基于应激颗粒的中药神经保护研究设想
陈 畅,罗婉君,段飞鹏,王寓平,胥雄飞,杨萌楚,姜金铸,谢永艳,黄锦伟*,黄丽萍*
·综述·
基于应激颗粒的中药神经保护研究设想
陈 畅1, 2, 3,罗婉君2,段飞鹏1,王寓平2,胥雄飞2,杨萌楚2,姜金铸3,谢永艳1,黄锦伟2*,黄丽萍1*
1. 江西中医药大学药学院,江西 南昌 330004 2. 澳门科技大学中药质量研究国家重点实验室,中国 澳门 999078 3. 中国中医科学院中药研究所,北京 100700
应激颗粒是存在于胞质的一种无膜致密颗粒,通过包裹mRNA、转录起始因子、RNA结合蛋白等翻译起始阶段的重要元件,使细胞在遭受不利刺激时出现短暂的蛋白翻译阻滞,从而起到细胞保护作用。研究发现,应激颗粒广泛分布于海马和皮层的神经元细胞、星形胶质细胞、小胶质细胞和浦肯野细胞等神经细胞中,生物过程涉及蛋白异常聚集、突触的成熟和可塑性、免疫调节、抗氧化应激反应、抑制细胞凋亡等,在急性缺血性脑卒中和神经退行性疾病的发生发展中发挥着重要作用。尤其是其多样化的物质组成和广泛参与的生物过程,为中医证候生物学及“同病异治”“整体调节”等中医药传统理论和治法的研究提供了崭新的思路和视野。对应激颗粒的基本特征、神经保护及研究方法等进行系统的梳理,同时以急性缺血性脑卒中为范例,尝试将应激颗粒这一现代生物学现象纳入到中医药现代研究中,提出研究设想和思路,以期用科学的语言阐释传统中医药相关理论与治法,也为行业同仁的相关研究提供参考。
应激颗粒;脑缺血;RNA结合蛋白;Ras-GTPase活化蛋白SH3结构域结合蛋白1;神经保护
当细胞受到不利环境(如紫外线照射、病毒感染、亚砷酸盐刺激、热休克、缺氧、饥饿)胁迫时,可出现短暂的蛋白质翻译阻滞,形成由翻译起始阶段mRNA等组成的一种致密性颗粒状聚集体。当胁迫消失后,该聚集体可解聚并释放包裹的mRNA及相关蛋白,使细胞能够继续正常的蛋白质翻译过程。这种致密的颗粒状聚集体即为应激颗粒。应激颗粒的形成是细胞应对不利刺激时的一种自我保护机制,提高了细胞在不利环境下生存的能力。近年来,研究发现应激颗粒参与了细胞的翻译调控、蛋白质错误折叠等生物学过程,尤其是其动态而快速的形成和解聚、调节蛋白异常聚集的特性,为急性缺血性脑卒中及神经退行性疾病的治疗提供了一种创新的思路。
1 应激颗粒的形成
应激颗粒的形成分为经典和非经典2种途径[1]。经典途径是应激颗粒形成的主要方式,依赖于真核翻译起始因子2α(eukaryotic initiation factor 2α,eIF2α)的磷酸化,非经典途径与破坏eIF4F复合物有关[2]。在哺乳动物细胞中,目前已知至少有5种激酶[3],包括蛋白激酶R(protein kinase R,PKR)、PKR样内质网激酶(PKR-like endoplasmic reticulum kinase,PERK)、一般性控制非抑制性蛋白2(general control non-derepressible 2,GCN2)、血红素调节抑制剂激酶(heme-regulated inhibitor kinase,HRI)和Z-DNA激酶[4],可在细胞受到不同的刺激时激活,促进eIF2α的磷酸化,进而抑制eIF2与三磷酸鸟苷(guanosine triphosphate,GTP)结合,影响eIF2-GTP-tRNAMet复合物的形成,导致蛋白翻译的暂时阻滞。在这一阶段,翻译起始阶段的mRNA、翻译起始因子、40S核糖体亚基和RNA结合蛋白等通过微管运输的方式[5],以“核优先”方式或“液-液相分离优先”方式进行装配[6],逐渐聚集并最终形成成熟的应激颗粒。在组成应激颗粒的组分中,超过50%为RNA结合蛋白[7],虽然目前还未完全阐明这些蛋白各自的功能,但其中的Ras-GTPase活化蛋白SH3结构域结合蛋白1(GTPase-activating protein SH3 domain binding protein 1,G3BP1)及T细胞胞质内抗原1(T-cell intracellular antigen 1,TIA1)对应激颗粒的聚集起关键作用[8]。研究表明,缺乏G3BP1将不能形成应激颗粒[9-11],即便在没有应激的情况下,G3BP1也能诱导应激颗粒的形成[8]。因此G3BP1也是被广泛用于应激颗粒形成的关键标志性蛋白[12]。
2 应激颗粒的解聚及影响因素
