Gastric mucosal precancerous lesions in Helicobacter pylori-infected pediatric patients in central China: A single-center, retrospective investigation
Miao Yu, Jing Ma, Xiao-Xia Song, Qiao-Qiao Shao, Xue-Chun Yu, Muhammad Noman Khan, Ya-Bin Qi, Ruo-Bing Hu, Pei-Ru Wei, Wei Xiao, Bai-Ling Jia, Yan-Bo Cheng, Ling-Fei Kong, Chuan-Liang Chen, Song-Ze Ding
Abstract
Key Words: Helicobacter pylori; Gastric cancer; Precancerous lesions; Inflammation; Children and adolescents
INTRODUCTION
Helicobacter pylori(H. pylori) has infected about 50% of the Chinese population and is responsible for 90%of non-cardia gastric cancer incidences. It is reported that one-third of asymptomatic or healthy children have serologically-confirmedH. pyloriinfection worldwide[1-3]. Although mostH. pylori-infected children do not have obvious clinical symptoms, studies have shown that children with gastrointestinal symptoms have a higher rate ofH. pyloriinfection, and the infection rates are affected by socioeconomic status, living habit, sanitary conditions, geographic region,etc.[4,5]. Despite the overall infection rate has slowly declined in China, the infection rate in rural areas is still much higher over that of city residents[6,7].
A recent meta-analysis in China in 2022 has indicated thatH. pyloriprevalence in children and adolescents is 28%[7]. Infection byH. pyloriis closely associated with gastric mucosal inflammation and extra-gastrointestinal diseases[8-10]. A small portion of infected patients will follow Correa’s cascade and develop severe gastric mucosal lesions, such as atrophic gastritis, intestinal metaplasia (IM), and gastric cancer[11].H. pyloriCagA has been shown to be associated with gastric mucosal precancerous lesions in several epidemiological studies[5,12].
One 2006 review summarized the prevalence of gastric mucosal precancerous lesions in children and adolescents, ranging from 0% to 72% inH. pylori-infected patients[13]. Prevalence of gastric atrophy in different populations may also vary depending on different geographic/genetic origins or environmental factors in the pediatric population. For example, one Tunisian study in 2009 found that atrophic gastritis accounted for 9.3% (32/354) of enrolled children; of the 32 children with atrophic gastritis, 30 were infected withH. pylori[14]. In 2014, a Mexican study of 82 children with chronic gastritis found that 8.5% (7/82) children had antral atrophy and 6.1% (5/82) had IM; among the 7 children with atrophy, 6 hadH. pyloriinfection[15]. Other studies carried out in different countries reported gastric atrophy was present in 4.4% to 10.7% ofH. pylori-positive children[16,17]. However, large-scale investigation is lacking in this area.
H. pylorihas the characteristics of family cluster infection and is transmissible among family members, including spouses and siblings[8,9,18]. The infected parents, especially mothers, play a major role in its transmission[19-21]. MostH. pyloriinfection is acquired during childhood and adolescents,and the infection will persist for decades unless proper treatment is offered. BecauseH. pyloriinfection usually does not cause or only causes mild gastric mucosal lesions in children and adolescents[22-24],the consequences were thought not to be as important as they were in adulthood. Hence, very few studies have focused on the relationships betweenH. pyloriinfection and gastric mucosa precancerous lesions in children and adolescents. Recent reports have begun to show that gastric mucosal atrophy and IM are also found in children and even in very young children, as mentioned above[13-15].
In this work, we retrospectively investigated a cohort ofH. pylori-infected symptomatic pediatric inpatients on gastric mucosal inflammation status, incidence and pattern of gastric mucosal precancerous lesions in a major hospital in central China, which is an area with a high prevalence ofH. pyloriand gastric cancer (H. pyloriinfection rate is 49.6%[6], and gastric cancer incidence is 42.52/100000[25]).Studies in this area will be helpful to understand the pattern and prevalence of gastric mucosal precancerous lesions in pediatric patients and provide evidence for futureH. pylorieradication recommendation and related disease prevention.
