Family-based Helicobacter pylori infection status and transmission pattern in central China, and its clinical implications for related disease prevention
Xue-Chun Yu, Qiao-Qiao Shao, Jing Ma,Miao Yu, Chen Zhang, Lei Lei, Yang Zhou, Wen-Chao Chen, WeiZhang, Xin-Hui Fang,Yuan-Zeng Zhu, Gang Wu, Xue-Mei Wang, Shuang-Yin Han, Pei-Chun Sun, Song-ZeDing
Abstract
Key Words: Helicobacter pylori; Atrophic gastritis; Family clustering infection; Gastric cancer; Gastrin;Pepsinogen
INTRODUCTION
ChronicHelicobacter pylori(H. pylori) infection is the major cause of chronic gastritis, peptic ulcers, and gastric cancer (GC), and is also closely associated with a number of extra-gastrointestinal (GI) diseases[1-3].H. pyloriinfection rate in China is about 50%, but the infection rate varies widely in different regions due to economic development, age, lifestyle habit, and sanitary conditions[4-6].H. pylorihas characteristics of family cluster infection[7]. MostH. pyloriinfections are acquired during childhood and adolescence, and infection will persist for decades unless proper treatment is offered.
Mounting evidence has demonstrated that transmission ofH. pyloriis mainly by oral-oral and fecaloral routes, and water sources[8,9], and intra-familial spread is the major source ofH. pyloritransmission[10-12]. The infected parent, especially the mother, is thought to play an important role in its transmission[9,11]. When parents are infected withH. pylori, the infection rates of their children markedly increase; spread has also been demonstrated between spouses and among siblings[13-17].Therefore, diagnosis and treatment of the whole family have important clinical implications for preventing related diseases[18]. Recently, the notion of “family-basedH. pyloriinfection control and management” has been introduced to China as a practical strategy to curbH. pyloriintra-familial transmission[3]. However, relatively few studies have been performed to investigate the family-based infection status, related factors, and pattern of intrafamilial transmission in the general population.
Type I [cytotoxin-associated protein-positive (CagA+), vacuolating cytotoxin-positive (VacA+)]H.pyloriinfection causes severe gastric inflammation and is prone to induce carcinogenesis[19,20]. Our previous study of 3572 patients admitted to the hospital showed that theH. pyloriinfection rate was 75.9% in this area of central China. The infection rate was further confirmed by investigation of 523 endoscopy-confirmed patients (76.9%), of whom 72.4% (291/402) had type IH. pyloriinfection and 27.5% had type IIH. pyloriinfection. Importantly, 88.4% of GC patients wereH. pylori-positive, of whom 84.2% had type I infection; only 11.6% of GC patients wereH. pylori-negative[21]. At present, the genotype of family based-H. pyloriinfection in the healthy household is unclear, as well as its relationship with GC epidemiological markers such as gastrin-17 (G-17), pepsinogen (PG) level, and PG I/II ratio (PGR).
Henan province in central China is one of the high-risk areas forH. pyloriand GC, with anH. pyloriinfection rate of 49.6%[22] and GC incidence of 42.52/100000[23]. The capital city, Zhengzhou, has a population of 12 million, but there has been no large-scale family-basedH. pyloriintrafamilial transmission survey, and the factors that affectH. pylorispread and cause disease are also unclear.
Therefore, we investigated family-basedH. pyloriinfection status, factors related to bacteria spread,bacteria genotype, and patterns of transmission for the residents in this area, and analyzed their impact on GC epidemiological markers including G-17, PGI, PGII, and PGR. The results of this study will provide information onH. pyloriinfection status in the household and help to refine eradication strategies for the prevention of related diseases.
MATERIALS AND METHODS
Study population and data collection
From September 2020 to April 2021, blood samples and questionnaires were collected from family members of 10 selected communities in greater Zhengzhou area; each community enrolled 20-30 families, with all members participating. The 10 communities were selected based on high, middle, and low living standards to prevent biased selection of the population; these included two high-income communities, six middle-class communities, and two communities originating from rural areas. Specifically, the study included two communities located in Guancheng District, namely Lufu Pavilion and Houjiadong Street communities; three communities located in Jinshui District, namely Chengbei Road,Jiagang, and Huilong Digital City communities; four communities in New East Zhengzhou District,namely Hanhai Qingyu, Zhengzhou Academy of Aviation Administration, Henan University of Finance, Economics and Law, and Nanxi Fudi communities; and one community in Central Zhengzhou District, namely Kowloon City community.
A total of 282 families (family size ≥ 2 persons) including 772 individuals participated in the survey.Inclusion criteria were: Family members being long-term residents living in Zhengzhou area, with no age limit; all family members being willing to participate by providing blood samples and filling out the questionnaire; at least 2 people composing the family unit, but with no limitation on how many people are living in the same household; and all family members being willing to provide written informed consent. An infected family was defined as a household with various family members infected withH.pylori, ranging from only 1 person to all family members being infected, and a family could be composed of only a couple, with or without children. Exclusion criteria were: Pregnant and breastfeeding females; people with mental illness; or people who refused to fill out the questionnaire or sign the consent form.