显微观察发现,尽管应激颗粒是一个胞质的无膜结构,但其也有1个相对密集的“核”及1个具有潜在流动性的“壳”,并呈现出液体一样,组分快速交换的特点[7,13]。当细胞所处的不利环境因素消失或减弱时,细胞中大部分的应激颗粒可在数分钟内同时解聚并消失,其解聚的过程表现为溶解而非分解为碎片[14-15]。应激颗粒解聚后,一方面,释放的mRNA及与翻译相关的主要元件回到核糖体,重新启动正常的蛋白翻译过程;另一方面,应激颗粒的组分还可与胞质中的另一种核糖核蛋白体,即P小体进行物质交换,mRNA进入P小体发生降解或参与其他生物过程,RNA结合蛋白等则通过自噬的途径予以清除[16-17]。
一些蛋白的磷酸化、甲基化修饰均能影响应激颗粒的形成和解聚[18]。如G3BP1的磷酸化破坏了它形成多聚体的能力,而去磷酸化[8]和去甲基化[19]则有利于促进应激颗粒的组装。其他如生长因子受体结合蛋白7和双特异性酪氨酸磷酸化调节激酶3的磷酸化,也可促进应激颗粒的解聚。另外,三磷酸腺苷(adenosine triphosphate,ATP)可驱动部分蛋白质组分的交换,ATP酶可通过影响微管运输的细胞转运,以及分子伴侣和RNA解螺旋酶的交互作用[5],参与到应激颗粒的运动中,暗示ATP可能参与驱动应激颗粒的形成[7],在应激颗粒的形成和解聚中扮演着重要的角色。此外,肌动蛋白调节蛋白[20]、热休克蛋白70等分子伴侣[21]及调节微管功能的相关蛋白如驱动蛋白、动力蛋白、组蛋白脱乙酰基酶6、RhoA/ROCK1,也参与了应激颗粒的形成和解聚过程[15,22-24]。
3 应激颗粒的功能
应激颗粒的形成,一方面促进了某些组分的“聚集”,增强了细胞的天然免疫应答等生物过程[25-27];另一方面也可通过“隔离”相关信号分子,抑制细胞信号通路的转导[28-29]。尽管目前对应激颗粒的功能并不完全清楚,但已有证据表明,其可调节以下细胞生物学过程。
3.1 抗氧化
G3BP1及其绑定的配体泛素特异性蛋白酶10(ubiquitin-specific protease 10,USP10)在应激颗粒的抗氧化作用中发挥着重要作用。研究表明,单独的USP10并不具有抵抗过氧化氢导致的氧化损伤作用,而是在形成应激颗粒后才能发挥此项功能[30]。在非应激状态下,USP10表达受到G3BP1的抑制,当应激颗粒形成后,G3BP1对USP10的抑制减弱,进而发挥抗氧化功能。
3.2 抗凋亡
应激颗粒的抗凋亡作用一方面与其抗氧化作用有关[30],同时也可通过“隔离”诱导细胞凋亡的关键信号分子予以实现。研究表明,应激颗粒除可通过抑制雷帕霉素复合物1通路的细胞凋亡外[31],还可通过招募并“隔离”活化的蛋白激酶C1受体等关键信号分子,使其不能与其他因子相结合,进而抑制了p38和c-Jun氨基末端激酶的激活,降低了细胞凋亡的发生[32]。此外,过表达的Fas活化丝氨酸/苏氨酸激酶可与应激颗粒中的重要RNA结合蛋白TIA1相互作用,降低TIA1对某些抗凋亡蛋白mRNA的抑制,从而促进这些抗凋亡蛋白的表达[33]。
3.3 参与免疫调节
cGAS/string天然免疫信号通路可识别由于衰老或损伤等所产生的胞质DNA碎片,启动I型干扰素表达,帮助细胞进行免疫防御。研究显示,G3BP1可通过帮助cGAS聚集发生相分离,增强其对DNA的敏感性和结合能力,进而促进cGAS的激活[34]。G3BP1缺失将导致cGAS不能有效结合DNA,从而抑制cGAS介导的I型干扰素的产生[35]。除此之外,应激颗粒的聚集可抑制肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)介导的核因子-κB通路的活化[36];组成蛋白CUGBP2及HuR可调控前炎症刺激物环氧合酶-2的表达等[37-38]。
4 应激颗粒与神经保护
4.1 应激颗粒与缺血性脑卒中
急性缺血性脑卒中后损伤部位蛋白翻译的暂时阻滞与应激颗粒的聚集关系密切[39],在脑缺血动物模型的大脑皮层和海马均可观察到大量应激颗粒的产生。研究表明[40],大脑皮层应激颗粒的形成在脑缺血再灌注后6 h达到峰值,在再灌注后24 h下降,而细胞凋亡水平和脑梗死体积在再灌注后6 h最低,24 h最高。提示应激颗粒的形成与凋亡水平呈负相关趋势,再灌注24 h是研究应激颗粒形成与细胞凋亡水平相关性的最佳观测时间点,同时也表明应激颗粒具有动态结构和抗凋亡作用。体外细胞实验也证明,通过促进糖氧剥夺/复氧的PC细胞应激颗粒的生成,可明显抑制细胞的凋亡水平[41-43]。除此之外,脑缺血可导致海马CA3区、hilar区、齿状回等神经元eIF2α迅速磷酸化,应激颗粒明显增加[44]。然而海马CA1区相对难以形成应激颗粒[45-46],因此当血流恢复灌注后,除CA1区外其他脑区的蛋白合成大多可逐步恢复[47-48],这也可以解释为何有的研究表明CA1区比CA3区对缺血更为敏感[49-50]。脑缺血后应激颗粒的形成也可通过不依赖于eIF2α磷酸化的途径产生,该过程与eIF4F复合物有关[51]。