MATERIALS AND METHODS
Study population and patient enrollment
From August 1, 2018 to July 31, 2021, 4258 pediatric patients with an age less than 18-years-old who had upper gastrointestinal symptoms were admitted to the Department of Pediatrics, People’s Hospital of Zhengzhou University; among them, 1852 underwent upper gastrointestinal endoscopy examination due to disease complaints, such as abdominal pain, abdominal distension, nausea, vomiting, hiccups,heartburn,etc. Patients who had taken biopsy specimens from the gastric mucosa for histopathological examination and rapid urease test were enrolled into the study. Exclusion criteria were as follows: (1)Use of antibiotics, bismuth salts, proton pump inhibitors, H2-receptor blockers, immunosuppressants and steroids over the past 1 mo; (2) History of gastrointestinal surgery; (3) Active upper gastrointestinal bleeding; and (4) Idiopathic inflammatory bowel disease.
The study finally enrolled 1015 eligible children and adolescents. Patient information was collected only for the purpose of disease analysis and were kept confidential. As this is a retrospective study, no informed consent was required. The research protocol was approved by the Ethics Committee of People’s Hospital of Zhengzhou University (2019-KY-No. 10). The flow chart of study design and patients’ enrollment is shown in Figure 1. Patients were classified into four groups according to age: (1)1-4 years; (2) 5-8 years; (3) 9-12 years; and (4) 13-18 years.
Endoscopic and histological evaluation of patients
At least two biopsy specimens were collected from the antrum or angulus during endoscopic examination. One biopsy sample was used for immediate rapid urease test. The other was oriented,fixed in formalin and embedded in paraffin blocks. After samples were sectioned, hematoxylin and eosin staining was applied for histological analysis.H. pyloriinfection was detected by immunohistochemical staining. Due to technique limitations, all patients were not given13C-urea breath test,serologicalH. pyloriantibody test or stool antigen test to determineH. pyloriinfection status at the time of their tests. Therefore, a patient was consideredH. pylori-infected if either histological staining or rapid urease test was positive or if both were positive. Conversely, a patient was considered to beH. pyloriuninfected if either histological staining or rapid urease test was negative or if both were negative.
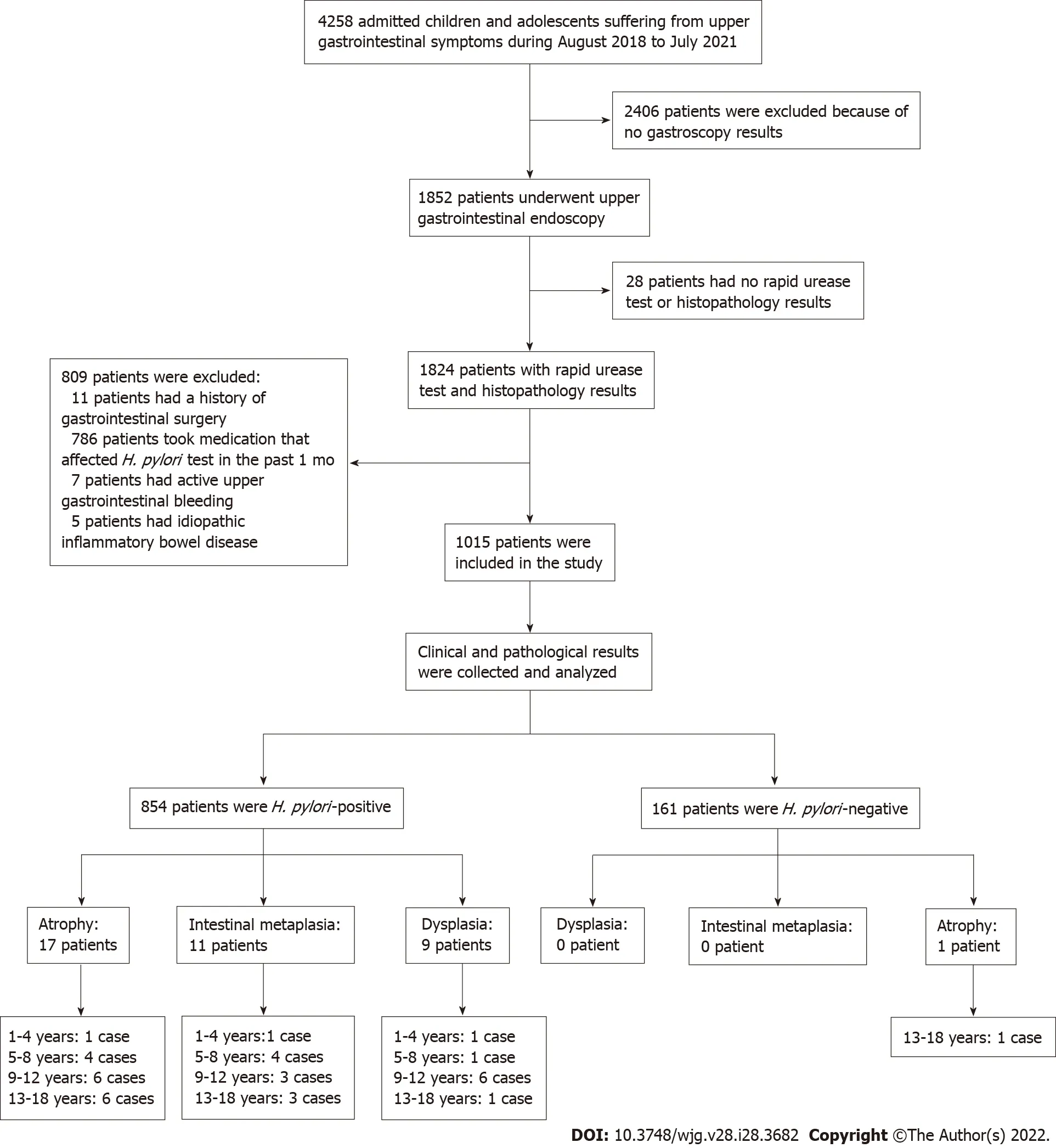