This study was approved by the Ethics Committee of People’s Hospital of Zhengzhou University(No. 53, 2021). All subjects provided written informed consent; for minor subjects, written informed consent was given by their legal guardian. This study was registered in the China Clinical Trial Registry(www.chictr.org.cn; No. ChiCTR2100052950), and the protocol is freely available from the website after registration.
Subject enrollment and questionnaire
Before and during enrollment, an introduction brochure or information booklet for the study was distributed to the community center staff, who were responsible for distributing and helping recruit community family members for onsite registration. A registration website was also open to community members for whole family-based registration; registration for only a single individual was declined. A questionnaire was filled out either online or onsite by each of the participating family members. Blood samples were collected from each participating member forH. pylori, gastrin, and PG analyses; if necessary,13C-urea breath test (UBT) was subsequently performed by appointment.
Based on the purpose of this study, questionnaire included the following 17 items: Age; sex; family ethic; number of family members; professions; marriage status; socioeconomic data; dining history;living habits; lifestyle; disease history; medication history; presence of GI symptoms;H. pylorieradication history; history of gastroscopy; infection history of other family members; and treatment history (Table 1).
H. pylori infection status, gastrin, and PG analyses
Three milliliters of fasting venous blood were collected from all subjects in the morning. Blood samples were centrifuged at 1000 ×gfor 10 min, (80-2 centrifuge; Jiangsu Zhongda Instrument Technology Co.,Ltd., Jiangsu, China), and samples were either analyzed on ice on the same day or stored at -80 °C for subsequent analyses. Serum anti-H. pyloriantibodies [detecting CagA, VacA, UreA, UreBvia H. pylorienzyme-linked immunosorbent assay (ELISA) kit (Blot Biotech Co., Ltd., Shenzhen, Guangdong,China)] and G-17, PGI, PGII levels, as well as PGR (viaPGI, PGII, G-17 ELISA kits; Biohit Biotechnology,Helsinki, Finland) were measured by an ELISA kit following manufacturer’s instructions as previously reported[22]. If patients had previously undergoneH. pylorieradication therapy, an additional13C-UBT was performed to obtain their current infection status (13C-UBT Diagnostic Kit; Beijing Boran Pharmaceutical Co., Ltd., Beijing, China).
Statistical analyses
Data were analyzed using SPSS for Windows version 25 (IBM Corp, Armonk, NY, United States).Continuous variables are expressed as mean ± SD, whereas categorical variables are described as percentages or frequencies. The measurement data were compared byt-test, and the enumeration data were compared byχ2test or Fisher’s exact test.P< 0.05 was considered statistically significant.
RESULTS
Demographic information of the enrolled families
As shown in Table 1, a total of 282 families including 772 members participated in this study. Among them, 419 hadH. pyloriinfection, giving an overall infection rate of 54.27% (419/772); among the infected individuals, 328 (42.49%, 328/772) were infected with type I strains and 91 (11.79%, 91/772)were infected with type II strains. Type I strains accounted for 78.28% (328/419) of cases, and type II strains accounted for 21.7% (91/419) of infected individuals (Figure 1A).
In total, 330 (42.75%) of the study participants were male, with an average age of 44.56 ± 20.19 years,and 442 (57.25%) were female, with an average age of 45.95 ± 18.74 years (P> 0.05). The age range of the enrolled individuals was 3 years to 90 years, with the youngest and oldest infected individuals aged 5 years and 87 years, respectively.
As shown in Figure 2, stratified age andH. pylorigenotype infection were further analyzed, type I strains was the dominant strains for all age groups. The infection rates of individuals under the age of 18 were 23.26% (20/86), and the age groups of 51-60 and 61-70 years had the highest infection rates of 63.01% (92/146) and 65.95% (93/141), respectively. Compared with age groups under 18-years-old, the infection rate was significantly higher in groups above 18-years-old (P< 0.05), but there was no difference in infection rates among groups above 18-years-old (P> 0.05).
Among questionnaire variables (Table 1), annual household income, history of smoking, history of alcohol consumption, dining location, presence of GI symptoms, and family history of gastric disease and GC did not affect infection rates (P> 0.05), but individuals with a higher education level showed significantly lower infection rates (P< 0.05).
H. pylori infection status of the enrolled families
The average family size of the study cohort was 2.74 personsperhouseholds, and family size ranged from as few as 2 personsperfamily to as many as 6perfamily (Figure 1B). In this survey, 2- and 3-person households accounted for 80.85% (228/282) of the families enrolled.
