Potassium-competitive acid blockers and gastroesophageal reflux disease
Wattana Leowattana,Tawithep Leowattana
Abstract Proton pump inhibitors (PPIs), the most commonly used antisecretory medications in the management of reflux illness, virtually eliminate elective surgery for ulcer disease, and relegate anti-reflux surgery to patients with gastroesophageal reflux disease (GERD) who are inadequately managed by medical therapy.However, PPI medications still leave some therapeutic demands of GERD unmet.Furthermore, up to 40%-55% of daily PPI users have chronic symptoms, due to PPI refractoriness. Potassium-competitive acid blockers (P-CABs) transcend many of the problems and limits of PPIs, delivering quick, powerful, and extended acid suppression and allowing for treatment of numerous unmet needs. Recently, it has become clear that compromised mucosal integrity plays a role in the etiology of GERD. As a result, esophageal mucosal protection has emerged as a novel and potential treatment approach. An increasing body of research demonstrates that when P-CABs are used as primary drugs or add-on drugs (to regular treatment),they provide a considerable extra benefit, particularly in alleviating symptoms that do not respond to PPI therapy.
Key Words: Potassium-competitive acid blocker; Gastroesophageal reflux disease; Proton pump inhibitors; Treatment outcome; Proton pump inhibitor-refractory patients;Esophageal mucosal resistance
INTRODUCTION
Gastroesophageal reflux, the reflux of stomach contents into the esophagus, usually occurs after large and fatty meals. Nonetheless, under normal settings, efficient esophageal cleaning mechanisms return the bulk of the refluxed material to the stomach, and symptoms do not occur. However, when stomach reflux is severe or physically aggressive, it causes symptoms and difficulties, as well as a decrease in quality of life, thus creating gastroesophageal reflux disease (GERD)[1,2]. Since the 1990s, the prevalence of GERD has grown consistently, particularly in East Asia and North America (2.5%-7.8%, and 10%-20%, respectively)[3-5]. Heartburn and regurgitation are common esophageal symptoms, accompanied by consequent chest discomfort and dysphagia. Chronic cough, hoarseness of voice, and asthma are other extra-esophageal symptoms that have been linked to GERD in population-based research. The three GERD phenotypes are erosive esophagitis (EE), non-erosive reflux disease (NERD), and Barrett's esophagitis. Their responses to therapy vary greatly. Of note, the majority (70%) of GERD patients have an endoscopically normal mucosa and are regarded as having NERD.
Pharmacologic, endoscopic, and surgical options for GERD treatment are now available. The most often recommended drugs for GERD treatment in clinical practice are proton pump inhibitors (PPIs)and histamine-2 receptor antagonists. Other, less potent agents typically used to treat mild or intermittent symptoms or as adjunct therapies include antacids, sucralfate, baclofen, alginate, and prokinetics[6,7]. The goals of GERD management include symptom relief, esophageal inflammation healing, esophageal inflammation maintenance and prevention, and quality of life enhancement. PPIs have long been thought to be the basis of GERD treatment. Because of their significant and consistent antisecretory action, PPIs have proven particularly useful in treating EE, managing symptoms, and preventing GERD-related complications such as esophageal ulcers, esophageal bleeding, and peptic stricture. Overall, PPIs have been regarded as a relatively safe class of drugs, with many patients taking them for an extended time. Despite the success of PPIs in addressing many aspects of GERD, there are still many unmet needs[8-10]. Advanced EE, NERD, overnight heartburn, maintenance therapy, and refractory GERD are among them.
Furthermore, PPIs are ineffective for postprandial heartburn and are currently not recommended for atypical or extra-esophageal GERD presentations as well as GERD complications[11,12]. Importantly,prolonged PPI medications have been linked to side effects, generating worries among physicians and patients alike about their safety profile. This review looks at the development and quality of present and potential acid suppression medications, focusing on the potassium-competitive acid blocker (P-CAB)class and exploring their clinical studies.