Figure 1 Flow chart of patient enrollment on gastric mucosal precancerous lesions in children and adolescents in central China. There were 4258 patients with upper gastrointestinal symptoms under 18 year of age, 2406 patients were excluded for no gastroscopy examination, and the remaining 1852 children had underwent upper gastrointestinal endoscopy. Among these patients, 809 were excluded due to either surgery, medication, bleeding or idiopathic inflammatory bowel disease, and 28 patients were excluded due to no rapid urease test or histopathology test; the final enrolled patient number was 1015. In 854 Helicobacter pylori (H. pylori)-positive patients, 17 patients had atrophy, 11 patients had intestinal metaplasia and 9 patients had dysplasia, and only 1 of the 161 H.pylori-uninfected patients had atrophic gastritis. H. pylori: Helicobacter pylori.
Histological variables (the activity of inflammation, lymphocyte and neutrophil granulocyte infiltration, glandular atrophy, IM and hyperplasia) were analyzed according to visual analog scale of the updated Sydney system[26]. Gastric mucosa changes were classified into absent, mild, moderate and severe levels according to the histological situation of each sample. Severity of lesions was determined by the most severe lesion in each patient. Atrophy of gastric mucosa was indicated by the destruction and reduction in gastric glands of the stomach. IM is a condition in which cells that create the lining of the stomach are changed or replaced by intestinal cells. In many cases, when gastric atrophy and IM coexist, they were allocated into either the atrophy or IM category based on the major pathological presentations of the specific patients. Dysplasia refers to a proliferative lesion, abnormal hyperplasia of the gastric gland basement membrane epithelium and abnormal changes in morphology and structure.These specimens were evaluated by two pathologists in a blinded fashion.
Statistical analyses
Data were analyzed using SPSS for Windows version 25 (IBM Corp., Armonk, NY, United States).Continuous variables were described as mean ± SD or median (interquartile range), while categorical variables were described as percentages or frequencies. Theχ2test or Fisher’s exact test were used to compare pathological changes of gastric mucosa, including activity of inflammation, presence of precancerous lesions and endoscopic pattern of gastric mucosa between patients withH. pyloriinfection and those withoutH. pyloriinfection. Grade data were analyzed using Wilcoxon signed-rank test, including degrees of inflammatory cell infiltration. APvalue less than 0.05 (derived from two-tailed tests) was considered statistically significant.