As shown in Figure 1C-F,H. pylori-infected individuals were distributed in 246 of the 282 families with varying numbers of members infected, ranging from only 1 person to all family members infected.The family infection rate was 87.23% with at least 1 person infected in a family unit (246/282), in 12.77%of the 282 households, no family members were infected (36/282) (Figure 1C). In 67 of the 246 infected families, all members were infected (27.24%, 67/246), among these 67 all member-infected households,46 households were infected with the same type I strains (68.66%, 46/67), 1 household was infected with type II strains (1.49%, 1/67), and 20 households had mixed type I and II strain infection (29.85%, 20/67)(Figure 1D). The data of the stratified family member infection rate of 282 households were shown in Figure 1E. In 53.66% (132/246) of families with at least 2 members infected, 59.09% (78/132) of these families were infected with type I strains, 5.30% (7/132) were infected with type II strains, and 35.61%(47/132) were infected with mixed type I and II strains (Figure 1F).
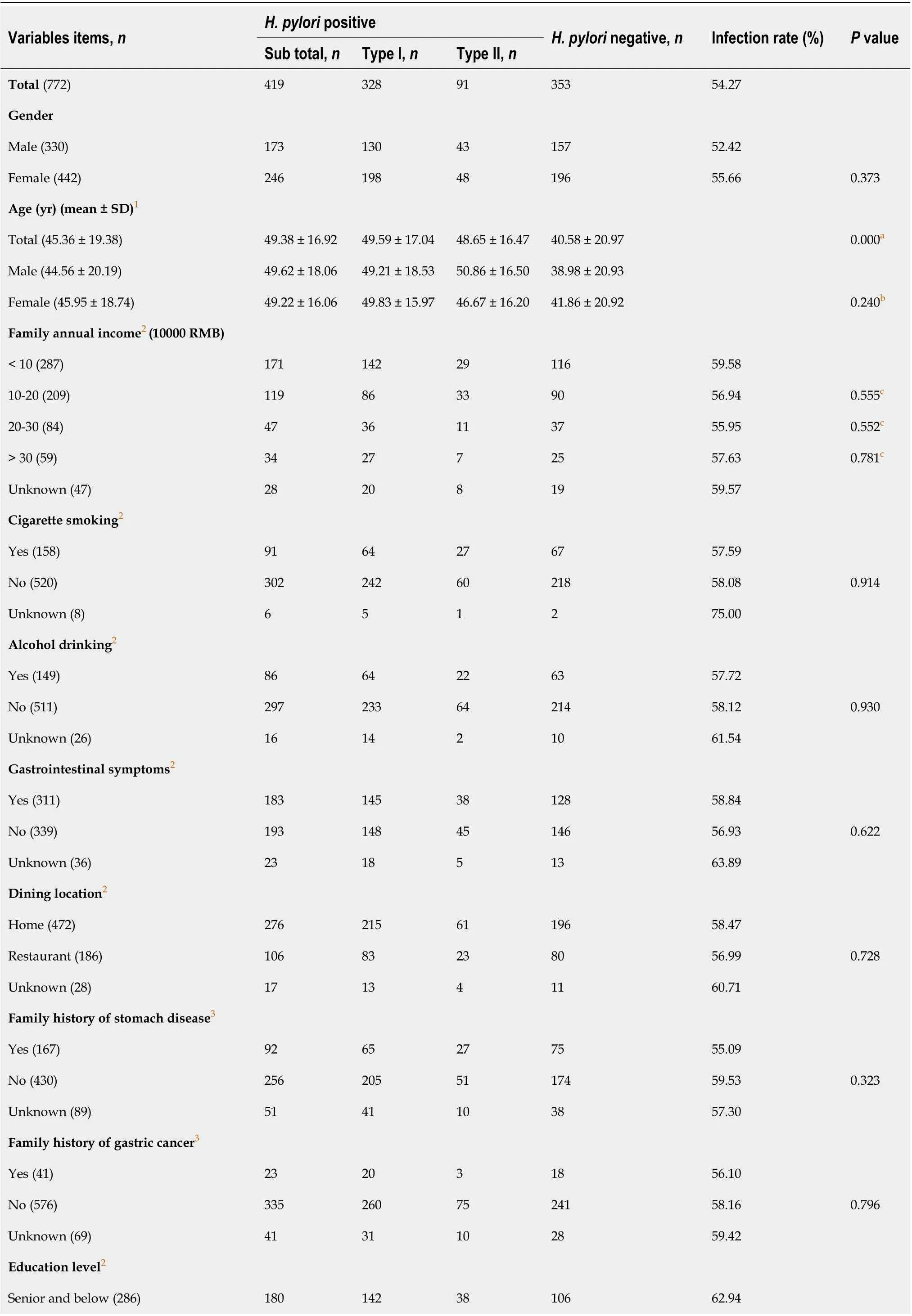
Table 1 Demography information and Helicobacter pylori infection status of 772 subjects
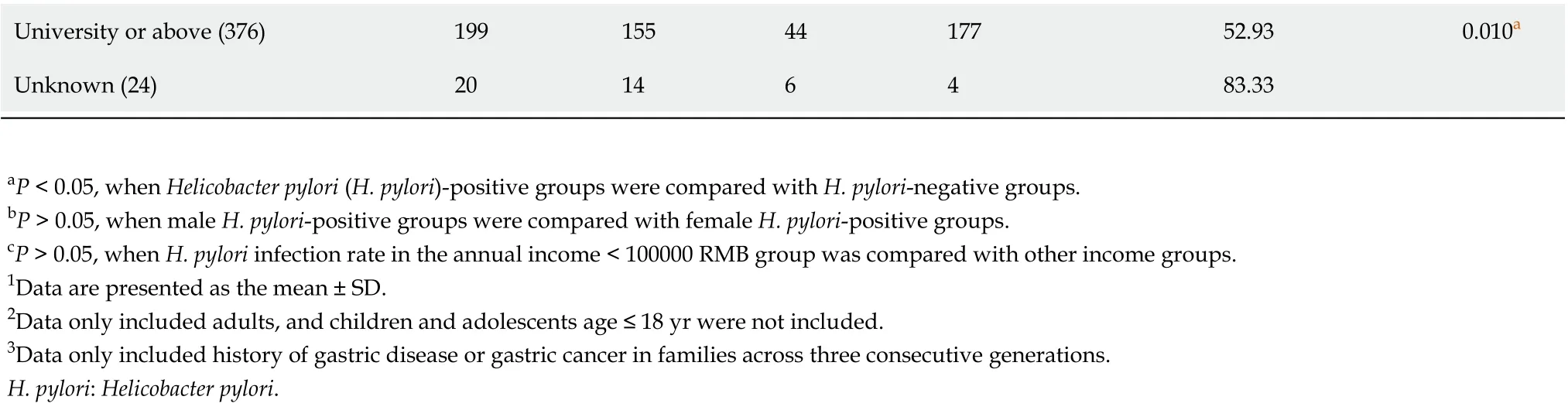
University or above (376)1991554417752.930.010a Unknown (24)20146483.33 aP < 0.05, when Helicobacter pylori (H. pylori)-positive groups were compared with H. pylori-negative groups.bP > 0.05, when male H. pylori-positive groups were compared with female H. pylori-positive groups.cP > 0.05, when H. pylori infection rate in the annual income < 100000 RMB group was compared with other income groups.1Data are presented as the mean ± SD.2Data only included adults, and children and adolescents age ≤ 18 yr were not included.3Data only included history of gastric disease or gastric cancer in families across three consecutive generations.H. pylori: Helicobacter pylori.
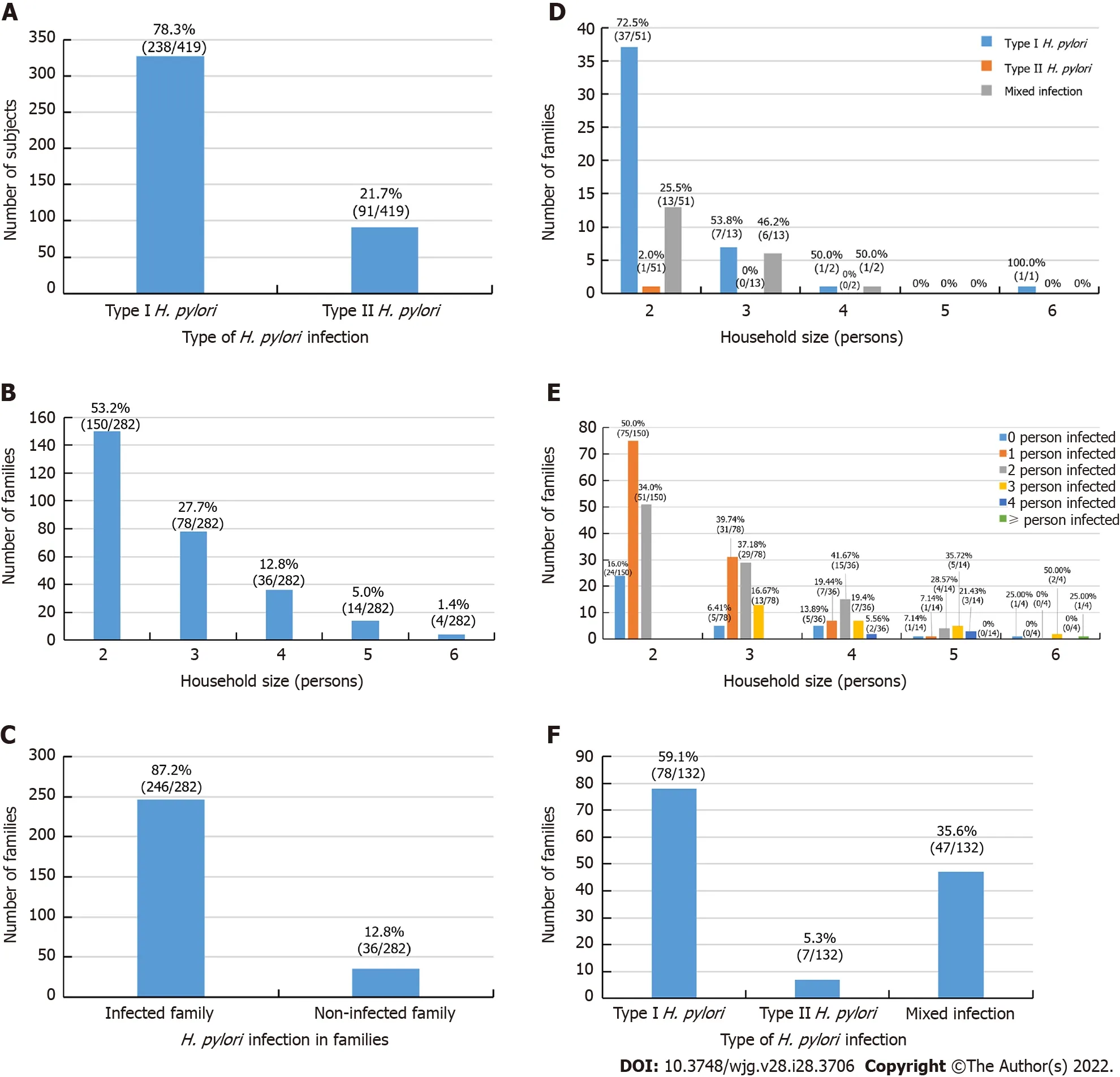