ROLE OF POTASSIUM ION IN ACID SECRETION
Gastric acid is essential for food and water purification and digestion. Gastric juice has an extremely low pH (pH = 1) because parietal cells in the oxyntic mucosa of the stomach secrete H+and Cl-ions to make hydrochloric acid. These cells secrete 1-2 L of hydrochloric acid every day. The action of gastric H+/K+-ATPase in the apical membrane of parietal cells promotes a very high concentration of H+in the lumen compared to plasma. The potassium ion plays an essential role in activating gastric H+/K+-ATPase and is required for the enzyme to function. At rest, H+/K+-ATPase is confined to tubulovesicular regions of a parietal cell with low K+concentrations and membranes that are impermeable to K+. As a result, the enzyme is incapable of activating and transporting H+ions. When the parietal cell is stimulated, the tubulovesicular components merge with the cell’s apical membrane. After being exposed to K+-containing luminal fluid, the H+/K+-ATPase enzyme can begin to exchange H+for K+[13-15]. Due to its important involvement in the production of gastric juice, K+is a possible therapeutic target for acid blockers. One technique is to block the K+channels that allow ions to flow through the apical membrane of parietal cells, while another is to compete with K+at the level of the parietal cells' H+/K+-ATPase.
P-CABs
The surface of the stomach H+/K+-ATPase (the proton pump) is exposed to the extremely acidic parietal cell canaliculus, which has a high affinity for K+during acid secretion. Given the significance of the cation for enzyme activity, drugs that compete for K+binding have the potential to inhibit acid secretion.The mechanism of action of P-CAB is based on this premise. P-CABs were initially developed in the 1980s and have been studied by many pharmaceutical companies worldwide due to their ability to block the proton pump quickly, efficiently, and reversibly. Schering-Plough was one of those companies that developed a prototype P-CAB, SCH28080. Although the mechanism of action was not fully known at that time, this drug was discovered to lower stomach acid output in humans[16,17]. SCH28080 was later demonstrated to inhibit gastric H+/K+-ATPase by competing with K+[18,19]. However, clinical development of SCH28080 was halted due to hepatotoxicity produced by repeated treatment. This condition sparked research into several kinds of SCH28080 derivatives, such as imidazopyridine derivatives (BY841), imidazo-naphthyridine derivatives (soraprazan), imidazo-thienopyridines (SPI-447), quinolone derivatives (SK&F96067)[20-23], pyrrolo-pyridazine derivatives (CS-526), pyrimidine derivatives (revaprazan), and pyrrole derivatives [vonoprazanm (VPZ)][24-26].
These novel antisecretory medicines vary from PPIs in that they compete with K+and cause a dosedependent selective and reversible inhibition of the proton pump. Because they aren't prodrugs that need to be activated in parietal cells like PPIs, they start working right away, and control of acid secretion begins within the first day of treatment after starting the first dose. In addition, their dissociation from the proton pump is sluggish, and they can stay in the stomach mucosa for up to 24 h.As a result, unlike PPIs, which are less effective at night, their acid inhibitory action persists throughout the day and night[27,28]. Table 1 compares the key differences in the mechanisms of action of P-CABs and PPIs.
ACTION MECHANISM OF P-CABs
P-CABs are classified into many chemical classes (Figure 1). They are a varied class of medications while having a similar mechanism of action. P-CABs are lipophilic, weak bases with limited pH stability and high pKa values. They may concentrate in acidic environments because of the combination of these properties. In the parietal cell canaliculi (pH = 1), a P-CAB with pKa of 6.0 would be 100000-fold larger than in the plasma (pH = 7.4).In vitroandin vivoinvestigations using AZD0865 and revaprazan showed a concentrated P-CAB in the gastric mucosa[29-31]. By binding ionically to the enzyme, P-CAB inhibits gastric H+/K+-ATPase and prevents further activation by K+. P-CABs are expected to bind at or near the K+binding site, preventing the K+from accessing the site (Figure 2). Although these novel drugs exhibit quick and potent antisecretory action, not all of their attractive pharmacodynamic features have translated into therapeutic advantages due to their hepatotoxicity and insufficient efficacy. In addition,linaprazan (AZD0865) failed to outperform standard-dose PPIs in the treatment of peptic ulcers and reflux esophagitis (RE)[32,33]. Soraprazan (BY359) and CS526 (R105366) have fulfilled their proof of effectiveness and safety goals in phase II tests, but data on these medicines have not been published[34].