4.2 应激颗粒与神经退行性疾病
神经退行性疾病的发生除与应激颗粒关键蛋白G3BP1关系密切外[52],还与一些蛋白的异常聚集有关。如β淀粉样蛋白、tau蛋白、α突触核蛋白(α-synuclein)、TAR DNA结合蛋白-43(TAR DNA binding protein-43,TDP-43)及FUS的异常聚集被认为是阿尔茨海默病、帕金森症、肌萎缩性脊髓侧索硬化症等神经退行性疾病的重要病理标志。这在形态学上与应激颗粒形成过程中,RNA结合蛋白高度聚集形成致密颗粒非常相似,这一病理过程已被证明与应激颗粒形成有关[53]。研究表明,内质网内未正确折叠蛋白的积累,可激活位于内质网的激酶PERK,引起eIF2α磷酸化,进而促进应激颗粒形成[54]。报道显示,用siRNA敲减TDP-43后[55],应激颗粒形成减少,提示TDP-43参与了应激颗粒的生成[56-58]。与神经退行性疾病相关的许多蛋白,如tau[59]、FUS[60]、ataxin-2[61]、运动神经元生存蛋白[62]、血管稳定蛋白[63]等,在应激发生时,也可被招募到应激颗粒中参与应激颗粒的形成[64]。
在连接应激颗粒与神经退行性疾病的RNA结合蛋白中,TDP-43的研究相对更为深入。TDB-43是一种DNA和RNA结合蛋白,病理性TDP-43被认为是额颞叶变性和肌萎缩侧索硬化症的主要病理标志物,后来也发现其参与了阿尔茨海默病、帕金森症及亨廷顿病等神经退行性疾病的病理过程[65-66]。正常情况下,应激发生后,位于细胞核的TDP-43迅速转移到胞质中,参与应激颗粒的形成并起细胞保护作用[57,67]。然而病理状态下,目前已知最少有4种情况可导致TDP-43的异常聚集,进而表现出TDP-43蛋白病的特征。①RNA结合功能缺陷的TDP-43因不能被募集到应激颗粒中,而在胞质中形成磷酸化和P62阳性TDP-43颗粒[68]。②当应激消失后,病理性的TDP-43聚集物并不随着应激颗粒的解体而解聚[69]。③应激颗粒异常解体时,TDP-43从应激颗粒释放至胞质,并发生磷酸化,进而发生液-液相分离形成病理性聚集体[70-71]。④TDP-43突变导致胞质TDP-43永久性聚集,进而增加了应激颗粒的稳定性,阻碍了应激颗粒本身的动态物质交换过程[72]。
深入研究发现,富含甘氨酸、天冬酰胺、谷氨酰胺和酪氨酸的朊蛋白样结构域[73-74],重点调节了TDP-43的剪接活性及与应激颗粒等的相互作用。
4.3 应激颗粒参与其他神经保护功能
G3BP1和TIA1是形成应激颗粒的关键RNA结合蛋白,基因敲除或其功能异常均会影响应激颗粒的正常聚集。G3BP1高表达于大脑海马、额叶皮层、小脑浦肯野细胞等部位[75]。基因敲除后,小鼠脑组织海马区钙离子稳态失衡,神经突触传递功能障碍,皮层及内囊神经元大量细胞凋亡,小脑浦肯野细胞数量明显减少,可出现记忆力减退及运动功能障碍等症状[75-78]。G3BP1复合物还可在小鼠脑组织中参与到内含子保留的转录调控中,并进一步调节谷氨酸神经元的相关功能,影响神经突触可塑性[78]。另外,TIA1的降低或敲除可促进小胶质细胞的增殖、活化与吞噬,白细胞介素-1β及TNF-α释放增多,氧化应激水平提高,神经突触丢失增加,加重了tau蛋白疾病发展[79]。富甘氨酸序列的冷诱导RNA结合蛋白也可参与应激颗粒的形成[80]。研究表明,在原代大鼠海马神经元细胞模型中,亚低温处理可通过上调冷诱导RNA结合蛋白的表达,抑制细胞内氧自由基的生成,从而直接或间接地抑制了氧自由基诱导的神经元细胞凋亡,进而起到海马神经元的保护作用[81]。
5 应激颗粒的观察和检测
对应激颗粒标签蛋白如G3BP1或TIA1的检测,是当前表征应激颗粒的主要手段[82]。在细胞水平,采用荧光显微镜或激光共聚焦显微镜观测技术,以免疫荧光法检测TIA1或G3BP1,已被用于观察毒胡萝卜素诱导海马神经元HT22细胞[83]及缺氧诱导的人小胶质HMC3细胞[84]应激颗粒的形成。若采用G3BP1与微管关联蛋白等神经细胞标记蛋白共同观察,还可考察应激颗粒的细胞定位[75]。为增加对应激颗粒检测的准确性,有研究同时采用了G3BP1及TIA1 2种标签蛋白,对缺氧/复氧的原代皮层神经元细胞或神经细胞PC12中的应激颗粒进行表征[43]。除此之外,以红色荧光蛋白、绿色荧光蛋白分别对G3BP1、TIA1进行标记,还可动态观察细胞受亚砷酸盐刺激后应激颗粒的形成过程[46,84]。在组织水平,除可采用免疫组化法对TIA1等标签蛋白进行定量的方法外[40],也可用免疫荧光法,以G3BP1或TIA1为标记,对大鼠脑缺血后缺血组织的应激颗粒进行形态学表征[41]。
