]. To date, radical surgery combined with albendazole treatment has been considered the best option for AE patients[10,11]. However, it is difficult to perform radical hepatectomyin vivowhen AE lesion invades the hepatocaval confluence[12]. Generally, only 35%-40%of patients can be treated with conventional radical resection[7]. Fortunately,ex vivoliver resection and autotransplantation (ELRA) can better realize the radical resection of end-stage hepatic AE with severely compromised outflow tract, and reconstruction of the affected vessels[13].
Retrohepatic inferior vena cava (RHIVC) is often severely invaded by AE lesions, and resection of the invaded duct segment often presents a variety of irregular shapes, resulting in different inferior vena cava (IVC) reconstruction methods and the complex process. Proper RHIVC reconstruction may simplify graft liver implantation, which is a key element to postoperative recovery. To the best of our knowledge, there have been few studies on the reconstruction of IVC in ELRA worldwide[14,15], and no clear consensus has been reached on the optimal strategy. This research reported our experience with 114 hepatic AE patients using different IVC reconstruction methods in ELRA. This is the study with the largest sample size to date, which provides a quantitative strategy of IVC reconstruction for end-stage hepatic AE patients with RHIVC infiltration.
MATERlALS AND METHODS
Patients
A total of 114 patients with end-stage hepatic AE who received ELRA at the First Affiliated Hospital of Xinjiang Medical University between August 2010 and December 2020 were retrospectively analyzed.All patients were divided into three groups: Group A was the repaired and reconstructed group (n= 64)that sought reconstruction of the original RHIVC by self-suture or vascular patch, group B was the RHIVC replacement group (n= 43) that completely replaced the RHIVC with substitute vessels, and group C was the RHIVC resection without reconstruction group (n= 7) that excised the invaded RHIVC segment without reconstruction (Table 1).
Medical ethical approval
This study was approved by the Ethics Committee of First Affiliated Hospital of Xinjiang Medical University and conducted in accordance with the Helsinki Declaration[16]. Written and signed informed consent was obtained from all patients or their legal custodians.
Preoperative assessment
All subjects underwent comprehensive preoperative evaluation, including abdominal computed tomography angiography (CTA) and hepatic magnetic resonance imaging, to assess the location of the lesion, the extent of vessel and bile duct involvement. For patients whose CTA results suggested RHIVC severe stenosis or occlusion, IVC digital subtraction angiography was also performed. The predicted autograft liver mass (GLM) was detected by a three-dimensional reconstruction system.
Surgical procedures
More details of the ELRA procedures are reported in our previous study[13]. Here, we focused on the reconstruction of IVC. First, we ligated and resected the IVC between the upward side of the confluence of three hepatic veins and the site at 1 cm above the confluence of renal vein, and removed it together with the liver. Then the RHIVC was reconstructedin vitroindividually depending on the defect of lumen wall after radical resection of AE lesion. When the defect was less than 120° of the lumen circumference, it was considered that directly suturing the defect of wall did not affect vascular tension or patency, and thus self-suture repair was performed. When the defect was more than 120° of the circumference, we repaired the lumen defect with vascular patches such as ligamentum teres hepatis and internal jugular veins, because direct suturing might lead to stenosis in this case. Moreover, the RHIVC was replaced in patients with extensive defect and being unable to obtain adequate vascular patches.The hepatic veins of autograft liver were anastomosed with an alternative vessel. In this case, the position and angle of anastomosis were relatively freely controlled, which facilitated the increased reflux of hepatic outflow and further improved the recovery of autograft liver function (Figure 1).
Uniquely, for some patients in this study, the preoperative CTA and IVC venography showed that the RHIVC segment was completely occluded, and suitably compensated by the collateral branches.During the operation, we further verified whether the RHIVC was completely occluded and whether the collateral circulation was sufficient. Finally, for the 7 patients, the AE infiltrated segment of IVC was resected without any reconstruction, the proximal end of IVC was anastomosed with autogenous hepatic vein, and the distal end was closed directly (Figure 1).