RESULTS
Patients’ demographic characteristics and clinical data
A total of 1852 selected patients were further examined from a pool of 4258 pediatric ward patients(Figure 1). Then, 809 patients were excluded due to surgery, medication, idiopathic inflammatory bowel disease or bleeding, and 28 more patients were excluded because of no rapid urease test or histopathological test. The remaining 1015 eligible patients were enrolled and analyzed. Patient demographic data were summarized in Table 1. Among the 1015 enrolled patients, 604 were males and 411 were females,with median age of 11-years-old, and 854 patients were infected byH. pylori, while 161 patients were not infected. The mean age ofH. pylori-infected patients was significantly older than those of non-infected subjects (11 yearsvs9 years,P< 0.05). There were no significant differences in sex distribution between theH pylori-infected and non-infected groups.
The clinical characteristics of patients are also presented in Table 1. For endoscopic manifestations,superficial gastritis was observed in 255 (29.86%) cases among theH. pylori-positive patients and 76(47.20%) cases among theH. pylori-negative patients (P< 0.05). There was no significant difference in the proportion of superficial gastritis with erosion, superficial gastritis with bile reflux and esophagitis regardless of the status ofH. pyloriinfection (P> 0.05). Antral nodularity was observed in 131 (15.34%)H. pylori-positive patients and 4 (2.48%)H. pylori-negative patients (P< 0.05). In bothH. pylori-infected and non-infected patients, most patients were clinically diagnosed with non-atrophic gastritis, and only a small percentage of patients developed atrophic gastritis or peptic ulcers.
H. pylori infection status in different age groups
In the 1-4 years, 5-8 years, 9-12 years and 13-18 years age groups (Table 2), theH. pyloriinfection rates were 40.74%, 78.39%, 88.05% and 89.60%, respectively. The trend of infection rate increasing as age increased was noted. Patient infection rates of the 5-8 years, 9-12 years and 13-18 years age groups were significantly higher than that of the 1-4 years age group. Infection rates of the 9-12 years and 13-18 years age groups were also significantly higher than that of the 5-8 years age group, but the infection rates had no significant difference between the 9-12 and 13-18 years age groups.
Gastric mucosal precancerous lesions in different age groups
Patient gastric mucosal precancerous lesions are shown in Table 3. Among the 854H. pylori-infected patients, 4.33% (37/854) had gastric mucosa precancerous lesions; among which, 17 patients had atrophy, 11 patients had IM, and 9 patients had dysplasia. Only 1 of the 161H. pylori-uninfected patients(0.62%) had atrophic gastritis. The incidence of precancerous lesions of gastric mucosa inH. pyloriinfected patients was significantly more than those uninfected patients (χ2= 5.178,P= 0.023). Among theH. pylori-positive patients, there were gastric mucosal precancerous lesions in each age group,whereas among theH. pylori-negative patients, only 1 patient with atrophic gastritis was found in the 13-18 years age group. Representative pictures of normal, gastritis, and gastric mucosal precancerous lesions are shown in Figure 2.
Active inflammation in H. pylori-infected and -uninfected patients
As described in Table 4, active inflammation was observed in 16.04% (137/854) of theH. pylori-infected patients and only 3.73% (6/161) of the -uninfected patients. A significant difference was noticed between these two groups (χ2= 16.975,P< 0.001). But in the 1-4 years age group, there was no significant difference in inflammatory activity between theH. pylori-infected and -uninfected patients(13.64%vs6.25%,χ2= 0.196,P= 0.658). Whereas in the 5-8 years, 9-12 years and 13-18 years age groups,the proportion of active inflammation in theH. pylori-positive patients was significantly higher than that in theH. pylori-negative patients (χ2= 4.901,P= 0.027;χ2= 3.987,P= 0.046;χ2= 6.012,P= 0.014).