尽管G3BP1和TIA1均可作为应激颗粒的标签蛋白,但在非应激状态下,TIA1主要位于细胞核内,应激发生后部分迁移到胞质中[85]。而G3BP1主要分散于细胞质中,荧光标记的G3BP1在应激发生后更容易观察到特异性的点状荧光[82,86]。因此有学者认为与TIA相比,G3BP1更适合作为应激颗粒的标志物[12]。
由于应激颗粒是一种无膜的亚细胞结构,且存在动态而迅速的物质交换过程,因此对其内部组成的研究还存在许多技术瓶颈[87]。目前相关的研究手段包括荧光漂白恢复技术[88]、高分辨显微技术[7]、蛋白组学分析[7]、差速离心及免疫纯化[24]、体外模拟[89]及数学建模[90]等。
6 基于应激颗粒的中医药现代研究设想
结构决定功能是当前生物学研究的基本认识。研究应激颗粒的组分构成对其功能的研究意义重大。报道显示,细胞受到不同的压力刺激时,所产生的应激颗粒组分不同[3]。如在酿酒的酵母中,eIF3在热应激诱导的应激颗粒中被发现,而葡萄糖缺乏诱导的应激颗粒中却无eIF3[91-93]。功能上看,同一类型细胞所产生的应激颗粒,也可能产生完全相反的生物效应。如在肿瘤细胞中,应激颗粒一方面可通过丝裂原活化蛋白激酶途径抑制肿瘤细胞凋亡[32];也可通过包裹住缺氧诱导因子-1α mRNA,使血管内皮细胞生长因子无法被激活,进而提高肿瘤的治疗效果[94]。
应激颗粒组成成分和功能的深入研究,为药物开发提供了一个全新的领域。在中药神经保护领域,尽管当前只有少数研究报道了中药(成分)可通过促进应激颗粒生成发挥脑保护作用[40,95],但应激颗粒介导的中药神经保护策略,已展示出创新的研究思路和广阔的研发前景,尤其是应激颗粒动态的物质组成与功能特征,更是与中医药的一些治疗理念不谋而合。首先,细胞受不同刺激导致的损伤,与传统中医认为的机体由于不同证候导致的疾病有诸多相似之处。如中医认为“中风”的基本病因有气虚、血瘀、痰湿、热毒等,这些中医学上的不同病因与细胞受到的不同环境刺激在损伤原理上非常相似。其次,应激颗粒受到不同刺激从而有不同的物质组成这一特性,一方面可为阐释中医的证候生物学提供科学载体和物质条件;同时采用不同的治法如补气、活血、化痰、清热等对应激颗粒不同的物质组成进行“纠偏”,也可为研究“同病异治”这一传统中医治法提供创新的思路和方法。另外,应激颗粒的形成,包裹和隔离了诸多细胞信号传递因子,对细胞的生物过程如凋亡、氧化应激、免疫调节等产生重大影响。尽管尚不清楚应激颗粒在不同情况下其确切的物质组成,但其对细胞生物过程的多方位调控,也为研究中药多成分如何调节机体的多个生物学效应,开创了一个全新的研究方向。以缺血性脑卒中为例,应激颗粒介导的中医证候生物学、“同病异治”及“整体调节”研究设想可简要概括为图1的研究思路和框架。
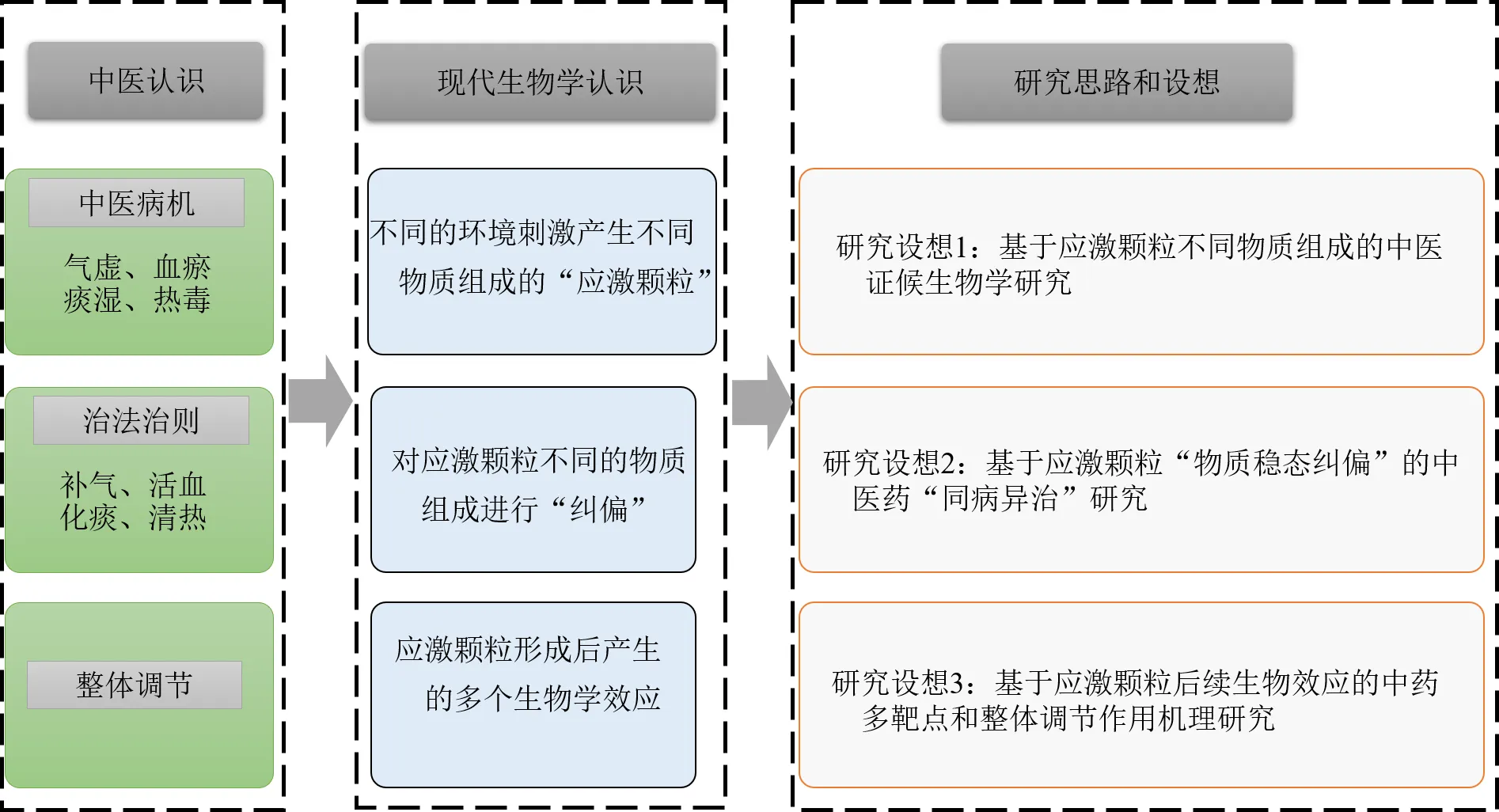
图1 基于应激颗粒的中医药现代研究设想(以缺血性脑卒中为例)
7 结语
当前对应激颗粒的研究和认识还不全面,相信随着现代生物学的进步,应激颗粒的物质组成和功能将逐渐被揭示,这为基于应激颗粒的创新药物研究开启了崭新的一页。尤其是当不利刺激发生时胞质成分快速聚集,刺激消失后迅速解聚的特性,为急性缺血性脑卒中等急性脑损伤的研究提供了良好的载体;其调控RNA蛋白的错误折叠和异常聚集,也为神经退行性疾病的研究开拓了新的思路和方法。
利益冲突 所有作者均声明不存在利益冲突
[1] Advani V M, Ivanov P. Stress Granule subtypes: An emerging link to neurodegeneration [J]., 2020, 77(23): 4827-4845.
[2] le Sage V, Cinti A, Amorim R,. Adapting the stress response: Viral subversion of the mTOR signaling pathway [J]., 2016, 8(6): 152.
[3] 陈新颖, 华子春, 殷武. 应激颗粒的形成与生物学意义 [J]. 中国细胞生物学学报, 2012, 34(8): 837-844.
[4] Chiang D C, Li Y, Ng S K. The role of the-DNA binding domain in innate immunity and stress granules [J]., 2021, 11: 625504.