Postoperative management and follow-up
After surgery, all patients received systemic anticoagulant therapy. Low-molecular-weight heparin was used in the 1stweek, and prothrombin time (PT) and international normalized ratio (INR) were closely observed. In the 2ndweek, oral tablets were substituted, and patients in group A and group C were recommended to withdraw these drugs 1 mo later when the PT and INR returned to normal levels.Patients in group B, however, needed to continue the medication for a long term and closely observe the coagulation index regularly. Each patient was regularly followed up, and serological tests, liver function tests and CTA scans were performed every 3-6 mo after discharge. Postoperative complications were assessed according to the Clavein-Dindo classification system[17]. All patients were administered with albendazole (10 mg/kg/d) routinely for 2 years[18].
Statistical analyses
The statistical software IBM SPSS 22.0 (IBM Corp, Armonk, NY, United States) was used for dataanalysis. The normally distributed continuous variables were expressed as means ± SD, whereas the abnormally distributed ones were expressed as medians and analyzed by analysis of variance. Survival curve was plotted by the Kaplan-Meier method.P< 0.05 was considered statistically significant.

Table 1 Clinical data of 114 alveolar echinococcosis patients treated by ex vivo liver resection and autotransplantation
RESULTS
Patient characteristics
Altogether 114 patients were treated by ELRA, including 50 males (43.9%) and 64 females (56.1%), with an average age of 36.2 ± 11.8 (range, 15-64) years. Preoperative PTCD or ERCP was performed in 25 patients to reduce the bilirubin levels and biliary obstructions. There were 21 patients with a previous history of hepatectomy. Albendazole therapy was administered to 27 patients. The average GLM was 828.3 ± 250.8 g. There were no significant differences in basic clinical data among the three groups (P>0.05; Table 1).
Intraoperative outcomes
Intraoperative reassessment confirmed that AE lesions invaded the RHIVC in all patients, and RHIVC repair or reconstruction was needed. Among them, the self-suture repairing was performed in 52 patients with a defective RHIVC lumen less than 120° of circumference after R0 resection. The lumen defect was repaired with autologous vascular patches in 12 patients whose defect was 120°-180° of wall circumference. Meanwhile, 43 patients had defect exceeding 180° of circumference with extensive range and difficulty in reestablishing with autogenous patches. For these cases, the RHIVC was replaced with artificial vascular grafts (n= 38) or allogeneic vascular (n= 5). In another 7 patients, preoperative imaging examination indicated that the RHIVC segment was occluded and a collateral circulation network was formed. During the operation, stable systemic hemodynamics with no obvious intestinal congestion was found after the IVC was ligated. Finally, the RHIVC was resected without reconstruction (Figure 2). The average operation time was 16.7 ± 2.9 h for group A, 15.5 ± 3.1 h for group B, and 16.9 ± 4.1 h for group C (P= 0.56), and the average anhepatic phase was 418.4 ± 108.3, 383.9 ±117.0, and 337.4 ± 108.7 min in groups A, B and C (P= 0.41; Table 2).

Table 2 lntraoperative outcomes for 114 alveolar echinococcosis patients

Figure 1 Schematic diagrams of retrohepatic inferior vena cava reconstruction methods. A-C: Hepatic alveolar echinococcosis (AE) lesion invades the hepatocaval confluence; D: Retrohepatic inferior vena cava (RHIVC) is invaded by the AE lesion (yellow arrow); E: RHIVC is extensive invaded (yellow arrow); F:RHIVC completely occluded (yellow arrow), and compensation by the collateral branches (black arrow); G: Self-repairing reconstruction method, showed vascular patch repair (red arrow) and self-suture repair (yellow arrow); H: RHIVC replacement method, showed the RHIVC after replacement (red arrow); I: RHIVC resection without reconstruction method, showed IVC anastomoses with graft liver hepatic vein (yellow arrow) and adrenal vein (red arrow), the collateral circulation branches after operation (black arrow).
Postoperative and follow-up outcomes
All patients were followed up with a median duration of 52 (range, 12-125) mo, except for 1 patient who was lost to follow-up at 6 mo after the operation. No intraoperative death occurred. The 30 d mortality rate was 7.0% (8/114) and another 7 patients died within 90 d (15/114). Among all subjects, the IVCrelated complication rates were 17.5% (11/63) in group A and 16.3% (7/43) in group B. IVC stenosis was found in 12 patients (10.5%), and thrombus was formed in 6 patients (5.3%). Twenty-two patients experienced grade III or higher complications according to the Clavin-Dindo classification system, and the complication rates were 17.2%, 16.3% and 57.1%, in the three groups (Table 3). The averagepostoperative hospital stay was 32.3 ± 19.8 d for group A, 26.7 ± 18.2 d for group B and 51.3 ± 29.4 d for group C (P= 0.03). Obviously, patients in group B obviously required a shorter postoperative hospital stay than the other two groups.
