Combination approaches in hepatocellular carcinoma: How systemic treatment can benefit candidates to locoregional modalities
Leonardo Gomes da Fonseca,Raphael L C Araujo
Abstract The management of hepatocellular carcinoma (HCC) is challenging because most patients have underlying cirrhosis, and the treatment provides, historically, a limited impact on the natural history of patients with advanced-stage disease.Additionally, recurrence rates are high for those patients who receive local and locoregional modalities, such as surgical (resection and transplantation) or imageguided (ablation and intra-arterial) therapies. Translational research has led to new concepts that are reshaping the current clinical practice. Substantial advancements were achieved in the understanding of the hallmarks that drive hepatocarcinogenesis. This has primed a successful incorporation of novel agents with different targets, such as anti-angiogenic drugs, targeted-therapies, and immune-checkpoint inhibitors. Although clinical trials have proven efficacy of systemic agents in advanced stage disease, there is no conclusive evidence to support their use in combination with loco-regional therapy. While novel local modalities are being incorporated (e.g., radioembolization, microwave ablation,and irreversible electroporation), emerging data indicate that locoregional treatments may induce tumor microenvironment changes, such as hyperexpression of growth factors, release of tumor antigens, infiltration of cytotoxic lymphocytes, and modulation of adaptative and innate immune response. Past trials that evaluated the use of antiangiogenic drugs in the adjuvant setting after ablation or chemoembolization fail to demonstrate a substantial improvement.Current efforts are directed to investigate the role of immunotherapy-based regimens in this context. The present review aims to describe the current landscape of systemic and locoregional treatments for HCC, present evidence to support combination approaches, and address future perspectives.
Key Words: Liver cancer; Hepatocellular carcinoma; Immunotherapy; Systemic therapy; Ablation;Embolization
INTRODUCTION
Hepatocellular carcinoma (HCC) is a highly fatal disease, representing the fourth cause of cancer-related mortality worldwide[1]. This situation is attributed to the challenging management of patients with HCCper sein concomitance with an underlying liver disease and cirrhosis-related complications.Moreover, a significant proportion of patients are diagnosed with advanced stage disease, not amenable to curative options. Finally, even patients who are treated with local therapies, such as surgery and ablation, present high rates of recurrence, reaching up to 70% in 5 years[2].
Treatment options for HCC are selected based on liver function, performance status, and tumor burden (size, number of lesions, metastatic spread, and vascular invasion). These patient- and tumorcentered characteristics define the stage of the cancer, each with a different prognosis[3]. Thus, clinical practice guidelines according to both tumor presentation and liver function integrate the available evidence based on well-delineated clinical trials and recommend treatment strategies for each stage[2,4](Figure 1).
Liver resection, local ablation, and liver transplantation are recommended for very-early and earlystage disease and are considered curative-intent treatments for HCC. Intra-arterial therapies, such as transarterial chemoembolization (TACE), are indicated for cases classified as intermediate stage(multinodular liver disease beyond Milan-criteria with preserved liver function), and systemic treatments are used for patients with advanced stage disease[3]. Therapeutic management of HCC is evolving rapidly with the incorporation of novel locoregional techniques and the increasing number of systemic agents tested in clinical trials, including immunotherapy. Each treatment approach is directed to treat HCC at different stages, and there is a lack of evidence to support the use of combined systemic and locoregional treatments. Therefore, the biological rational behind combination strategies and the urgent need to improve outcomes for this lethal disease prime intense research activity in both basic and clinical fields. This review aims to address the current landscape of systemic and locoregional treatments and the future perspectives regarding combined approaches.
SYSTEMIC TREATMENT FOR HCC: CURRENT LANDSCAPE AND PERSPECTIVES
Systemic treatment is recommended for patients with advanced stage disease (preserved liver function,performance status 0-2, metastatic spread, and/or macrovascular invasion) and for patients in earlier stages who have contra-indications or progressed after locoregional modalities according to the concept of treatment stage migration[3].