[5] Nadezhdina E S, Lomakin A J, Shpilman A A,. Microtubules govern stress granule mobility and dynamics [J]., 2010, 1803(3): 361-371.
[6] Protter D S W, Parker R. Principles and properties of stress granules [J]., 2016, 26(9): 668-679.
[7] Jain S, Wheeler J R, Walters R W,. ATPase-modulated stress granules contain a diverse proteome and substructure [J]., 2016, 164(3): 487-498.
[8] Tourrière H, Chebli K, Zekri L,. The RasGAP-associated endoribonuclease G3BP assembles stress granules [J]., 2003, 160(6): 823-831.
[9] Wang I, Hennig J, Jagtap P K A,. Structure, dynamics and RNA binding of the multi-domain splicing factor TIA-1 [J]., 2014, 42(9): 5949-5966.
[10] Bittencourt L F F, Negreiros-Lima G L, Sousa L P,. G3BP1 knockdown sensitizes U87 glioblastoma cell line to Bortezomib by inhibiting stress granules assembly and potentializing apoptosis [J]., 2019, 144(3): 463-473.
[11] Aulas A, Caron G, Gkogkas C G,. G3BP1 promotes stress-induced RNA granule interactions to preserve polyadenylated mRNA [J]., 2015, 209(1): 73-84.
[12] 邵琪, 屈阳, 朱子晨, 等. 应用G3BP1稳转细胞系监测应激状态下的应激颗粒形成 [J]. 畜牧兽医学报, 2020, 51(9): 2275-2283.
[13] Souquere S, Mollet S, Kress M,. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells [J]., 2009, 122(Pt 20): 3619-3626.
[14] Anderson P, Kedersha N. Stress granules: The Tao of RNA triage [J]., 2008, 33(3): 141-150.
[15] Kolobova E, Efimov A, Kaverina I,. Microtubule-dependent association of AKAP350A and CCAR1 with RNA stress granules [J]., 2009, 315(3): 542-555.
[16] Kedersha N, Chen S, Gilks N,. Evidence that ternary complex (eIF2-GTP-tRNAiMet)-deficient preinitiation complexes are core constituents of mammalian stress granules [J]., 2002, 13(1): 195-210.
[17] Kedersha N, Anderson P. Stress granules: Sites of mRNA triage that regulate mRNA stability and translatability [J]., 2002, 30(Pt 6): 963-969.
[18] Hofmann S, Kedersha N, Anderson P,. Molecular mechanisms of stress granule assembly and disassembly [J]., 2021, 1868(1): 118876.