PHARMACOKINETICS AND PHARMACODYNAMICS OF P-CABs
P-CABs reach peak plasma concentrations quickly after oral administration, in part because they are acid-stable and may be administered as immediate-release formulations. In healthy men, a single dose of revaprazan (100 to 200 mg) resulted in peak plasma concentrations at 1.7 to 1.8 h, which declined to a mean elimination time (T1/2) of 2.2 h to 2.4 h with repeated administration. The pharmacokinetics and concentration-time profiles of revaprazan were comparable following repeated administration (on day 7) to those reported after the initial dosage on day 1[35]. In 2004, Yuet al[36] conducted a single-blind,dose-rising, parallel-group, randomized, placebo-controlled trial in 46 healthy subjects. In the singledose research, plasma concentrations of revaprazan attained peak values 1.3 h to 2.5 h after administration and thereafter decreased mono-exponentially with a terminal T1/2 of 2.2 h to 2.4 h in dosage groups up to 200 mg. Revaprazan has linear pharmacokinetic properties, with negligible accumulation after numerous doses. The onset of pharmacological effects was quick, with the greatest benefits shown on the first day of treatment with repeated doses. They concluded that revaprazan was safe, welltolerated, and efficiently suppressed acid secretion by increasing intragastric pH in a dose-dependent manner.
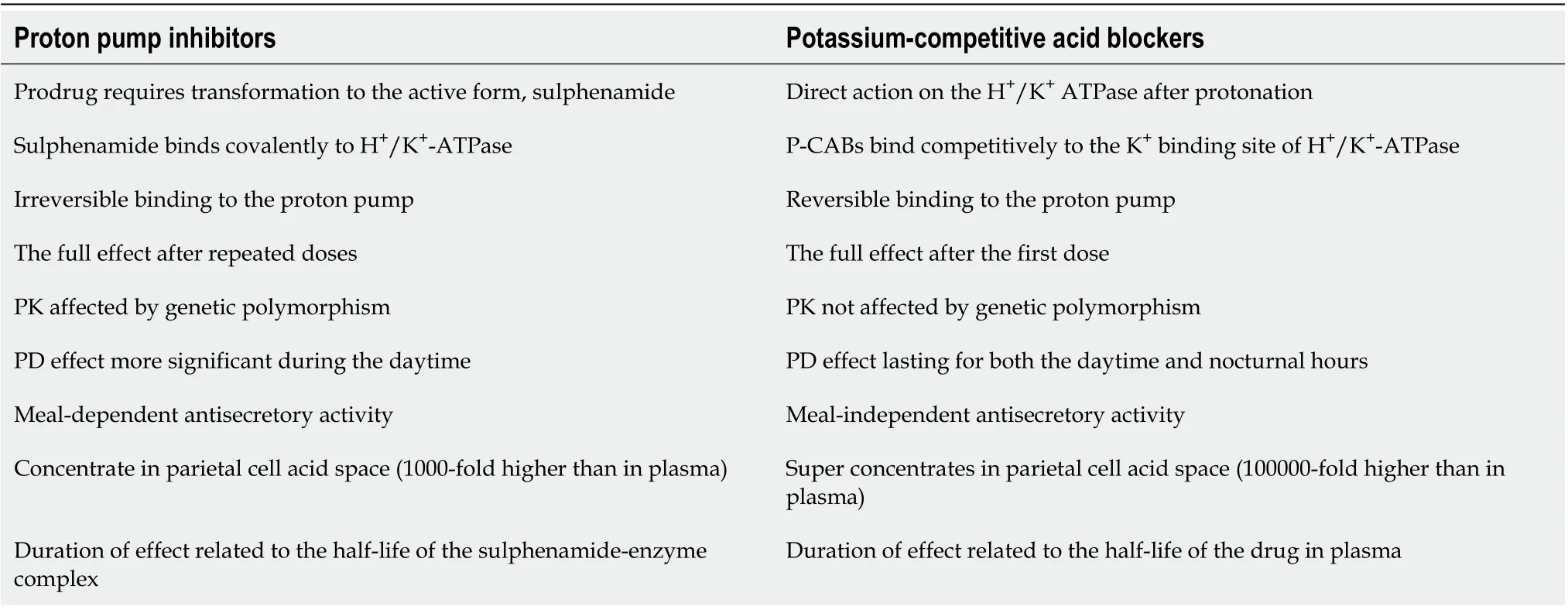
Table 1 Main differences in the mechanisms of action between proton pump inhibitors and potassium-competitive acid blockers[12]

Figure 1 Differences in chemical classes and chemical structures of potassium-competitive acid blockers[12]. P-CAB: Potassium-competitive acid blocker.