Figure 1 Helicobacter pylori infection status of the 282 enrolled families. Values above each column are the case number and percentages of each group. A: Genotype pattern of the 419 infected subjects in the enrolled families; B: Distribution pattern of the 282 enrolled families ranged from 2-6 people; C: General Helicobacter pylori (H. pylori) infection status of 282 families; D: Genotype pattern of the 67 all member-infected families from the enrolled 282 families; E: Distribution pattern of the H. pylori infection status of the 282 enrolled families; F: Genotype pattern of the 132 H. pylori-infected families with ≥ 2 persons infected from the 282 enrolled families. Infected family: At least 1 person in a family was infected; Non-infected family: All members in a family were not infected; Type I H. pylori: H. pylori infection with type I strains; Type II H. pylori: H. pylori infection with type II strains; Mixed infection: H. pylori infection with type I and type II strains.
H. pylori infection status between couples
H. pyloriinfection status between couples is shown in Figure 3. In all, 244 of the 282 families had both spouses, and infection rate of both spouses was 34.84% (85/244); further, 17.21% (42/244) couples were not infected, and 47.95% (117/244) had only a single spouse infection (Figure 3A). Among 117 families with infection of only 1 spouse, 75.21% (88/117) were infected with type I strains and 24.79% (29/117)were infected with type II strains (Figure 3B). Of these spouses, the husband was infected in 49.57%(58/117) of cases and the wife was infected in 50.53% (59/117) of cases (P> 0.05), they were further stratified into type I and type II strains infections (Figure 3C). Furthermore, among the 85 families with both husband and wife co-infected withH. pylori, 68.24% (58/85) were infected with the same type of strain, of whom 63.53% (54/85) were infected with type I strains, 4.71% (4/85) were infected with type II strains, and 31.76% (27/85) had mixed type I and type II infection (Figure 3D). Significantly more couples were infected with the same type of strain than with mixed strains (P< 0.05). In addition, with the increase in marriage duration, infection rate of both husband and wife was significantly increased (r= 0.98,P< 0.05; Figure 3E).