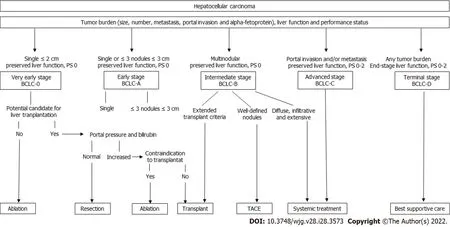
Figure 1 Treatment algorithm for hepatocellular carcinoma based on level-1 evidence. The algorithm establishes five stages according to liver function, tumor burden, and performance status. Each stage is linked to first-line treatment recommendation, although individual decisions can be defined according to patient profile and available treatment options. Adapted from Ref. [3]. BCLC: Barcelona clinic liver cancer; TACE: Transarterial chemoembolization.
The natural history of advanced HCC is poor, with a median overall survival of 4-8 mo without active treatments[5]. Since 2008, the use of agents that target hallmarks of hepatocarcinogenesis are gradually improving prognosis and achieving substantial clinical benefit for HCC patients. Sorafenib, a multikinase inhibitor, has been shown to improve overall survival over placebo in the SHARP and Asia-Pacific trials and became the first approved drug for advanced HCC[6,7]. After almost a decade, other drugs of the same class were tested with positive results in phase III trials, such as lenvatinib (noninferior to sorafenib in first-line)[8], regorafenib (superior over placebo in second-line for patients who tolerated sorafenib)[9], and cabozantinib (superior over placebo after sorafenib failure)[10]. These drugs share common targets, such as vascular endothelial growth factor receptor (VEGFR) and plateletderived growth factor receptor, while some of them present, individually, more direct actions against specific targets, such as the case of lenvatinib against fibroblast growth factor receptor, and cabozantinib against mesenchymal-epithelial transition (MET) receptor. Additionally, ramucirumab, a monoclonal antibody against VEGFR, was shown to improve overall survival over placebo in patients who present with alpha-fetoprotein (AFP) ≥ 400 ng/mL after sorafenib failure and is also approved for clinical use[11]. Generally, these drugs present antiangiogenic activity and promote clinical benefit by delaying tumor progression rather than inducing a substantial reduction in tumor burden. Trials with these drugs are consistent in showing a statically significant, but modest, increase in overall survival, with survivals around 11-13 mo in the first line, a response rate of less than 10% (except for lenvatinib, with a response rate of 18.8% in the REFLECT trial), and a range of class-related adverse events, such as fatigue, arterial hypertension, skin reaction, and diarrhea[12].
In the past years, the advent of immune-based treatments, such as immune checkpoint inhibitors,brought huge advances in HCC management. The biological background for the use of immunotherapy in HCC is based on the remarkable immunotolerance of the liver due to the high antigenic load derived from the enteral-portal circulation. Additionally, HCC develops in a microenvironment of chronic inflammation and underlying liver disease. Low infiltration of CD8 + T lymphocytes, responsible for the antitumor immune response, and a marked presence of exhausted lymphocytes and regulatory T lymphocytes are described in HCC, contributing to an immunosuppressive and procarcinogenic microenvironment[13,14]
The immune checkpoint inhibitors aimed at boosting anti-tumoral immunity in the priming phase by the recognition of antigens presented by dendritic cells to lymphocytes (anti-CTLA4 agents) and in the effector phase of T-CD8 cytotoxic cells against tumor cells (anti-PD1, anti-PDL1) were tested in phase I/II trials showing durable response rates of around 15%-20%. Therefore, pembrolizumab and nivolumab (anti-PD1 drugs) received approval for use in second-line treatments[15,16]. However,comparative trials showed that immune checkpoint inhibitors as monotherapy did not improve outcomes comparing to the available treatments. In a phase III trial, nivolumab was not proven to improve survival compared to sorafenib in the first line[17] and pembrolizumab did not achieve statistical superiority over placebo in the second-line[18].