In 2015, Sakuraiet al[37] conducted 2 phase I, single rising-dose, randomized, double-blind, placebocontrolled trials in 84 volunteers from Japan using 1-120 mg VPZ and 63 healthy males from the United Kingdom using 1-40 mg VPZ to evaluate the pharmacokinetics, pharmacodynamics, safety, and tolerability. They discovered that VPZ plasma concentration-time profiles exhibited fast absorption at all doses, with a median Tmaxof up to 2 h. T1/2was predicted to be up to 9 h. The acid suppression effect was dose-dependent, with a 24-h intragastric pH 4 holding time for 40 mg VPZ being 92% in Japanese males and 87% in British males. They determined that single oral doses of 20-120 mg VPZ suppressed gastric acid production in healthy male participants in a quick, deep, and 24-h manner. Furthermore,VPZ was well tolerated at all dosages tested, suggesting that it might be used as an alternate therapy for acid-related diseases.

Figure 2 Potassium-competitive acid blockers inhibit gastric H+/K+-ATPase by binding ionically to the enzyme and prevent further activation by the K+[7]. P-CAB: Potassium-competitive acid blocker.
Recently, Hanet al[38] performed a phase I, randomized, placebo-controlled study in 56 healthy volunteers withoutHelicobacter pyloriinfection to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of tegoprazan (TPZ). They found that TPZ was well tolerated. The majority of the adverse events were mild and resolved without any long-term consequences. On day 7, despite multiple dosages of TPZ, there was no accumulation in the plasma. The pharmacodynamics study found that TPZ suppressed gastric acid rapidly in a dose-dependent manner. They concluded that TPZ was well tolerated and demonstrated rapid and potent gastric acid suppression.
Sunwooet al[39] conducted a randomized, double-blind, double-dummy, placebo-controlled study to evaluate a single ascending dose and multiple ascending doses of fexuprazan (FPZ) in 120 healthy male subjects withoutHelicobacter pylori(H. pylori) infection. They discovered that FPZ was well tolerated and suppressed gastric acid output for 24 h after delivery. In the multiple ascending doses trial, FPZ plasma concentrations grew in a dose-proportional way, but it did not appreciably accumulate in the plasma in the single ascending dosage study. They concluded that FPZ showed an immediate, long-lasting gastric acid suppression effect and was safe.
In 2020, Hwanget al[40] conducted a single- and multiple-dose, randomized, double-blind, placebocontrolled trial to elucidate the pharmacodynamics, pharmacokinetics, and safety of FPZ among healthy volunteers of Korean, Caucasian, and Japanese descent. FPZ (40, 60, or 80 mg for Koreans; 40 or 80 mg for Caucasians; 20, 40, or 80 mg for Japanese) or a placebo were given to ten participants in each group at random. Gastric acid suppression was shown to be consistent across ethnic groups. After successive doses of 40 mg, the mean percentages of time when the intragastric pH was over 4 in Korean,Caucasian, and Japanese participants were 64.3 percent, 62.8 percent, and 70.3 percent, respectively.Furthermore, the 80 mg dose that could effectively suppress gastric secretion was 94.8%, 90.6%, and 90.6% for the Korean, Caucasian, and Japanese subjects, respectively. They determined that FPZ suppressed stomach acid in the same way in Korean, Caucasian, and Japanese patients, and that the pharmacokinetic and pharmacodynamic correlations, as well as the safety, were the same in all three ethnic groups. The FPZ could be utilized regardless of ethnicity.