Kaplan-Meier survival analysis curve and log-rank test were used to observe the postoperative survival for the three groups based on the follow-up results. The survival rates of group A and group B were quite close, whereas that of group C was lower, but the differences were not statistically significant(P= 0.81). The early periods after surgery, especially in the 1stmonth, witnessed the high mortality rate(Figure 3).
DlSCUSSlON
Hepatic AE is also known as “parasitic cancer,” which is ascribed to its tumor-like characteristics with infiltration of vessels or biliary structures and distant metastasis[19]. The insidious onset and slow progression of AE usually contribute to the delayed diagnosis, and hepatic veins and RHIVC are often invaded by the time when treatment is sought in more than half of the patients, thus depriving them of the opportunity for traditional surgical resection[20,21]. In 1986, the Liver Transplantation Center of Bezancon Hospital in France was the first to report allogeneic liver transplantation (ALT) for an endstage hepatic AE patient[22]. Since then, more successful clinical cases and treatment experience with liver transplantation for end-stage hepatic AE have been reported worldwide[23-26]. However, a shortage of donor livers and a significant recurrence rate have limited the further development of ALT for hepatic AE[24,27]. In 2010, our center first applied ELRA to manage a patient with end-stage hepatic AE[28]. Compared with ALT, this technique requires no graft donor or immunosuppressive treatment,and is more affordable[29,30]. In particular, it is more suitable for patients with severe invasion of RHIVC and intrahepatic veins whose are unresectable by traditional surgical methods. Notably, the corresponding reconstruction of autograft liver RHIVC remains the most challenging step in the ELRA procedures, since no uniform fixed pattern is available and individual design is required. To date, very few studies have investigated the reconstruction of IVC in ELRA worldwide (Table 4), and no clear consensus has been reached on the optimal reconstruction strategy. In this study, we analyzed the largest series of end-stage hepatic AE cases with seriously infringed RHIVC who received ELRA treatment, and discussed the safety and effectiveness of three different IVC reconstruction methods.
Reconstruction options for the RHIVC are assessed individually from preoperative evaluation and intraoperative reassessment of vascular defect. In our center, the repairing and reconstruction method was used when the defect in the RHIVC was less than 180° of the lumen circumference after radical resection of the lesion. The average operation time and anhepatic phase of this pattern were relativelylonger than the other two methods, which was attributed to the diversity and complexity of this approach. RHIVC stenosis occurred in 10 patients, but only 1 developed lower limb edema, and the symptoms disappeared after balloon dilation. Meanwhile, no clinical symptoms were found in the other 9 patients. It seemed that IVC stenosis after surgery might be related to the longer cold ischemia time and tunica intimal injury during the reconstruction process. In this regard, strict management of the reconstruction time and shortening the cold ischemia phase as much as possible are the key factors for preventing IVC stenosis[31]. Based on previous reports[32,33] and our experience, the ligamentum teres hepatis is applied most often as a vascular patch, due to its convenience and strong plasticity.Postoperative patency and prognosis of these patients are satisfactory, suggesting that reconstruction with autogenous materials may be the optimal choice for RHIVC, although the process is complex and demanding.