Characteristics of inflammatory cell infiltration in H. pylori-infected and -uninfected groups
The degree of neutrophil granulocyte infiltration with differentH. pyloriinfection status is shown in Table 5. For the 854 infected patients, the proportions of absent, mild and moderate neutrophil granulocyte infiltration were 83.96%, 13.58% and 2.46%, respectively. While for the -uninfected patients,they were 96.27%, 3.73% and 0%, respectively. Compared withH. pylori-infected patients, the prevalence of neutrophil granulocyte infiltration in gastric mucosa was significantly higher than that in uninfected patients (Z= 4.319,P< 0.001). This difference betweenH. pylori-positive and -negative patients was also indicated in different age groups, with the exception of the 1-4 years age group.

Table 1 Demographic and clinical data of patients

Table 2 Helicobacter pylori infection status in different age groups
As for lymphocyte infiltration, patients withH. pyloriinfection had more severe lymphocyte infiltration than uninfected patients (Z= 3.997,P< 0.001). In theH. pylori-positive group, 40.98%, 50.59% and 8.43% of mild, moderate and marked lymphocyte infiltration, respectively, were found, while in theH.pylori-negative group, the rates were 56.52%, 40.99% and 2.48%, respectively. InH. pylori-infected patients, the 9-12 years and 13-18 years age groups also showed more severe lymphocyte infiltration compared to the uninfected patients (Z= 2.539,P= 0.011;Z= 2.164,P= 0.030), but this difference was not significant between the 1-4 years and 5-8 years age groups (Z= 0.570,P= 0.569;Z= 1.737,P= 0.082)(Table 6). Representative pictures of gastric mucosal inflammatory cell infiltration are shown in Figure 2.

Table 3 Precancerous lesions in the gastric mucosa in children and adolescents

Table 4 Comparison of active inflammation between Helicobacter pylori-positive and -negative patients

Table 5 Degree of neutrophil granulocyte infiltration in Helicobacter pylori-positive and -negative patients
DISCUSSION
In the current study, we investigated gastric mucosal inflammation and precancerous lesions in pediatric patients who were hospitalized for gastrointestinal symptoms. Among the 1015 children in our study who underwent upper gastrointestinal endoscopy, gastric mucosa precancerous lesions occurredin 4.33% ofH. pylori-infected children, in which the proportions of atrophy, IM and dysplasia were 1.99%, 1.29%, 1.05%, respectively, significantly higher than findings fromH. pylori-negative patients.

Table 6 Degree of lymphocyte infiltration in Helicobacter pylori-positive and -negative patients
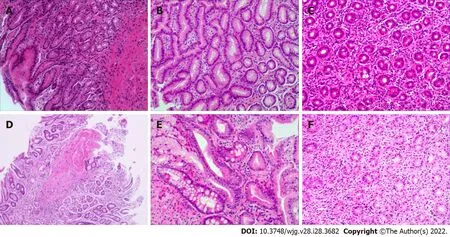
Figure 2 Representative pictures of normal and pathological gastric mucosa manifestations in children and adolescents. A: Normal gastric mucosa; B: Chronic inflammation of the gastric mucosa; C: Active inflammation of the gastric mucosa; D: Gastric mucosal atrophy; E: Intestinal metaplasia; F: Mild dysplasia. Hematoxylin and eosin, × 400.
Under endoscopy evaluation,H. pylori-positive children presented more nodular gastritis, which is an important feature ofH. pyloriinfection in children. But, the proportions of esophagitis, chronic superficial gastritis with erosions, chronic superficial gastritis with bile reflux and esophagitis were not significantly different between the infected and non-infected groups. The results also showed that children withH. pyloriinfection had a significantly higher incidence of gastric mucosal inflammation,and the degrees of neutrophil and lymphocyte infiltration were also much more serious than inH.pylori-negative children. These results are in line with previous studies that showed various degrees of inflammation inH. pylori-infected pediatric patients. For example, in 2017, Broideet al[27] showed that monocyte and neutrophil infiltration was more severe in the infected children group with chronic gastritis in Israel. One retrospective study of 196 children in Japan in 2006 also reached a similar conclusion[17].