X842 is a linaprazan prodrug that is currently under development. X842 has a T1/2of 10 h and enables improved intragastric pH regulation over 24 h. Linaprazan was established in a phase I study that used a single dose or multiple escalating dosage design to assess the drug’s safety and tolerability in healthy volunteers as the primary endpoint[41]. X842 was shown to be safe and well tolerated by the participants in the study. During the trial, no severe or significant adverse events were recorded. When both pharmacodynamics and pharmacokinetics were evaluated, precise dose linearity was identified.The mean median intragastric pH at each X842 dosage was never less than 4.
P-CABs EFFICACY IN GERD
VPZ efficacy in GERD
In 2015, Ashidaet al[42] undertook a parallel-group, dose-ranging, multicenter, randomized, doubleblind trial in 732 patients with EE to assess the effectiveness and safety of VPZvslansoprazole (LPZ).The eligible EE individuals were randomized 1:1:1:1:1 to undergo an 8-wk therapy with LPZ 30 mg,VPZ 5 mg, VPZ 10 mg, VPZ 20 mg, and VPZ 40 mg. They discovered that with VPZ 5, 10, 20, and 40 mg,and LPZ 30 mg, the proportion of healed EE individuals at week 4 was 92.3%, 92.5%, 94.4%, 97.0% and 93.2%, respectively. When corrected for baseline Los Angeles (LA) grades A/B and C/D, all VPZ regimens were non-inferior to LPZ 30 mg therapy. With VPZ 5, 10, 20, 40 mg, and LPZ 30 mg, the proportions of healed EE individuals were 87.3%, 86.4%, 100%, 96.0%, and 87.0%, respectively, for LA grades C/D. The incidence of adverse events was the same in all groups. They indicated that VPZ was efficacious and comparable to LPZ in the treatment of EE. For severe EE (LA grades C/D), they advised VPZ doses of 20 mg or more.
Furthermore, Ashidaet al[43] performed another parallel-group comparison, multicenter,randomized, double-blind trial to evaluate the non-inferiority, long-term efficacy, and safety as maintenance therapy of VPZ 20 mg compared with LPZ 30 mg in 409 endoscopically confirmed EE patients (LA grades A-D). They discovered that the proportion of patients with healed EE up to week 8 for VPZ (203/205) and 95.5% for LPZ (190/199) was 99.0% for VPZ (203/205) and 95.5% for LPZ(190/199), demonstrating the non-inferiority of VPZ 20 mg therapy. There were a few EE recurrences (<10%) for long-term maintenance evaluation in patients treated with VPZ 10 mg or 20 mg. They stated that the comparative trial demonstrated the non-inferiority of VPZ 20 mg to LPZ 30 mg in EE, and that VPZ was well tolerated and efficacious among long-term maintenance EE patients (Table 2).
In 2017, Iwakiriet al[44] conducted a randomized, double-blind, multicenter trial of VPZ 20 mg or 40 mg in 19 patients with PPI-resistant (LPZ 30 mg) EE to measure stomach and esophageal pH over 24 h.Patients with endoscopically proven PPI-resistant EE received VPZ 20 mg (9 cases) or VPZ 40 mg (10 cases) for 8 wk after a 7- to 14-d run-in period with LPZ 30 mg therapy. The percentage of stomach pH 4[pH 4 holding time ratio (HTR)] increased significantly in both groups from baseline: In the 20 mg group, it increased from 73.21% to 96.46%, and in the 40 mg group, it increased from 69.97% to 100.00%.There were no significant increases in esophageal pH 4 HTRs from baseline. After 8 wk of treatment, the healing rate in patients with baseline EE grades A-D was 60.0% in the 20 mg group and 71.4% in the 40 mg group. They concluded that VPZ 20 mg and 40 mg substantially suppressed gastric acid secretion over 24 h with considerably higher gastric pH at 4 HTR, resulting in an EE repair rate greater than 60%.
In the same year, Yamashitaet al[45] conducted a study to evaluate the effects of VPZ and PPIs in 8 RE patients using multichannel intraluminal impedance-pH. They found that the mucosal lesions in 7/8 patients (87.5%) with persistent gastric mucosal injury after completing an 8-wk standard PPI therapy had entirely healed after VPZ therapy. From 26.5% to 78.0%, there was a considerable rise in stomach pH > 4 HTR. In addition, a decrease in esophageal pH of 4 HTR was seen, although it was not statistically significant. The overall number of reflux events, comprising acid and proximal reflux episodes, as well as the time it took for acid to clear, were both dramatically reduced. They concluded that VPZ should be used in patients with PPI-refractory RE.