Parental infection and infection status of children and adolescents
In 51 families with both parents and children younger than 18 years of age, as shown in Table 2, the infection rate of children was 23.08% (6/26) when both parents wereH. pylori-infected; however, when both parents were not infected, the infection rate of children was 18.18% (2/11) (P> 0.05). When only mother was infected, the infection rate of children was 45.45% (5/11); no child was infected (0/9) when only father was infected (P> 0.05).
Table 3 shows infection status of the 51 families comprising both parents and children, for a total of 190 individuals. Among these family members, the infection rates were as follows: Father, 62.75%(32/51); mother, 62.75% (32/51); grandfather, 50.00% (2/4); grandmother, 66.67% (8/12); maternal grandfather, 25.00% (1/4); maternal grandmother, 37.50% (3/8); and other relatives, 66.67% (2/3).
Comparison of G-17, PGI, and PGII levels, and PGR with different types of H. pylori infection
To determine the impact ofH. pyloriinfection on common GC epidemiological markers (e.g.,G-17, PGI,PGII, and PGR) in healthy households, we assayed their levels duringH. pyloriinfection. As shown in Table 4, compared toH. pylori-negative groups, PGI levels were significantly higher and PGR was significantly lower inH. pyloritype I infections compared withH. pylori-negative groups (P< 0.05).However, there were no differences in G-17, PGII level, and PGR between type IIH. pyloriinfection andH. pylori-negative groups (P> 0.05). The levels of G-17, PGI, and PGII were significantly higher, and PGR was significantly lower inH. pylori-infected groups than inH. pylori-negative groups (P< 0.05).