The above results indicate thatH. pyloriinfection is closely related to gastric mucosal inflammation,which might be the basis for development of gastric precancerous lesions, as both basic experiments and clinical investigations have confirmed that certain bacterial components, such as CagA and VacA, have detrimental effects on gastric epithelial cells. In addition, CagA is a bacterial oncoprotein, which can cause epithelial cell oncogenic transformationin vivo[28-30]. Therefore, future experiments for more detailed analysis of theH. pyloricomponent on the pathogenesis of gastric precancerous lesions in children will be very helpful to understand the carcinogenesis and provide more rational for precise intervention and prevention.
The significance of this study is that it provides evidence to demonstrate thatH. pyloriinfection is associated with the occurrence of precancerous lesions in pediatric patients, and these lesions increase with increase in age. Furthermore, the pediatric population might present in a similar way to adults withH. pyloriinfection, suggesting a healthcare threat to the pediatric patients. As eradication ofH.pylorihas been shown to prevent the development of atrophic gastritis, IM and gastric malignancy in adults[31,32], it is expected that proper intervention in pediatric patients could prevent the development of future diseases. Future investigation on the rationale and health economic evaluation would be required to provide more supporting evidence.
These results are also in line with previous reports. For example, in 2006, a study of 131H. pyloriinfected children in Japan showed that the proportion of gastric antrum atrophy was 10.7%[17]. In a 2020 study in Romania, theH. pyloriinfection rate was 33.06% (82/248); among 82 infected children, 9(11%) had atrophic gastritis and 2 (2.4%) had IM[33]. A survey of 1634 children in China in 2014 found that atrophic gastritis accounted for 4.4% (23/524 cases) of theH. pylori-infected children[16]. However,one Austrian study in 2011 found gastric atrophy was rare (1/84) in children infected withH. pylori[34].In addition, no gastric atrophy was found in 66 French children infected withH. pyloriin 2009[35], and no precancerous lesions were found in 132 gastric biopsies from 22 symptomaticH. pylori-infected children in a 2009 study in Brazil[36].
AsH. pylori-induced disease presentations are mostly present in adulthood, the presence of precancerous lesions in children unveiled a critical issue that was largely neglected previously[13,15,37].Although there are still controversies on the significance and consequence ofH. pylori-induced serious gastric mucosal lesions in children[13], current consensus reports from the Asia-Pacific region[38],China[8,39] and Japan[18] have indicated thatH. pylorishould be eradicated when such conditions are present, and their family members should also receive screening and treatment if confirmed positive. It is hypothesized that gastric mucosal atrophy and IM in children and adolescents might be present in a similar way to adultH. pyloriinfection and is probably more common in areas with highH. pyloriinfection rates than previously thought.
One of the major shortfalls of this retrospective study is that the rapid urease test was the primary test used to detectH. pyloriinfection because13C-urea breath test, serologicalH. pyloriantibody tests and stool antigen tests were not available for more accurate diagnosis. This could result in the underestimation ofH. pyloriinfection rates or have false negative results in the uninfected children group. Two perplexing results might be attributed to these drawbacks as there are 8 duodenal ulcer patients(Table 1) and few patients had active gastric inflammation (Tables 4 and 5) in theH. pylori-negative group. Due to the strong relationship ofH. pyloriin causing these diseases, it is unlikely these patients were not infected byH. pylori. Furthermore, it is likely because of the limitation of the rapid urease test,which is unable to detect patchy distribution ofH. pyloriin the stomach. Therefore, future studies will be required to confirm these discrepancies. Even with these drawbacks, the overall conclusions and significance of the investigation were not affected.