In 2018, Ashidaet al[46] conducted a study to compare VPZ 10 mg (n= 202) and 20 mg (n= 204) with LPZ 15 mg (n= 201) as maintenance treatment in 607 patients with healed EE. EE recurrence rates with LPZ 15 mg, VPZ 10 mg, and VPZ 20 mg were 16.8%, 5.1%, and 2.0%, respectively, throughout a 24-wk maintenance period. VPZ was shown to be non-inferior to 15 mg LPZ. The 10 mg and 20 mg VPZ were significantly lower than the LPZ at 15 mg. The EE recurrence rate, on the other hand, did not change substantially between the two VPZ dosages. They determined that VPZ 10 mg and 20 mg were noninferior to LPZ 15 mg as maintenance treatment for individuals with healed EE.
A retrospective cohort study that recruited 55 patients with symptomatic GERD (NERD = 30, EE = 25)treated with VPZ 10 mg who had been followed for more than 1 year was conducted by Shinozakiet al[47]. They discovered that taking VPZ 10 mg for one month relieved GERD symptoms in 89% of patients and was maintained in 82% after one year without further treatment. In 47% of cases, 1-year maintenance treatment resulted in long-term relief of GERD symptoms. Nine of the forty-nine responders experienced a return of GERD symptoms, while VPZ dosage escalation relieved symptoms in 67% (6/9) of patients. Postprandial discomfort, postprandial distress, constipation, and diarrhea significantly decreased after 1 m and remained stable after a year. After 1 year of therapy, the endoscopic healing rate of EE was 95%. They concluded that 1 year of VPZ therapy reduces GERD symptoms significantly, and endoscopic healing of EE is good. VPZ is an efficient and beneficial longterm treatment for GERD.
In 2019, Oshimaet al[48] conducted a randomized, placebo-controlled experiment in 32 patients with endoscopically proven EE who had heartburn at least once a week to see how quickly VPZ and LPZ relieve heartburn. For 14 d, the patients were given either VPZ 20 mg or LPZ 30 mg before breakfast.They found that heartburn was relieved earlier with VPZ than with LPZ. Furthermore, on day 1, VPZ and LPZ totally cured heartburn in 31.3% and 12.5% of patients, respectively. With VPZ medication,significantly more patients experienced full nighttime heartburn resolution compared with LPZ treatment. They concluded that during the first week of therapy, VPZ provided more prolonged heartburn relief than LPZ.
Recently, Akiyamaet al[49] studied the efficacy of VPZ 20 mg in 13 patients with PPI-refractory GERD who exhibited continued pathological esophageal acid exposure (EAE). Among 13 patients who were observed by multichannel intraluminal impedance-pH at baseline (PPI treatment) and after VPZ 20 mg therapy, the median gastric acid exposure times of the VPZ group were lower than those of the PPI group, both during daytime and nocturnal observations. Furthermore, during the 24-h monitoring period, VPZ 20 mg treatment resulted in lower median EAE values (4.5%) than PPI therapy (10.6%).
EAE normalization occurred in 46% of VPZ-treated people, and it was connected to complete stomach acid reduction (P= 0.005). Reflux symptoms (P= 0.01) and EE (P= 0.01) improved after switching to VPZ 20 mg. They concluded that VPZ 20 mg outperforms PPIs in stomach acid suppression, EAE control, symptom alleviation, and esophagitis healing in patients with PPI-refractory GERD.

Table 2 Clinical studies regarding the efficacy of potassium-competitive acid blocker in gastroesophageal reflux disease treatment
Mizunoet al[50] carried out a study to assess the effectiveness of VPZ 10 mg as a maintenance treatment for healed RE in 50 patients who completed 48-wk maintenance therapy. Maintenance therapy with VPZ 10 mg at 48 wk avoided the recurrence of esophageal mucosal breaches in 43 of 50 patients (86.0%). Throughout the 48-wk maintenance therapy, the symptomatic non-relapse rate for acid reflux-related symptom score on the frequency scale for GERD symptoms and acid reflux score on the gastrointestinal symptom rating scale was 70.0% and 72.0%, respectively. They determined that VPZ 10 mg is clinically efficacious for the long-term maintenance of healed RE.