Table 4 Literature summary of inferior vena cava reconstruction in ex vivo liver resection and autotransplantation
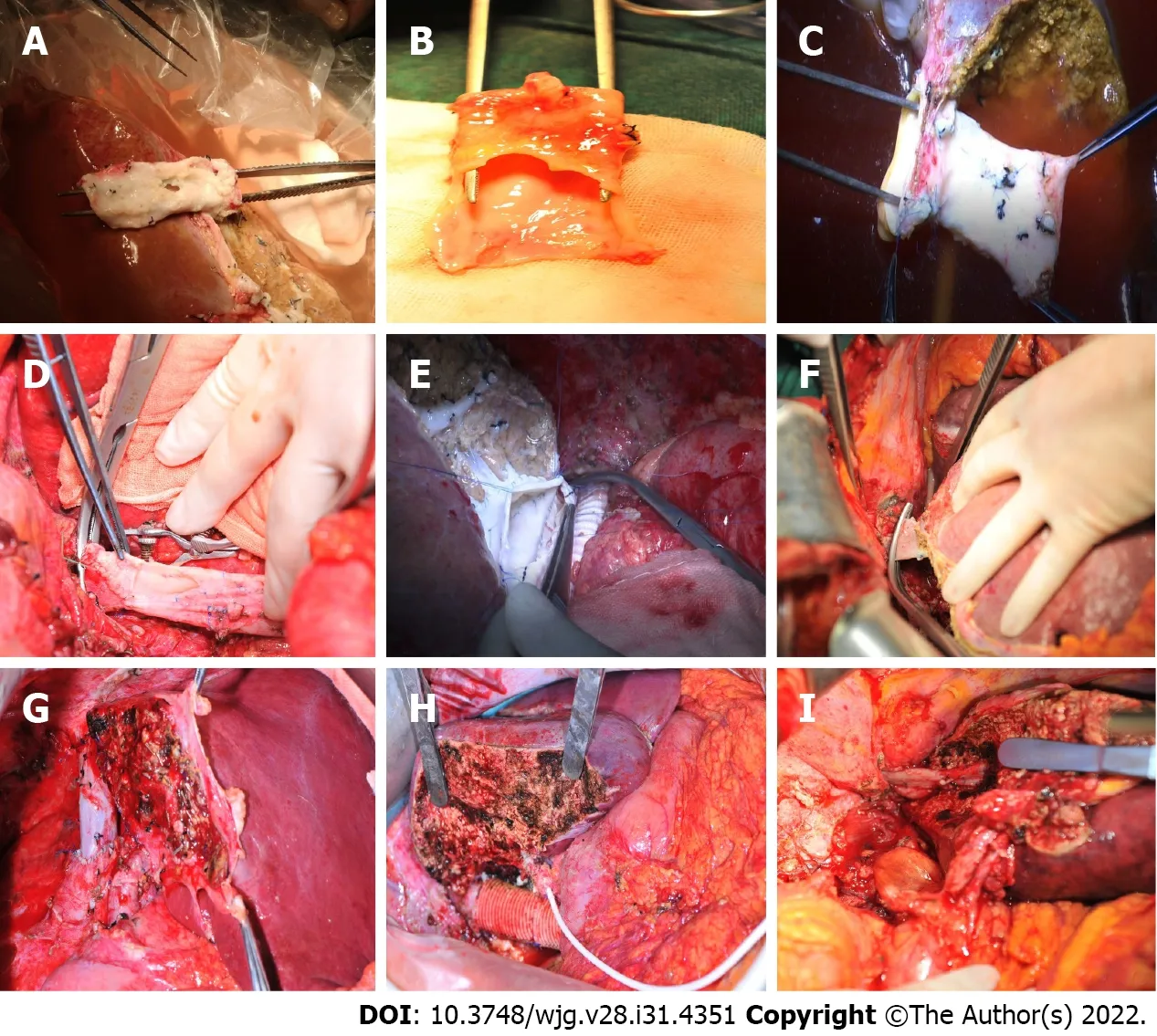
Figure 2 lntraoperative procedures. A: The infiltrated retrohepatic inferior vena cava (RHIVC); B: The large defect of RHIVC after radical resection; C: The RHIVC with self-suture repairing; D: The original RHIVC anastomoses with hepatic vein; E: The artificial blood vessel anastomoses with graft liver hepatic vein; F:The suprahepatic IVC anastomoses with left hepatic vein; G: The autologous RHIVC after reconstruction; H: Artificial RHIVC; I: Anastomotic stoma of suprahepatic IVC and hepatic vein.
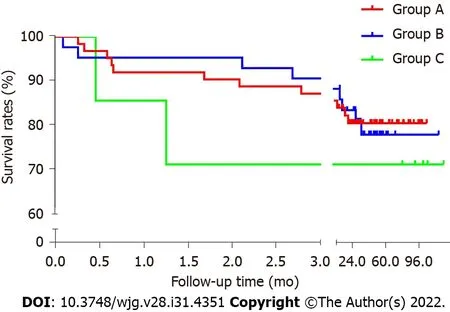
Figure 3 Kaplan-Meier survival analysis curve.