[19] Tsai W C, Gayatri S, Reineke L C,. Arginine demethylation of G3BP1 promotes stress granule assembly [J]., 2016, 291(43): 22671-22685.
[20] Figley M D, Bieri G, Kolaitis R M,. Profilin 1 associates with stress granules and ALS-linked mutations alter stress granule dynamics [J]., 2014, 34(24): 8083-8097.
[21] Walters R W, Muhlrad D, Garcia J,. Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in[J]., 2015, 21(9): 1660-1671.
[22] Li C H, Ohn T, Ivanov P,. eIF5A promotes translation elongation, polysome disassembly and stress granule assembly [J]., 2010, 5(4): e9942.
[23] Bartoli K M, Bishop D L, Saunders W S. The role of molecular microtubule motors and the microtubule cytoskeleton in stress granule dynamics [J]., 2011, 2011: 939848.
[24] Kwon S, Zhang Y, Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response [J]., 2007, 21(24): 3381-3394.
[25] Reineke L C, Lloyd R E. Diversion of stress granules and P-bodies during viral infection [J]., 2013, 436(2): 255-267.
[26] Reineke L C, Kedersha N, Langereis M A,. Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1 [J]., 2015, 6(2): e02486.
[27] Reineke L C, Lloyd R E. The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses [J]., 2015, 89(5): 2575-2589.
[28] Wippich F, Bodenmiller B, Trajkovska M G,. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling [J]., 2013, 152(4): 791-805.
[29] Leung A K L, Vyas S, Rood J E,. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm [J]., 2011, 42(4): 489-499.
[30] Takahashi M, Higuchi M, Matsuki H,. Stress granules inhibit apoptosis by reducing reactive oxygen species production [J]., 2013, 33(4): 815-829.
[31] Thedieck K, Holzwarth B, Prentzell M T,. Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells [J]., 2013, 154(4): 859-874.
[32] Arimoto K, Fukuda H, Imajoh-Ohmi S,. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways [J]., 2008, 10(11): 1324-1332.
[33] Tian Q, Taupin J, Elledge S,. Fas-activated serine/threonine kinase (FAST) phosphorylates TIA-1 during Fas-mediated apoptosis [J]., 1995, 182(3): 865-874.
[34] 薛文. G3BP1促进胞质DNA感受器cGAS介导的抗病毒免疫[D]. 北京: 军事医学科学院, 2020.
[35] Liu Z S, Cai H, Xue W,. G3BP1 promotes DNA binding and activation of cGAS [J]., 2019, 20(1): 18-28.
[36] Kim W J, Back S H, Kim V,. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions [J]., 2005, 25(6): 2450-2462.
[37] Moraes K C M, Monteiro C J, Pacheco-Soares C. A novel function for CUGBP2 in controlling the pro-inflammatory stimulus in H9c2 cells: Subcellular trafficking of messenger molecules [J]., 2013, 37(10): 1129-1138.
[38] Feng S, Chen W, Cao D,. Involvement of Na+, K+-ATPase and its inhibitors in HuR-mediated cytokine mRNA stabilization in lung epithelial cells [J]., 2011, 68(1): 109-124.
[39] Monique L. Mechanisms of translation arrest following focal brain ischemia [D]. Detroit: Wayne State University, 2011.
[40] 司文文. 左旋樟脑通过表观调控应激颗粒生成抗中风损伤研究 [D]. 广州: 广州中医药大学, 2019.
[41] Si W W, Li Y, Ye S Y,. Methyltransferase 3 mediated miRNA m6A methylation promotes stress granule formation in the early stage of acute ischemic stroke [J]., 2020, 13: 103.
[42] Si W W, Ye S Y, Ren Z X,. miR‑335 promotes stress granule formation to inhibit apoptosis by targeting ROCK2 in acute ischemic stroke [J]., 2019, 43(3): 1452-1466.
[43] Si W W, Li Z, Huang Z F,. RNA binding protein motif 3 inhibits oxygen-glucose deprivation/reoxygenation-induced apoptosis through promoting stress granules formation in PC12 cells and rat primary cortical neurons [J]., 2020, 14: 559384.
[44] Jamison J T, Kayali F, Rudolph J,. Persistent redistribution of poly-adenylated mRNAs correlates with translation arrest and cell death following global brain ischemia and reperfusion [J]., 2008, 154(2): 504-520.
[45] Kayali F, Montie H L, Rafols J A,. Prolonged translation arrest in reperfused hippocampal cornu ammonis 1 is mediated by stress granules [J]., 2005, 134(4): 1223-1245.
[46] Ohshima D, Arimoto-Matsuzaki K, Tomida T,. Spatio-temporal dynamics and mechanisms of stress granule assembly [J]., 2015, 11(6): e1004326.
[47] Thilmann R, Xie Y, Kleihues P,. Persistent inhibition of protein synthesis precedes delayed neuronal death in postischemic gerbil hippocampus [J]., 1986, 71(1/2): 88-93.
[48] Araki T, Kato H, Inoue T,. Regional impairment of protein synthesis following brief cerebral ischemia in the gerbil [J]., 1990, 79(5): 501-505.
[49] Crepel V, Epsztein J, Ben-Ari Y. Ischemia induces short- and long-term remodeling of synaptic activity in the hippocampus [J]., 2003, 7(4): 401-407.
[50] Lehotský J, Burda J, Danielisová V,. Ischemic tolerance: The mechanisms of neuroprotective strategy [J]., 2009, 292(12): 2002-2012.