In 2020, Xiaoet al[51] conducted a phase III, double-blind, multicenter study in 468 endoscopically confirmed EE patients and randomly assigned them to take either VPZ 20 mg (238) or LPZ 30 mg (230)once daily for 8 wk. They found that the 8-wk EE healing rates in the VPZ and LPZ groups were 92.4%and 91.3%, respectively. Moreover, in patients with a baseline LA classification of C/D, VPZ had higher 2-wk, 4-wk, and 8-wk EE healing rates than LPZ. Overall, the rates of EE healing appeared to be greater with VPZ than with LPZ. They concluded that VPZ is not inferior to LPZ in EE healing at 8 wk, and the two treatment groups had identical safety results.
Last year, Okanobuet al[52] performed a randomized control study to evaluate the efficacy of VPZ 10 mg (n= 36) compared with VPZ 20 mg (n= 37) in 73 patients with EE. The patients were given each dose for four weeks as an initial treatment, followed by eight weeks of maintenance therapy with VPZ 10 mg. The endoscopic healing rates of the 20 mg and 10 mg groups were 94.6% and 94.4%, respectively,after four wk. In both treatment groups, the frequency scale for GERD symptoms decreased significantly, from 13 to 4 and 14 to 3, respectively, in the 20 mg and 10 mg groups. The scores have plummeted to 2 after 12 wk. They determined that after 4 wk and throughout the experiment, VPZ 10 mg medication had a similar therapeutic response to VPZ 20 mg treatment in terms of mucosal repair and symptom reduction. These results were also the same in LA classification grade A/B patients but not in grade C/D patients.
A prospective, multicenter, open-label, randomized cross-over trial with two periods was conducted by Matsudaet al[53] to clarify the efficacy and superiority of VPZ 10 mg every other day over LPZ 15 mg in the maintenance management of 122 erosive GERD patients. They observed that 93.6% of the VPZ group and 82.1% of the LPZ group had well-controlled symptoms, with a significant difference (P=0.003) using McNemar's test. The VPZ-LPZ and LPZ-VPZ groups, respectively, had 96.7% and 80.0% of patients well managed throughout the first four weeks (P= 0.007). For the second 4 wk, 94.4% of patients in the VPZ-LPZ and 76.7% of patients in the LPZ-VPZ groups were well controlled following 6 consecutive days a week (P= 0.009). They found that taking VPZ 10 mg every other day decreased GERD symptoms considerably, as indicated by the frequency scale for the gastrointestinal symptom rating scale and GERD symptoms.
TPZ efficacy in GERD
Leeet al[54] conducted a parallel-group, multicenter, randomized, double-blind comparative trial in 302 Korean patients with endoscopically confirmed EE in 2019. The patients were randomly assigned to receive TPZ 50 mg, TPZ 100 mg, or EPZ 40 mg for 4 wk or 8 wk. They confirmed that at week 8, the cumulative healing rates for TPZ 50 mg, TPZ 100 mg, and EPZ 40 mg were 98.9% (91/92), 98.9%(90/91), and 98.9% (87/88), respectively. Both TPZ dosages were non-inferior to EPZ 40 mg, and TPZ was well tolerated.
Kimet al[55] undertook a multicenter, double-blind, placebo-controlled, phase III trial in 324 Korean patients with NERD in 2021 to investigate the safety and effectiveness characteristics of TPZ relative to placebo. They were randomized into three groups with TPZ 50 mg, TPZ 100 mg, and placebo once daily for 4 wk. After 4 wk of therapy with TPZ 50 mg, TPZ 100 mg, or placebo, 42.5% (45/106), 48.5% (48/99),and 24.2% (24/99) of patients achieved full relief of significant symptoms. TPZ 50 mg and 100 mg performed better than the placebo by a substantial statistical difference. Both the TPZ and placebo groups had significantly greater percentages of heartburn-free days and full-resolution rates of heartburn. Furthermore, no apparent change in the occurrence of treatment-emergent adverse events was observed.