DISCUSSION
H. pyloriinfection rates vary greatly among different countries and regions[24]. Although numerous studies have demonstrated that intrafamilial transmission is one of the most important sources ofH.pylorispread[2,18,24], there are few studies on the characteristics and pattern of family-basedH. pyloriinfection for disease prevention and control[9,25,26]. Therefore, focusing on family-basedH. pyloriinfection control and management would be a novel approach to reduce the related diseases and GC burden in a society.
In this work, we analyzedH. pyloriinfection status in a total of 772 individuals from 282 families in the Zhengzhou area. The results showed that despite an overall infection rate of only 54.27%, in as high as 87.23% of the surveyed families (246/282), there was at least 1 person infected, and in 27.07%(67/246) of these infected families, all family members were infected; further, 34.55% (85/246) of these families were infected with the same type of strain. Therefore, this study provides new evidence showing the importance of family-basedH. pyloriinfection control, which has substantive public health implications, and suggests that intrafamilial infection is a major source ofH. pyloritransmission. Thus,preventing intrafamilial spread is critical to eliminate the source of infection in order to prevent the related diseases.
Over the past several decades, the social and family structure in China has changed dramatically. The latest national statistics[27] (2020) revealed that the nation has a population of 1.41 billion and 492 million families with an average family size of 2.62 persons/family, which is much smaller than it was in 1990, when China had 1.13 billion citizens and 278.6 million families, with an average family size of 4.05 persons/family[27]. Due to the previous nationwide “one-children-per-family policy” (between 1982 and 2016), most families in China only have 1 child and two generations. As these children do not have siblings, transmission among siblings within a family unit did not appear to be the major route of transmission in the current analysis.H. pylorispread from parent or grandparent to children is probably more important for bacteria transmission. Although the current results provide a snapshot ofH. pyloriinfection status in the general public, a nationwide large-scale investigation is needed to explore the nationwide infection status and develop policies for related disease prevention.

Table 2 Parental infection and infection status of children and adolescents in 51 families

Table 3 Helicobacter pylori infection status in 51 families with children and other relatives
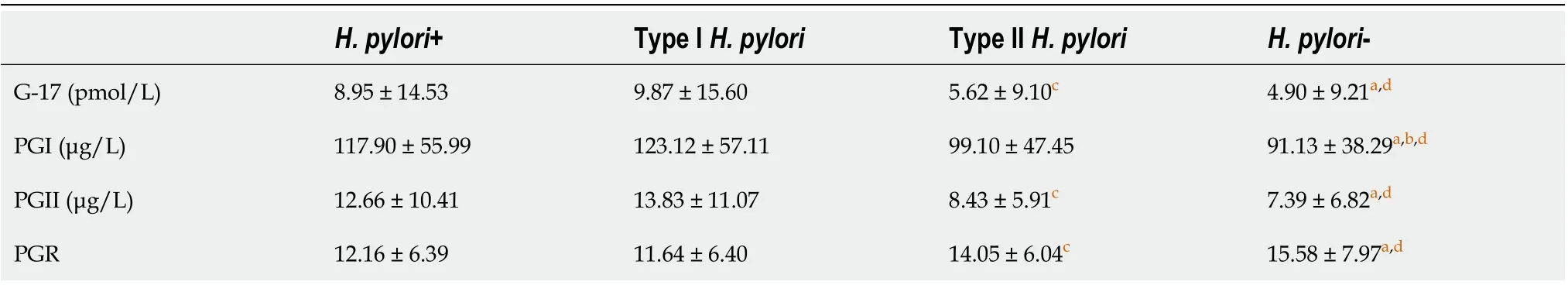
Table 4 Serum gastrin-17, pepsinogen I, pepsinogen II and pepsinogen I/II ratio levels in Helicobacter pylori-infected population
In single factor analysis, we noted that the highly infected age groups were between 31 years and 70 years, and infection rates increased along with age and duration of marriage. Annual household income, history of smoking, history of alcohol consumption, dining location, and family history of gastric disease or GC were not different between the infected and non-infected groups, but individuals with a higher education level showed a lower infection rate. A 2020 all-ages population-based crosssectional study[28] in Wuwei county in northwestern China showed that the prevalence ofH. pyloriinfection was closely associated with socioeconomic conditions, sanitary situations, dietary habits of the participants in the city. Boarding, eating at school, and drinking untreated water were main factors explaining the rising infection rate in junior-senior high school students. The results indicated that close contact is associated with increased infection risk. In addition, differences in geographic location, study population, lifestyle habit, and sanitary conditions are important factors that greatly contribute toH.pyloriinfection[22,29,30].