The use of combinations of agents with different mechanisms of action can improve results of immunotherapy through additive effect and because VEGF can enhance the immunosuppression of the tumor microenvironment by inhibiting the function of effector T cells, increasing the recruitment of regulatory T cells and myeloid-derived suppressor cells[19]. This combination was explored in the IMBRAVE150 trial, which randomized patients to receive sorafenibvsa combination of bevacizumab(an anti-VEGF antibody) and atezolizumab (an anti-PD-L1 antibody). This trial demonstrated a significant improvement in overall survival (19.2 movs13.4 mo), and also in progression-free survival and response rate[20,21]. IMBRAVE150 trial was a landmark in HCC management and marked the transition towards the use of combined systemic therapies (using dual-immunotherapy or antiangiogenic plus immunotherapy combinations). Several combinations showed encouraging results in phases I and II trials, such as lenvatinib plus pembrolizumab[22], pembrolizumab plus regorafenib[23],nivolumab plus ipilimumab[24],and nivolumab, ipilimumab, and cabozantinib[25]. These preliminary results suggested that combinations may yield response rates of more than 20% according to RECIST criteria, which seems to compare favorably to the rates with multikinase inhibitors. However, it is important to point out some limitations of early phase trials. Firstly, non-comparative trials are not adequate to draw definitive conclusions. Besides, the response rate is not a surrogate marker for survival benefit in HCC, even more considering that RECIST may not capture the spectrum and patterns of progression and response in patients under immunotherapy.
Recently, durvalumab and tremelimumab as a combination treatment was announced to yield survival benefit over sorafenib in a phase III trial and is expected to be incorporated in the first-line setting as an alternative to atezolizumab plus bevacizumab[26,27]. Currently, other phase III trials are awaiting results in the context of combination therapies for advanced HCC (Table 1).
ROLE OF LOCOREGIONAL THERAPIES IN HCC: IS THERE ROOM FOR IMPROVING OUTCOMES?
Liver resection is indicated in early HCC, regardless of the presence of cirrhosis, since the liver function remains compensated, and is rarely is does the patient have a clinically significant portal hypertension.However, the risk of liver decompensation after resection in patients with restricted hepatic functional reserve is a concern, especially in cases requiring major hepatectomies (3 or more segments)[2,28,29]. On the other side, liver transplantation has the advantage of treating both cirrhosis and offer curative-intent treatment for HCC. Thus, liver transplantation decreases both the risk of recurrence, since the most recurrence is in the liver, andde novolesions by removing the cirrhotic liver. But its application in clinical practice is hampered by organ shortage, complexity, heterogenous availability in different worldwide regions, and the risk of progression on waiting list[30].
In patients with liver-only disease not amenable to surgical modalities, locoregional interventional procedures play a key role. Locoregional treatment is defined as imaging-guided tumor directed procedures, and it is estimated that 50% of patients with HCC might receive any of these treatments during the course of the disease[31,32]. Basically, there are two groups of locoregional therapies: (1)Ablative therapies; or (2) Intra-arterial therapies. Although both modalities are consolidated with high level evidence, emerging approaches and techniques are being increasingly adopted and are expected to improve clinical outcomes. Besides, the risk of recurrence and progression in patients treated with locoregional therapies has led to intense research activity towards combination with systemic treatment[2,4].
ABLATIVE THERAPIES
Current evidence of ablative methods in HCC
Ablative therapies induce tumor destruction through different mechanisms according to the method:Chemical [percutaneous ethanol injection (PEI)], thermal [radiofrequency ablation (RFA), microwave(MWA), and cryoablation], and short pulses of high voltage (irreversible electroporation). In general,ablative techniques are considered a curative therapy for HCC < 2-3 cm and is associated with complete responses in 70%-90% of the cases, although recurrences occur in approximately 50% of the cases within 5 years[33-35].
The most used and recommended method is RFA, which has shown survival superiority over PEI in randomized trials[33]. Tumor size, number of nodules, and Child-Pugh class are associated with prognosis in patients treated with RFA[36-38]. Comparative studiesvsresection demonstrated that RFA seems to be inferior to surgery in tumors > 3 cm[39]. Limitations to RFA include proximity to large vessels (due to heat effect), size, and location.
MWA is another technique that has been increasingly used and has the advantage of achieving a faster heating over a larger volume and being less susceptible to heat sink effect. Most studies that addressed comparisons between RFA and MWA showed similar efficacy, with a trend toward best results with MWA in tumors > 3 cm[40-42].
Alternative techniques, such as IRE and cryoablation, are under active research, with a small series of studies showing encouraging results[43,44]. IRE has the advantage of causing less thermal damage to adjacent tissues. However, more data are still required for IRE and cryoablation in order to fully incorporate these techniques into clinical practice guidelines.