Generally, replacement of the RHIVC is indicated for patients those with a large defect after resection that cannot be repaired by suture or patch. In the present study, 38 patients had the RHIVC replaced with artificial vessels, and 5 were treated with the cryopreserved allogeneic vascular donated after citizen death (DCD). Due to the shortage of resources, rejection, high preservation requirements and time restrictions, DCD vessels are not the first choice for substitute material. In our center, the expanded polytetrafluoroethylene artificial vessel (Sokang Corp, Shanghai, China)[34] is generally preferred.RHIVC replacement with artificial vascular is not only convenient, but also effectively shortens the anhepatic phase and operation time. On the other hand, artificial vessels are associated with potential risk, including venous thrombosis, artificial vascular malformation, and distortion or stenosis of anastomoses caused by postoperative liver hyperplasia. In our research, thrombosis of artificial vessels occurred in 5 patients, among them, 2 died of liver failure due to simultaneous thrombosis of the IVC and portal vein. Another 3 patients developed lower limb or scrotal edema, and the symptoms recovered after symptomatic treatments, including thrombolysis and anticoagulation. In addition,stenosis of anastomoses was found in 2 patients. As there may not be any clinical means for decreasing the incidence of graft thrombosis and stenosis apart from anti-coagulation and anti-infective treatment,we recommend that replacement of RHIVC with artificial materials should be avoided when it can be reestablished with autogenous materials.
Hepatic AE behaves like a slow-growing “warm cancer,” and occlusion and severe stenosis of the IVC is a chronic process, which provides sufficient time for the establishment of collateral circulation.The obstructed blood flow can induce expansion of lumbar vein plexus, azygos vein and hemiazygos vein on both sides of the spine, as well as the formation of a functional collateral circulation network[32]Although the extent of vascular invasion is a vital indicator for the choice of reconstruction method, it remains controversial whether the IVC should be reconstructed for patients with adequate collateral circulation. Blairet al[35] proposed that, for low-grade retroperitoneal sarcoma, resection without reconstruction was performed after the establishment of collateral circulation. In addition, Hardwigseet al[36] also reported IVC resection without reconstruction in 6 patients with hepatic carcinoma. Unlike malignant tumors, the parasite grows relatively slow, which provides enough time for the organism to form collateral circulation. Among our subjects, 7 received RHIVC resection without reconstruction,since a sufficient collateral path was formed, but the outcomes were unsatisfactory. To be specific,postoperative complications occurred in all 7 patients, and their survival rate was only 71.1%. One patient died of liver failure on the 14thday after operation, and another one died of multiple organ failure caused by severe abdominal infection on the 38thday after operation. There is little previous information available concerning resection without reconstruction of the RHIVC in ELRA (Table 4).Before this study, Duet al[37] reported 8 patients receiving IVC resection without reconstruction, thus supporting this approach from the most reported cases at that time. In their subjects, 1 patient died of upper gastrointestinal bleeding, and 3 developed lower extremity or scrotal edema. Comparatively, the complication rate and postoperative hospital stay in our study were relatively high, which might be related to the immature collateral circulation or pathway destruction by excessive traction of diaphragm. It cannot be determined whether these poor outcomes are related to not providing reconstruction, and more studies are needed for further elucidation. To be sure, a comprehensive preoperative evaluation system and a meticulous intraoperative procedure are essential to the success of this method.