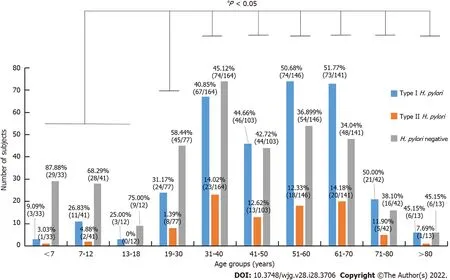
Figure 2 Helicobacter pylori infection status of 772 subjects in different age groups. Values above each column are the case number and its percentages in each specific age group. aP < 0.05 when Helicobacter pylori (H. pylori) infection rate in the age group of ≤ 18 yr was compared with other age groups.Type I H. pylori: H. pylori infection with type I strains; Type II H. pylori: H. pylori infection with type II strains.
Similar results were obtained from other regions, such as one community-based study[31] in Vietnam in 2017 on familial clustering in a multiple-generation population. The study showed that high monthly income, not regularly being fed chewed food, and being breastfed were protective factors againstH.pyloriinfection. Risk factors forH. pyloriinfection in children were not regularly handwashing after defecation,H. pylori-infected mother and grandfather, father’s occupation, mother’s education, and household size. Other factors such as number of siblings, infected fathers, regularly sharing a bed,group living, and antibiotic use were not found to be significant risk factors for infection.
H. pylori-infected family members are a possible source of continued transmission, which is an important health threat for uninfected family members[7,32,33]. A 2003 study[9] in the Stockholm area of Sweden using bacterial isolates for family-based DNA fingerprinting technique demonstrated a high proportion of shared strains among siblings, and between spouses, but also showed different strains in a portion of subjects (8%). Similar results were also reported by a 2009 study in Bangladesh[26]. In the current analysis, we found that among the 67 all member-infected households (Figure 1F), 68.66%(46/67) were infected withH. pyloritype I strains, 1.49% (1/67) were infected with type II strains, and 29.85% (20/67) had mixed type I and II strain infection. These data support the notion that intrafamilial transmission is the primary transmission route, but exogenous infection outside the family can occur,indicating that there may be multiple sources of transmission. In 244 couples comprising both husband and wife, 34.84% (85/244) of them were co-infected, and 68.24% (58/85) of households were infected with the same strain. With an increase of marriage duration, the infection rate of both husband and wife was significantly increased, suggesting that there was also cross-infection between husband and wife.
One unexpected result was that when both parents were infected, the infection rate of children was 23.08% (6/21), whereas when both parents were not infected, the infection rate of children was 18.18%(2/11), and the difference did not reach statistical significance. This result was slightly different from our previous concept that parentalH. pyloriinfection is an independent factor for infection in young children, and that mothers play an important role inH. pyloritransmission to their descendent[13-15,26]. However, the current results likely reflect the current infection status in this region, as the gradually improved living standard, sanitary condition, use of tap water, and avoiding chewing food to feed children over the past several decades in China’s urban family have resulted in a reduced infection rate.This is in line with the fact that the overallH. pyloriinfection rate is declining in most of China’s urban areas[3,22]. Another possibility is that the current cohort had relatively small numbers of children and adolescents, which may not have generated enough power for statistical significance. In addition, we were unable to perform bacterial DNA fingerprinting to confirm if the strains were identical, so the genotype that precise bacterial strains transmit within a family unit has yet to be determined. Future large-scale investigations are needed to confirm the current conclusion.

Figure 3 Helicobacter pylori infection status between 244 couples. Values above each column are the case number and percentages of each group. A:Helicobacter pylori (H. pylori) infection status of 244 couples; B: Type I and II H. pylori genotype status of the 117 couples with 1 spouse infected; C: H. pylori genotype and sex of 117 couples with 1 spouse infected; D: H. pylori genotype status in 85 couples with both spouses infected; E: Relationship between infection and marriage duration in 244 couples. The dashed line across the figure is the trendline of both spouses infected, r = 0.98. Non-infected: All members in a couple were not infected; one spouse infected: Only 1 in a couple was infected; Type I H. pylori: H. pylori infection with type I strains; Type II H. pylori: H. pylori infection with type II strains; Mixed infection: H. pylori infection with type I and type II strains.
Type IH. pyloristrain accounted for 78.28% of the infected population in this survey, similar to the results of our previous study in patients admitted to the hospital, which showed that type IH. pyloriinfection accounted for 72.4% (291/402) of the infected patients, and type II was 27.6%[21]. When compared toH. pylori-negative groups, G-17, PGI, and PGII levels were higher inH. pylori-infected groups. G-17, PGI, and PGII levels were significantly higher and PGR was significantly lower in the type IH. pylori-infected groups than inH. pylori-negative groups. The levels of G-17 and PG II were significantly higher, and PGR was significantly lower in type IH. pylori-infected groups than in type IIinfected groups. These results are in line with our previous endoscopy results from inpatients, and indicate that both type I and type IIH. pyloristrains increase G-17 Level, whereas only type IH. pyloriinfection affects PGI and PGII levels and the PGR in this geographic area[21].
Type I infection and reduced PGR are risk factors for gastric mucosal precancerous lesions and GC[19,21]. Therefore, these results have important clinical implications, as the abnormal expression of gastric markers was noted in a portion of individuals in healthy households infected withH. pyloribefore they sought medical examinations. It was unclear if this group of individuals had gastric mucosal precancerous lesions; thus, further examinations by endoscopy may be required for confirmation. The results of this study also provide another line of support showing that family-basedH. pyloriinfection control and management would be an important strategy for infection control and related disease prevention[3,18,34,35].