Combination of ablative and systemic treatment: rationale and current evidence
The high risk of local and distant recurrence after ablation indicates the need for adjuvant strategies to improve cure rates. Features, such as large tumors, multinodularity, and vascular invasion (macroscopicor microscopic), are significantly related to higher recurrence rates. Novel markers, such as genetic signatures, circulating microRNA, circulating non-coding RNA, circulating cell-free DNA, gut microbiota, and circulating tumor cells, have also been shown to predict the risk of recurrence[45-47]

Table 1 Clinical trials in advanced hepatocellular carcinoma with combination of systemic treatment
To evaluate the impact of multikinase inhibitors with antiangiogenic activity combined with ablation,the STORM trial randomized patients treated with resection (n= 900) or ablation (n= 214) to sorafenib or placebo for a total of 4 years. Unfortunately, there was no difference in recurrence-free survival between groups, with a median to time to recurrence of 33.3 mo in the sorafenib group and 33.7 mo for the placebo group (HR = 0.94,P= 0.26)[48]. Thus, the positive results obtained with sorafenib in advanced disease have not been reproduced in the context of adjuvant post-resection or ablation. Other drugs used in the advanced stage, such as cabozantinib, regorafenib, ramucirumab, and lenvatinib have not been evaluated in patients after ablation. In sum, VEGFR-directed therapies as monotherapy do not seem to improve the results of ablation.
Recently, the immune features of localized HCC and the response to local treatment are being investigated[49-51]. The concentration of intratumor CD3 + and CD8 + T cells and the expression of programmed death ligand 1 (PD-L1) by immune and tumor cells appear to be associated with HCC aggressiveness and risk of recurrence after curative treatments, such as ablation and surgery[13]. The high expression of PD1 can lead a condition of exhausted CD8 + impairing cytotoxicity and decreasing of pro-inflammatory cytokines production and anti-tumor ability[13].
Ablation can induce stimulate inflammatory and cytokine production within the treated site. The tumor debris released upon ablation represent a tumor antigen repertoire that can boost antitumoral immunologic response. Indeed, tumor antigens can be found in dendritic cells in lymph nodes following ablation[52]. This background indicates a potential role for immune checkpoint inhibitors directed to PD1 and PD-L1 in combination with ablative therapies (Figure 2). A phase I trial including 32 patients treated with tremelimumab and ablation showed accumulation of CD8+ cells, suggesting immune system activation[53]. Preliminary results of the NIVOLVE trial were presented in 2021. In this phase II single arm trial, 55 patients after surgery (n= 33) or ablation (n= 22) received nivolumab for 12 mo. The median recurrence-free survival was 26 mo. In this trial, the authors suggested that patients with tumor-infiltrating lymphocytes, positive PD-L1, and negative staining for beta-catenin tend to develop fewer recurrences[54]; however, larger and comparative studies are needed to confirm these findings. Currently, several active trials (recruiting or waiting results) are addressing the role of immune-checkpoint inhibitors in combination with anti-VEGFR agents or alone after curative treatments, such as ablation (Table 2).
INTRA-ARTERIAL THERAPIES
Current evidence of intra-arterial therapy in HCC
Intra-arterial therapies harness the arterial supply of HCC that differs from the liver parenchyma, which receives its blood supply predominantly from the portal vein. This group of therapies are composed of transarterial embolization (TAE), chemoembolization (TACE), and selective internal radiotherapy(SIRT) or radioembolization (TARE).
The mechanism of action of TAE is based on the obstruction of the blood supply causing ischemia,while TACE adds a cytotoxic agent (most commonly doxorubicin) and a carrier agent (commonly lipiodol). Drug-eluting bead (DEB)-TACE uses microspheres carrying cytotoxic agents which are associated with a sustained drug release and, theoretically, a lower systemic exposure to chemotherapy[55].

Table 2 Clinical trials approaching combination of systemic treatment and ablation enrolling, recruiting, or waiting for final results

Figure 2 Mechanisms of tumor microenvironment changes after locoregional therapies. After local interventions, tumor immune microenvironment may change with a pronounced release of tumor antigens, cytokines, lymphocyte infiltration, and hyperexpression of vascular endothelial growth factor. TACE:Transarterial chemoembolization; PD-L1: Programmed death ligand 1; VEGF: Vascular endothelial growth factor.