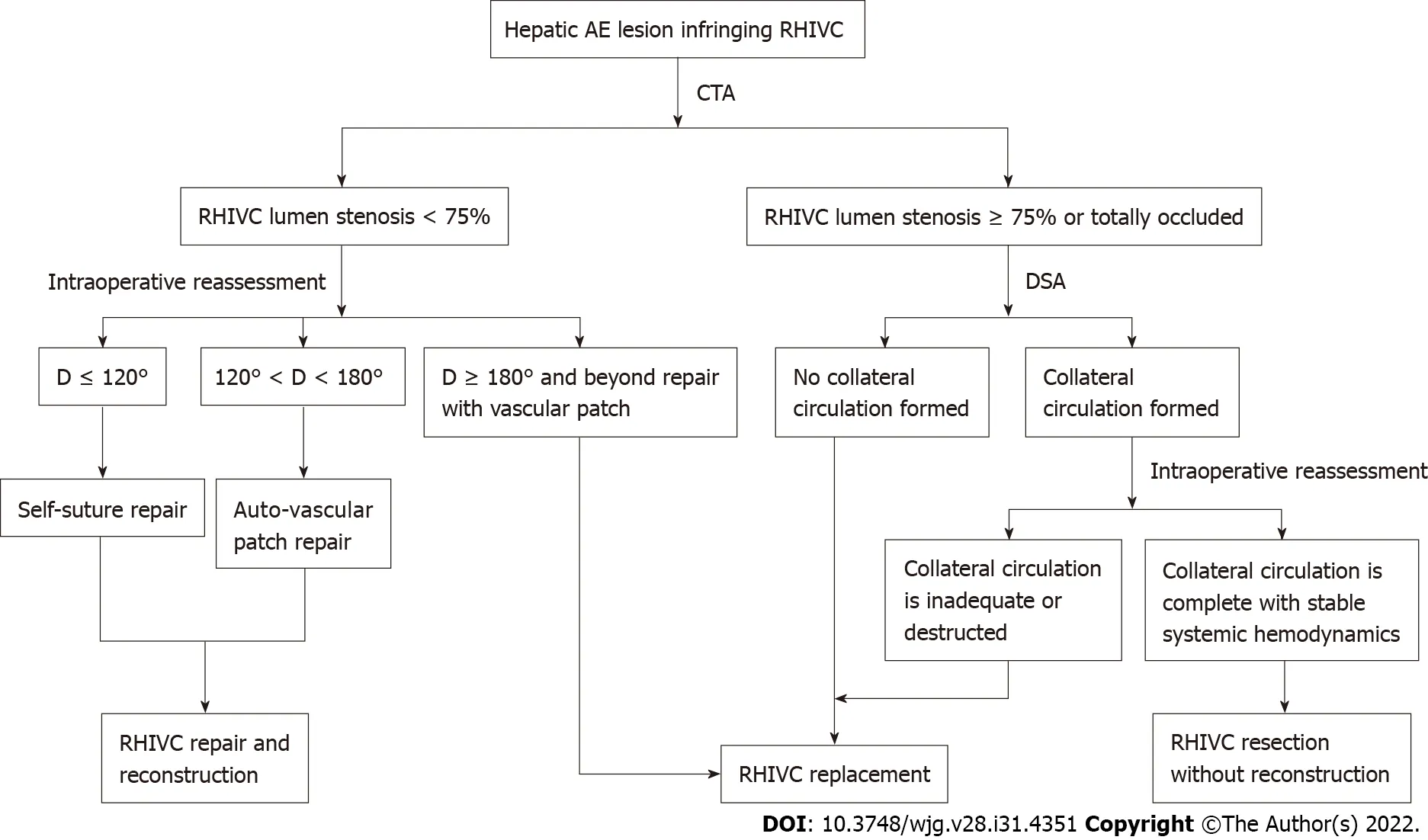
Figure 4 Strategy for retrohepatic inferior vena cava reconstruction in ex vivo liver resection and autotransplantation. CTA: Computed tomography angiography; D represents the defect of retrohepatic inferior vena cava lumen after resection; DSA: Digital subtraction angiography; RHIVC: Retrohepatic inferior vena cava.
Surgical planning based on a reasonable strategy can improve the success rate of operation and reduce related complications. In this study, although adverse outcomes were not entirely avoided, the postoperative complications and prognosis were acceptable, showing that our approaches were relatively safe and effective on end-stage hepatic AE with infiltrating outflow vessels of the liver. Based on the outcome of the present study and our experience, the following strategies are recommended for IVC reconstruction in hepatic AE patients treated by ELRA: (1) When the wall defect of the IVC after radical resection is less than 120° of the lumen circumference, the self-suture method can be used; (2) If the defect is 120°-180° of circumference, repair with autogenous vascular patches is the optimal choice;(3) When the defect has exceeded 180° of circumference and is difficult to repair by autologous patches,the RHIVC should be replaced. However, the strict anticoagulation therapy must be provided postoperatively; and (4) The RHIVC resection without reconstruction approaches are suitable when the IVC is completely blocked and the collateral circulation is fully formed (Figure 4). Although this study reports a retrospective single-center experience, it is also the largest cohort to specifically compare the different IVC reconstruction methods in ELRA, which can serve as a reference for IVC reconstruction in liver transplantation.