Although this pilot work provides novel information regarding family-basedH. pyloriinfection status, it had some limitations. First, the investigation was performed with a relatively small number of families, and in some groups, especially children and adolescents, the number of samples was not large enough to reach statistical significance; thus, future large-scale, multiple region sampling would provide more convincing data. Second, this was a cross-sectional study without data from endoscopy to confirmH. pyloriinfection-related disease status and pathological changes in gastric mucosa; therefore,some in-depth information was missing, and the work was performed in a Chinese setting, which may not be suitable to other areas. Third, type I and IIH. pylorigenotype concordance through antibody array analysis only provided a very general evaluation. As we did not obtain bacteria strain culture and DNA fingerprinting data,H. pyloriintrafamilial transmission was unable to be evaluated precisely to assess the heterogeneity ofH. pyloristrains within families. Thus, future studies are needed to evaluate theH. pyloriDNA fingerprinting pattern for more precise evaluation. Even with these limitations, this study provides novel points and information on family-basedH. pyloriinfection characteristics, which merit further large-scale exploration.
CONCLUSION
The current results provide snapshots of family-basedH. pyloriinfection status in central China. The high infection rate and coincidence of people infected withH. pyloriwithin a family unit indicate the status and pattern of intrafamilial transmission, which provide a novel option for family-basedH. pyloriinfection control and related disease prevention. The concept is applicable not only to Chinese residents but also to other communities with high infection rates.
ARTICLE HIGHLIGHTS


Research conclusions
H. pyloriinfection in healthy households is very common in central China, and poses an important health threat to uninfected family members. The intrafamilial infection status and patterns of transmission represent one important source ofH. pylorispread, and indicate the urgent need for family-based infection control and related disease prevention.
Research perspectives
The results of this study provide new information on family-basedH. pyloriinfection status in central China, and support the novel concept of family-basedH. pyloriinfection control and management. This notion is also likely to benefit otherH. pyloriand GC prevalent areas.
ACKNOWLEDGEMENTS
The authors are grateful to the staff of the Department of Gastroenterology and Hepatology, People’s Hospital of Zhengzhou University, for their valuable assistance in this work.
FOOTNOTES
Author contributions:Ding SZ, Wu G, Wang XM, Han SY and Sun PC conceived and designed the study; Yu XC,Shao QQ, Zhang C and Yu M searched and screened related literature; Yu XC, Shao QQ, Ma J, Lei L and Zhou Y performed the data extraction and quality assessment; Yu XC, Zhou Y, Chen WC, Zhang W, Fang XH and Zhu YZ analyzed the data; Yu XC and Ding SZ wrote the manuscript; all authors critically revised and approved the final version of the manuscript.
Supported byNational Natural Science Foundation of China, No. U1604174; Henan Provincial Government-Health and Family Planning Commission, No. 20170123 and No. SBGJ202002004; and Henan Provincial Government-Health and Family Planning Commission Research Innovative Talents Project, No. 51282.
Institutional review board statement:This study was reviewed and approved by the Ethics Committee of People’s Hospital of Zhengzhou University, 2021, No. 53.
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Xue-Chun Yu 0000-0003-4425-6019; Qiao-Qiao Shao 0000-0002-4900-9394; Jing Ma 0000-0003-1172-527X; Miao Yu 0000-0002-3587-2760; Chen Zhang 0000-0002-8810-6875; Lei Lei 0000-0002-6109-4229; Yang Zhou 0000-0002-5398-7764; Wen-Chao Chen 0000-0002-0642-0060; Wei Zhang 0000-0003-4620-6517; Xin-Hui Fang 0000-0001-5044-1320; Yuan-Zeng Zhu 0000-0001-9594-0916; Gang Wu 0000-0002-6627-0690; Xue-Mei Wang 0000-0003-0825-434X; Shuang-Yin Han 0000-0002-9907-7256; Pei-Chun Sun 0000-0001-7063-9537; Song-Ze Ding 0000-0002-4589-6942.
S-Editor:Fan JR
L-Editor:A
P-Editor:Li X
 World Journal of Gastroenterology2022年28期
World Journal of Gastroenterology2022年28期
- World Journal of Gastroenterology的其它文章
- Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma:Experimental and clinical scenarios
- Gut microbiota alteration and modulation in hepatitis B virus-related fibrosis and complications:Molecular mechanisms and therapeutic inventions
- Combination approaches in hepatocellular carcinoma: How systemic treatment can benefit candidates to locoregional modalities
- Update on endoscopic ultrasound-guided liver biopsy
- Non-alcoholic fatty liver disease-related hepatocellular carcinoma: Is there a role for immunotherapy?
- Potassium-competitive acid blockers and gastroesophageal reflux disease