FPZ efficacy in GERD
In a phase III, multicenter, randomized, double-blind trial, FPZ, the antisecretory action of a pyrrole derivative with a quick and full onset was tested. A total of 260 adult patients with endoscopically proven EE (LA grades A to D) were given either FPZ 40 mg or EPZ 40 mgperday. The cumulative proportion of patients with healed mucosal breaks, as evaluated by endoscopy, was the main outcome measure at week 8. The healing rate, symptoms, and quality of life were assessed at week 4, and it was discovered that FPZ was non-inferior to EPZ, with the groups having identical cumulative healing rates at 8 wk and equivalent rates at 4 wk. In individuals with moderate to severe heartburn, FPZ provided superior symptom alleviation that lasted throughout the night. The medication was well tolerated, with similar rates of side effects across treatment groups[56].
SAFETY OF P-CABs
Despite the fact that PPIs are one of the safest pharmacological classes available and have been used for almost 30 years, the number of papers on delayed-release PPI safety has skyrocketed, with many extensively discussed subjects appearing in high-profile journals[57,58]. Clinical studies and subsequent meta-analyses have revealed that VPZ has outstanding short and medium-term safety when compared to the performance of PPIs[42-53,59,60]. Because serum gastrin and pepsinogen I levels in healthy volunteers and GERD patients mirrored the antisecretory effect of VPZ, hypergastrinemia associated with long-term therapy may be a concern[61,62]. Clinical studies and subsequent meta-analyses have revealed that VPZ has outstanding short and medium-term safety when compared to the performance of PPIs[63]. Long-term PPI therapy has been associated with dysbiosis and changes in the gut microbiome, and similar changes have now been reported with VPZ[64-66]. Lipopolysaccharide biosynthesis proteins and lipopolysaccharide biosynthesis were the most dramatically elevated pathways in response to VPZ. They are most likely caused by an increase in intraluminal pH and are analogous toH.pyloriresponses to external pH changes. Because lipopolysaccharide is a potent immune response stimulator generated by Gram-negative bacteria, these data imply that VPZ may increase bacterial growth[67-69].
CONCLUSION
Many of the disadvantages and limitations of delayed-release PPIs are overcome by P-CABs. Mucosal healing in acid-related disorders is linked to the duration and degree of acid suppression, and also the length of treatment. Given the challenges in achieving good symptom management, particularly at night, with presently available delayed-release PPIs once daily, this novel family of medicines, P-CABs,delivers immediate, powerful, and extended acid suppression. They have the potential to address many of the unmet therapeutic needs in GERD, such as obtaining immediate heartburn relief and fast and specific healing of severe RE. The benefits of long-term acid suppression extend toH. pylorieradication,where intragastric pH control, especially at night, is crucial. Moreover, VPZ may be an optimum dual therapy as a straightforward, dependable, and successful first-line treatment for GERD. More thorough evaluations of VPZ, TPZ, and FPZ are needed, especially in Europe and North America. After extensive worldwide use of P-CABs, clinicians will justify whether it is effective, safe, and superior to currently available treatment with PPIs. In our opinion, VPZ or other P-CABs should be reserved for difficult-totreat acid-related illnesses and unmet requirements, where the benefit-to-risk ratio is predicted to be the best.
FOOTNOTES
Author contributions:Leowattana W wrote the paper; Leowattana T collected the data.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Togo
ORCID number:Wattana Leowattana 0000-0003-4257-2480; Tawithep Leowattana 0000-0003-2316-3585.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年28期
World Journal of Gastroenterology2022年28期
- World Journal of Gastroenterology的其它文章
- Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma:Experimental and clinical scenarios
- Gut microbiota alteration and modulation in hepatitis B virus-related fibrosis and complications:Molecular mechanisms and therapeutic inventions
- Combination approaches in hepatocellular carcinoma: How systemic treatment can benefit candidates to locoregional modalities
- Update on endoscopic ultrasound-guided liver biopsy
- Non-alcoholic fatty liver disease-related hepatocellular carcinoma: Is there a role for immunotherapy?
- Making the case for multidisciplinary pediatric aerodigestive programs