Based on randomized clinical trials of TACEvsno active treatment and also in a large meta-analysis,this is considered the standard of care for patients with intermediate-stage [Barcelona clinic liver cancer(BCLC)-B: Multinodular disease, preserved liver function, and performance status of 0][56,57]. In published cohorts with selected patients, the median overall survival with TACE reaches 48 mo[58].There is no definitive evidence for the superiority of TACE over TAE, although data from meta-analysis suggests that the former is associated with better outcomes[57]. A prospective trial demonstrated that DEB-TACE presents lower treatment-related toxicities and a trend towards a better objective response rate compared to conventional TACE[59].
Guidelines recommend that patients who present progression with infiltrative tumors, vascular invasion, metastatic spread, or absence of a significant response after two TACEs should be considered for systemic treatment[2,4]. The risk of liver deterioration should be carefully monitored after TACE due to the prognostic impact of liver decompensation in patients with HCC[60]. The decision to declare TACE failure and switch to systemic treatment is heterogenous in different parts of the world, and many scores have been proposed to help in this decision, although some of them still require further validation in a population treated strictly according to the BCLC algorithm[61-65]. For example, the hepatoma-arterial embolization (HAP) score includes albumin, AFP, bilirubin and tumor diameter and was concepted to predict prognosis before the first TACE in a heterogeneous cohort with around 30% of BCLC-C patients[64]. The Assessment for Retreatment with TACE (ART) score was designed to evaluate benefit of a 2ndTACE and assess Child-Pugh score, AST levels, and tumor response after the first TACE.The ART score was originally developed in a cohort that included around 20% of patients with impaired liver function (Child-Pugh B8 or more)[66].
With the availability of more effective systemic agents, the migration from TACE to systemic treatment is a key decision in the management of HCC and should not be delayed at a point of irreversible deterioration of liver function or performance status.
TARE has been increasingly studied in HCC and has become a common practice in many centers.Data comparing TACE to TARE demonstrated in patients with early or intermediate stage a longer time to progression favoring TARE, but no survival benefit[67]. Although encouraging, there is no solid evidence that TARE should became standard of care in patients eligible to intra-arterial therapies.
A recent single-arm retrospective study including patients with solitary HCC ≤ 8 cm reported objective response rates of 88.3% and a 3-years survival of 86.6%. In this study TARE served as a neoadjuvant therapy for transplantation or resection in 21% and 6.8% of patients, respectively[68].
In uncontrolled trials, TARE was suggested to be safe in patients with portal vein invasion[69].However, randomized trials in the setting of advanced stage HCC did not prove superiority of TARE over sorafenib in overall survival[70,71]. Therefore, TARE is not recommended as a standard therapy in patients classified as BCLC-C. There is a need to better explore the role of TARE in HCC in prospective and randomized trials, especially for subgroups of patients requiring downstaging, segmental portal vein thrombosis, and using rigorous dosimetry.
Combination of intra-arterial and systemic treatment: rationale and current evidence
Although TACE is the standard treatment for intermediate stage, only around 50% of patients present objective response rate and most of them will eventually present distant and local progression[72]. This has led to studies focused on improving the outcomes for HCC patients treated with TACE by combining multikinase inhibitors.
The rational for testing these combinations comes from the demonstration of increased intratumorally micro vessel density and VEGF expression in residual tumors after TACE, suggesting that TACE may stimulate tumor angiogenesis[73]. However, antiangiogenic drugs, such as sorafenib[74,75], brivanib[76], and bevacizumab[77], failed to demonstrated survival benefit in combination with TACE in controlled trials.
Recently, TACTIS trials randomized patients to TACE alone or combined with sorafenib and showed that sorafenib improved progression-free survival (25.2 movs13.5 mo;P= 0.006), but with no significant impact on overall survival (36.2 movs30.8 mo;P= 0.40)[78,79]. Currently, available evidence fails to encourage the use of multikinase inhibitors in combination with TACE as a standard practice,but there is a growing enthusiasm with the use of immunotherapy in this setting.