[51] Ayuso M I, Martínez-Alonso E, Regidor I,. Stress Granule induction after brain ischemia is independent of eukaryotic translation initiation factor (eIF)2α phosphorylation and is correlated with a decrease in eIF4B and eIF4E proteins [J]., 2016, 291(53): 27252-27264.
[52] Sidibé H, Dubinski A, Vande Velde C. The multi-functional RNA-binding protein G3BP1 and its potential implication in neurodegenerative disease [J]., 2021, 157(4): 944-962.
[53] Wolozin B, Ivanov P. Stress granules and neurodegeneration [J]., 2019, 20(11): 649-666.
[54] Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery [J]., 2011, 9(12): 860-875.
[55] McDonald K K, Aulas A, Destroismaisons L,. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1 [J]., 2011, 20(7): 1400-1410.
[56] Castellani R J, Gupta Y, Sheng B Y,. A novel origin for granulovacuolar degeneration in aging and Alzheimer’s disease: Parallels to stress granules [J]., 2011, 91(12): 1777-1786.
[57] Dormann D, Rodde R, Edbauer D,. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import [J]., 2010, 29(16): 2841-2857.
[58] Liu-Yesucevitz L, Bilgutay A, Zhang Y J,. Tar DNA binding protein-43 (TDP-43) associates with stress granules: Analysis of cultured cells and pathological brain tissue [J]., 2010, 5(10): e13250.
[59] Vanderweyde T, Yu H, Varnum M,. Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies [J]., 2012, 32(24): 8270-8283.
[60] Vance C, Rogelj B, Hortobágyi T,. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6 [J]., 2009, 323(5918): 1208-1211.
[61] Nonhoff U, Ralser M, Welzel F,. Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules [J]., 2007, 18(4): 1385-1396.
[62] Zou T, Yang X M, Pan D M,. SMN deficiency reduces cellular ability to form stress granules, sensitizing cells to stress [J]., 2011, 31(4): 541-550.
[63] Thiyagarajan N, Ferguson R, Subramanian V,. Structural and molecular insights into the mechanism of action of human angiogenin-ALS variants in neurons [J]., 2012, 3: 1121.
[64] Wolozin B. Regulated protein aggregation: Stress granules and neurodegeneration [J]., 2012, 7: 56.
[65] Warraich S T, Yang S, Nicholson G A,. TDP-43: A DNA and RNA binding protein with roles in neurodegenerative diseases [J]., 2010, 42(10): 1606-1609.
[66] 邓燕芬, 刘运海, 田田. 结合蛋白TDP-43与神经变性疾病 [J]. 中华神经科杂志, 2014, 47(3): 196-198.
[67] Ayala Y M, Zago P, D'Ambrogio A,. Structural determinants of the cellular localization and shuttling of TDP-43 [J]., 2008, 121(Pt 22): 3778-3785.
[68] Mann J R, Gleixner A M, Mauna J C,. RNA binding antagonizes neurotoxic phase transitions of TDP-43 [J]., 2019, 102(2): 321-338.
[69] Parker S J, Meyerowitz J, James J L,. Endogenous TDP-43 localized to stress granules can subsequently form protein aggregates [J]., 2012, 60(4): 415-424.
[70] Gasset-Rosa F, Lu S, Yu H Y,. Cytoplasmic TDP-43 de-mixing independent of stress granules drives inhibition of nuclear import, loss of nuclear TDP-43, and cell death [J]., 2019, 102(2): 339-357.
[71] McGurk L, Gomes E, Guo L,. Poly (ADP-ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization [J]., 2018, 71(5): 703-717.
[72] Li Y R, King O D, Shorter J,. Stress granules as crucibles of ALS pathogenesis [J]., 2013, 201(3): 361-372.
[73] Romano M, Buratti E, Romano G,. Evolutionarily conserved heterogeneous nuclear ribonucleoprotein (hnRNP) A/B proteins functionally interact with human andTAR DNA-binding protein 43 (TDP-43) [J]., 2014, 289(10): 7121-7130.
[74] 赵郴, 杜海宁. RNA结合蛋白与神经退行性疾病 [J]. 生命的化学, 2015, 35(2): 193-199.
[75] Martin S, Zekri L, Metz A,. Deficiency of G3BP1, the stress granules assembly factor, results in abnormal synaptic plasticity and calcium homeostasis in neurons [J]., 2013, 125(2): 175-184.
[76] Martin S, Tazi J. Visualization of G3BP stress granules dynamics in live primary cells [J]., 2014(87): 51197.
[77] Zekri L, Chebli K, Tourrière H,. Control of fetal growth and neonatal survival by the RasGAP-associated endoribonuclease G3BP [J]., 2005, 25(19): 8703-8716.
[78] Martin S, Bellora N, González-Vallinas J,. Preferential binding of a stable G3BP ribonucleoprotein complex to intron-retaining transcripts in mouse brain and modulation of their expression in the cerebellum [J]., 2016, 139(3): 349-368.
[79] LeBlang C J, Medalla M, Nicoletti N W,. Reduction of the RNA binding protein TIA1 exacerbates neuroinflammation in tauopathy [J]., 2020, 14: 285.
[80] de Leeuw F, Zhang T, Wauquier C,. The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor [J]., 2007, 313(20): 4130-4144.
[81] 李静辉, 张雪, 孟宇, 等. 亚低温状态下冷诱导RNA结合蛋白调节氧化还原系统对海马神经元的保护作用 [J]. 生理学报, 2015, 67(4): 386-392.