Although the current work provides novel points onH. pylori-induced gastric mucosal precancerous lesions in children, due to its retrospective nature, the investigation has limitations. First, this study only discussed the relationship betweenH. pyloriinfection and gastric mucosa precancerous lesions and was unable to analyze the effects of different genotypes of infectedH. pyloristrains.Therefore, more detailed information onH. pyloristrains are not available. Nonetheless, our previous work demonstrated that type IH. pylori(CagA- and VacA-positive) is the primary type ofH. pyloriinfection in this region[40]. It is expected that the pediatric population is similar to the adult population. Second, not all cases had biopsy specimens from both the gastric antrum and body. Some of the precancerous lesions andH.pyloriinfection may have been missed due to histopathological presentation of the stomach, and patchyH. pyloriinfection status in other places of the stomach were unable to be examined. In the future,multiple biopsies might be helpful to overcome these drawbacks. Third, this was a single-center,retrospective analysis, and all the children were hospitalized due to gastrointestinal symptoms, which may be the reason why the infection rate was much higher than among the general population. In the future, large-scale investigations in the general public and multi-center, prospective and randomized control analyses are needed to comprehensively assess the risk ofH. pyloriinfection and precancerous lesions in children and adolescents in order to understand the overall precancerous lesions ofH. pyloriinfected pediatric patients.
CONCLUSION
Our results show thatH. pylori-infected children have more active inflammation and inflammatory cell infiltration in the gastric mucosa, and infection rate increases with patient age. The incidence of precancerous lesions inH. pylori-infected patients is also significantly higher compared to that in -uninfected patients. Although the percentage is only 4.33%, it provides an alarming alert and call for further investigation and intervention for this population group.
ARTICLE HIGHLIGHTS

ACKNOWLEDGEMENTS
The authors are grateful to the staff of the Department of Gastroenterology and Hepatology, People’s Hospital of Zhengzhou University for their valuable assistance in this work.
FOOTNOTES
Author contributions: Yu M, Cheng YB, Jia BL, Kong LF, Chen CL and Ding SZ designed the research; Yu M, Song
XX, Ma J, Shao QQ, Yu XC, Khan MN, Qi YB, Hu RB, Wei PR and Xiao W collected the clinical data and performed
the experiments; Yu M analyzed the data; Yu M and Ding SZ wrote the paper; Ding SZ revised the article; all authors approved the final version of the manuscript.
Supported by the National Natural Science Foundation of China, No. U1604174; Henan Provincial Government-
Health and Family Planning Commission, No. 20170123 and No. SBGJ202002004; and Henan Provincial Government-Health and Family Planning Commission Research Innovative Talents Project, No. 51282.
Institutional review board statement:This study was reviewed and approved by the Ethics Committee of People’s Hospital of Zhengzhou University (2019-KY-No. 10).
Informed consent statement:The study was performed retrospectively. Patients were not required to give informed consent for the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:All authors declare that there are no conflicts of interests related to this work.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Miao Yu 0000-0002-3587-2760; Jing Ma 0000-0003-1172-527X; Xiao-Xia Song 0000-0003-4633-9862; Qiao-Qiao Shao 0000-0002-4900-9394; Xue-Chun Yu 0000-0003-4425-6019; Muhammad Noman Khan 0000-0003-4814-7768; Ya-Bin Qi 0000-0002-7736-4043; Ruo-Bing Hu 0000-0003-4735-1310; Pei-Ru Wei 0000-0001-6306-590X; Wei Xiao 0000-0002-6814-9261; Bai-Ling Jia 0000-0002-2920-2322; Yan-Bo Cheng 0000-0003-3203-4603; Ling-Fei Kong 0000-0003-1710-8344;Chuan-Liang Chen 0000-0002-5566-7297; Song-Ze Ding 0000-0002-4589-6942.
S-Editor:Yan JP
L-Editor:A
P-Editor:Yan JP
 World Journal of Gastroenterology2022年28期
World Journal of Gastroenterology2022年28期
- World Journal of Gastroenterology的其它文章
- Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma:Experimental and clinical scenarios
- Gut microbiota alteration and modulation in hepatitis B virus-related fibrosis and complications:Molecular mechanisms and therapeutic inventions
- Combination approaches in hepatocellular carcinoma: How systemic treatment can benefit candidates to locoregional modalities
- Update on endoscopic ultrasound-guided liver biopsy
- Non-alcoholic fatty liver disease-related hepatocellular carcinoma: Is there a role for immunotherapy?
- Potassium-competitive acid blockers and gastroesophageal reflux disease