Similar to ablative treatments, intra-arterial therapies may modulate innate and adaptative immunity by releasing cellular debris and inflammatory cytokines and inducing T-cell responses, what is suggested to be related to improved prognosis[80,81] (Figure 2). A recent study compared HCC tissue from patients who were previously submitted to TACE to patients not treated with TACE and found that the former had a lower density of immune-exhausted effector cytotoxic and T-regs with an upregulation of pro-inflammatory pathways[82]. Considering this background, strategies aimed at blocking negative regulators such as PD-1 and CTLA4 are promising and are being investigated in phase III clinical trials (Table 3).
TARE also seems to modulate immunological microenvironment in HCC. A study that compared findings before and after TARE showed that tumor-infiltrating lymphocytes after TARE exhibited signs of immune activation, such as higher expression of granzyme-B and infiltration of CD8+Tcells, NK cells,and antigen-presenting cells[83,84]. Initial data on the combination of nivolumab and SIRT showed favorable safety profile, but efficacy data are pending[85,86].
Besides the biological background, the use of systemic treatment in earlier stages (combined with locoregional treatment) may benefit those patients who would present clinical deterioration or liver function worsening during TACE-refractoriness and would not found fit to receive further systemic treatment in that situation.
CONCLUSION
The treatment landscape of HCC management evolved rapidly in the past years. The most relevant achievements took place in the management of advanced stage disease. The median overall survival in pivotal clinical trials shifted from 5-8 mo in placebo arms[6], to almost 20 mo with immunotherapy combinations[21]. Still, some questions remain to be answer regarding the identification of predictive biomarkers and the optimal sequence in first-, second-, and later lines[87-89].
On the other, the field evolved modestly in the setting of local and locoregional stages, comprising early and intermediate stages. Patients in the early stage are offered surgical resection, liver transplantation, or ablation. Due to organ shortage and strict criteria to transplant, surgery and ablation has been widely used. Unfortunately, their recurrence rates are high and determine results in long-term mortality[33-35]. Although attempts were made, no systemic treatment has improved outcomes in early stage. Robust evidence supports those immunological changes occur after tumor ablation and the use immunotherapy is encouraging.
Patients in the intermediate stage have historically been offered TACE with the aim to achieve tumor control. Similarly, most of them will eventually present local or distant progression and will beconsidered for systemic treatment. The concomitant use of immunotherapy-based systemic agents and intra-arterial therapies may enhance anti-tumor immune response and impact positively on the outcomes. Several clinical trials are eagerly awaited.

Table 3 Clinical trials approaching combination of systemic treatment and intra-arterial actually enrolling, recruiting or waiting for finalresults
The most promising ongoing trials approach the combination of TACE and immunotherapy, with the aim to significantly delay time-to-progression and, hopefully, increase cure rates for patients who achieve complete response with locoregional treatment. Similarly, trials that test adjuvant immunotherapy after ablation may increase cure rates by reducing the risk of recurrence and -de novotumors. It is likely that biomarkers and subsets of clinical features will emerge from these trials and will support the selection of patients that will derive more benefit from combination approaches.
Finally, it is key to consider that if active trials succeed in showing a better anti-tumor efficacy with combination of locoregional and systemic treatment, a longer survival of patients with HCC will be expected. Furthermore, this will highlight the burden of cirrhosis-related complications as a competing risk of mortality and the importance of multidisciplinary teams as the standard approach for HCC.
FOOTNOTES
Author contributions:Both authors performed the writing and prepared the figures and tables; Both authors designed the outline and coordinated the writing of the paper.
Conflict-of-interest statement:All authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Noncommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Brazil
ORCID number:Leonardo Gomes da Fonseca 0000-0002-0216-3618; Raphael L C Araujo 0000-0002-7834-5944.
S-Editor:Ma YJ
L-Editor:Filipodia
P-Editor:Ma YJ
 World Journal of Gastroenterology2022年28期
World Journal of Gastroenterology2022年28期
- World Journal of Gastroenterology的其它文章
- Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma:Experimental and clinical scenarios
- Gut microbiota alteration and modulation in hepatitis B virus-related fibrosis and complications:Molecular mechanisms and therapeutic inventions
- Update on endoscopic ultrasound-guided liver biopsy
- Non-alcoholic fatty liver disease-related hepatocellular carcinoma: Is there a role for immunotherapy?
- Potassium-competitive acid blockers and gastroesophageal reflux disease
- Making the case for multidisciplinary pediatric aerodigestive programs