[82] Guillén-Boixet J, Kopach A, Holehouse A S,. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation [J]., 2020, 181(2): 346-361.e17.
[83] Arimoto-Matsuzaki K, Saito H, Takekawa M. TIA1 oxidation inhibits stress granule assembly and sensitizes cells to stress-induced apoptosis [J]., 2016, 7: 10252.
[84] Voelz C, Habib P, Köberlein S,. Alteration of miRNA biogenesis regulating proteins in the human microglial cell line HMC-3 after ischemic stress [J]., 2021, 58(4): 1535-1549.
[85] Kedersha N L, Gupta M, Li W,. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules [J]., 1999, 147(7): 1431-1442.
[86] Sun Y J, Dong L N, Yu S Q,. Newcastle disease virus induces stable formation of bona fide stress granules to facilitate viral replication through manipulating host protein translation [J]., 2017, 31(4): 1337-1353.
[87] 潘昕祺, 王爽, 崔亚宁, 等. 应激颗粒研究进展及相关技术 [J]. 电子显微学报, 2019, 38(4): 420-428.
[88] Buchan J R, Parker R. Eukaryotic stress granules: The ins and outs of translation [J]., 2009, 36(6): 932-941.
[89] Elbaum-Garfinkle S, Kim Y, Szczepaniak K,. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics [J]., 2015, 112(23): 7189-7194.
[90] Kato M, Han T W, Xie S H,. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels [J]., 2012, 149(4): 753-767.
[91] Buchan J R, Muhlrad D, Parker R. P bodies promote stress granule assembly in[J]., 2008, 183(3): 441-455.
[92] Grousl T, Ivanov P, Frýdlová I,. Robust heat shock induces eIF2alpha-phosphorylation-independent assembly of stress granules containing eIF3 and 40ribosomal subunits in budding yeast,[J]., 2009, 122(Pt 12): 2078-2088.
[93] Hoyle N P, Castelli L M, Campbell S G,. Stress-dependent relocalization of translationally primed mRNPs to cytoplasmic granules that are kinetically and spatially distinct from P-bodies [J]., 2007, 179(1): 65-74.
[94] Moeller B J, Cao Y T, Li C Y,. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules [J]., 2004, 5(5): 429-441.
[95] 任振兴. 左旋樟脑通过miR-140-HnrnpA1轴促进急性脑缺血应激颗粒形成的机制机制研究 [D]. 广州: 广州中医药大学, 2018.
Inspiration of neuroprotection of traditional Chinese medicine (TCM) based on stress granules
CHEN Chang1, 2, 3, Betty Yuen Kwan Law2, DUAN Fei-peng1, WANG Yu-ping2, XU Xiong-fei2, YANG Meng-chu2, JIANG Jing-zhu3, XIE Yong-yan1, Vincent Kam Wai Wong2, HUANG Li-ping1
1. School of Pharmacy, Jiangxi University of Chinese Medicine, Nanchang 330004, China 2. State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau 999078, China 3. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China
Stress granules (SG) are membrane-free dense granules present in the cytoplasm that act as cytoprotective agents by wrapping mRNA, transcription initiation factors, and RNA-binding proteins and other important components of the translation initiation phase, resulting in a transient protein translation block when cells are subjected to adverse stimuli. This cellular process is a self-protection mechanism for cells. It was found that SG were widely distributed in neurons, astrocytes, microglia, purkinje cell in the hippocampus and cortex, and the biological processes involve abnormal protein aggregation, synaptic maturation and plasticity, immune regulation, antioxidant stress response, and inhibition of apoptosis. For instance, SG participate the misfolding and abnormal accumulation of the essential protein, synaptic maturation and plasticity, it also exhibits immunoregulation, anti-oxidation and prevention of cell apoptosis in neuronal cells, so as to contribute to the neuronal function recovery. Accordingly, SG are considered as an alternative approach for treatment of stroke and neurodegenerative disease. In particular, its diversified substance composition and widely involved biological process provide a new vision for the study of TCM syndrome biology and the traditional treatments of TCM such as “different treatment of the same disease” and “holistic regulation”. Here, the principal character, neuroprotective functions and related research methods are systematically reviewed. At the same time, taking acute ischemic stroke as an example, the modern biological phenomenon of SG is tried to incorporate into the modern research of TCM, and research inspiration and ideas are put forward in order to explain the relevant theories and treatments of TCM in a scientific way and provide reference for other researchers.
stress granules; cerebral ischemia; RNA-binding proteins; G3BP1; neuroprotection
R285
A
0253 - 2670(2022)16 - 5185 - 08
10.7501/j.issn.0253-2670.2022.16.028
2022-02-18
国家自然科学基金资助项目(82060759);国家自然科学基金资助项目(81660713);澳门科技发展基金资助项目(0048/2018/A2);中国中医科学院科技创新工程项目(CI2021A00916,CI2021A04404,CI2021A04405);江西中医药大学研究生境外访学基金资助项目
陈 畅,副研究员,硕士生导师,研究方向为中药神经药理学。E-mail: cchen@icmm.ac.cn
黄丽萍,教授,博士生导师,研究方向为中药神经药理学。E-mail:jxnchlp@163.com
黄锦伟,教授,博士生导师,研究方向为中药药理学。E-mail: kawwong@must.edu.mo
[责任编辑 崔艳丽]