CONCLUSlON
In short, ELRA can be considered a safe and feasible option for the end-stage hepatic AE patients with RHIVC infiltration. The RHIVC reconstruction approaches should be selected appropriately according to the lumen defect degree after radical resection. It should be emphasized that RHIVC resection without reconstruction should be considered with caution, and RHIVC reestablishment may be more beneficial for patients with poor compensation. Regrettably, the strategy does not consider the length of the wall defect, since it is related to the location, angle, elasticity and tension of the vessel, all of which are hard to be quantified without a larger dataset of cases. Therefore, prospective, multicenter studies with long-term follow-up are necessary to further evaluate and improve the strategy presented here.
ARTlCLE HlGHLlGHTS


Research motivation
There are no clear consensus has been reached on the strategy for retrohepatic inferior vena cava(RHIVC) reconstruction in ELRA.
Research objectives
To provide a strategy of RHIVC reconstruction for end-stage hepatic AE patients with hepatocaval confluence infiltration.
Research methods
The clinical data of 114 patients, including the operation time, anhepatic phase, intraoperative blood loss, complications and postoperative hospital stay, were analyzed and the patients were routinely followed up.
Research results
We found a lower survival rate in group C (resection without reconstruction method) than in groups A(self-suture repairing method) and B (replacement method). Also, the complications rate was higher in group C.
Research conclusions
The RHIVC reconstruction methods should be selected appropriately depending on the defect degree of AE lesions in IVC lumen.
Research perspectives
Our strategies can serve as a reference for IVC reconstruction in liver transplantation.
FOOTNOTES
Author contributions:Maimaitinijiati Y contributed to the conception and design, and drafting of the article;Maimaitinijiati Y and Ran B contributed to the acquisition of data, analysis, and interpretation of data; Jiang TM,Tuerganaili A, and Wen H contributed to the conception and design, and provision of study material; Jiang TM,Zhang RQ, Guo Q, and Wang ML contributed to the data collection; Shao YM did provision of study material; Zhang RQ analyzed the data; Wen H did final approval of the version to be submitted.
lnstitutional review board statement:The study was approved by the Human Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University and conducted in accordance with the Declaration of Helsinki. All data were analyzed anonymously.
lnformed consent statement:Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement:The authors have no conflicts of interest to declare.
Data sharing statement:The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Yusufukadier Maimaitinijiati 0000-0003-2069-9202; AJi Tuerganaili 0000-0001-6737-8874; Tie-Min Jiang 0000-0002-6100-1870; Bo Ran 0000-0002-0241-3410; Rui-Qing Zhang 0000-0003-3022-2555; Ying-Mei Shao 0000-0001-5154-345X; Qiang Guo 0000-0002-5943-4255; Mao-Lin Wang 0000-0001-8944-0894; Hao Wen 0000-0002-6431-9382.
S-Editor:Zhang H
L-Editor:Filipodia
P-Editor:Zhang H
 World Journal of Gastroenterology2022年31期
World Journal of Gastroenterology2022年31期
- World Journal of Gastroenterology的其它文章
- Duodenal-jejunal bypass reduces serum ceramides via inhibiting intestinal bile acid-farnesoid X receptor pathway
- Preoperative contrast-enhanced computed tomography-based radiomics model for overall survival prediction in hepatocellular carcinoma
- Prevalence and clinical characteristics of autoimmune liver disease in hospitalized patients with cirrhosis and acute decompensation in China
- Application of computed tomography-based radiomics in differential diagnosis of adenocarcinoma and squamous cell carcinoma at the esophagogastric junction
- Radiomics and nomogram of magnetic resonance imaging for preoperative prediction of microvascular invasion in small hepatocellular carcinoma
- Insights into induction of the immune response by the hepatitis B vaccine
