Modification of ash fusion behavior of high ash fusion temperature (AFT) coal by textile dyeing sludge addition and its mechanism
ZHAO Chao-yue , LI Feng-hai,,* , MA Ming-jie , LI Yang ,ZHAO Wei , ZHANG Xu-jing , FANG Yi-tian
(1. School of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo 454003, China;2. Department of Chemistry and Chemical Engineering, Heze University, Heze 274000, China;3. State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;4. Ten Mines of Pingdingshan Tianan Coal Co. Ltd., Pingdingshan 467021, China)
Abstract: To address the slagging problem during coal entrained-flow bed (EFB) gasification, the influences of textile dyeing sludge (TDS) addition on the fusing characteristics of high ash fusion temperature (AFT) coal were explored under a reducing atmosphere. And the change mechanisms were investigated by X-ray diffraction, Fourier Transform Infrared Spectroscopy (FT-IR) and FactSage calculation. The results showed that the flow temperature of high ash fusion temperature(AFT) coal decreased below 1380 °C when the TDS addition reached 20%-25%, which met the requirements of liquid-slag removal for EFB gasification. With the content of TDS increasing, the formations of low-melting minerals (e.g., hercynite,anorthite, and albite) decreased AFT. The bridging oxygen bonds of the network structure were destroyed by metal ions (e.g.,Fe2+, Ca2+, Na+), formation of much non-bridged oxygen (NBO) bonds relaxed the silicate network, thus decreasing the AFT.The formations of NBO bonds were confirmed by gradual decreases in the peak strengths of Si-O-Si and Si-O-Al bonds and intensified the vibration of Fe-O and Si-O-M ( M: Ca2+ or Na+) bonds. FactSage calculation results were in good agreement with the experimental ash fusion behavior.
Key words: ash fusion behaviors;textile dyeing sludge;high AFT coal;modification mechanisms
Abundant coal resource occupies the dominant position in Chinese total consumption[1,2]. Coal gasification technology is a broad application way in the coal industry because of its high efficiency and low pollution[3-5]. Different gasification methods (fixed-bed,fluidized-bed, and entrained-flow bed (EFB)) were adopted for the coals with different ash fusion characteristics[6]. EFB gasification was used widely because of its excellent technical performance, wide adaptability and high conversion ratio. Generally, it is required that the flow temperature (FT) for coal ash in the EFB gasification should be lower than 1380 °C and the viscosity of coal ash should be within 2.5-25 Pa·s under operating temperature. But high ash fusion temperature (AFT) coal (FT > 1500 °C) accounts for 57% for China[7], which will cause problems (slag blockage, fouling, deposition, etc.) during EFB gasification, thus shortening the service life of EFB gasifier[8]. The characteristics of fusibility and viscositytemperature of coal ash are the decisive factors to mitigate slag-hanging thickness and slag-discharging performance of EFB gasifier. The viscositytemperature behavior was critical for continuous operation and prediction of slag formation[9].Considering the costs and time, the coal AFT should be selected to mitigate slagging and fouling in the EFB gasifier[10,11]. The influence of coal ash composition on structural polymerization degree was fundamental to understanding slag properties[12]. Currently, researchers worldwide have carried out numerous examinations on the AFT regulation of coals. According to the acidic and alkaline components of coal ash, biomass[13,14], coal blending[15]and additive (e.g. phosphorus-based[16],calcium-based[17], iron-based[18]) were mainly used to modify AFT. However, straw is limited by seasons,and it contains high water content, which leads to high pretreatment cost[19]; coal with different ash content may be distributed unevenly, which may lead to high transportation costs on coal blending[20]; pure additive is relatively expensive in industrial practice[21]. In addition, the solid waste from the chemical industry with high content of additive compositions are economically attractive.
Recently, the combination of coals and solid waste to achieve rational utilization has attracted wide attention. Textile dyeing sludge (TDS) is produced from the treatment of textile dyeing water, which is one of the most polluted waste in current industrial process.The sludge contains a large amount of substances including organic pollutants, microbial pollutants and heavy metals. The traditional landfill and fertilizer are not in line with the current environmental protection treatment methods. How to reuse the waste in a circular economy has become an ever-increasing social concern.
In addition, as a kind of high ash waste biomass resource, TDS could be pyrolyzed and gasified to recover energy[22]. Ran et al.[23]found potential ecological risk index lowered than 14 after pyrolysis of TDS in the fluidized bed, thus reduced environmental toxicity of heavy metals in TDS. TDS could be copyrolyzed with coal[24], biomass (e.g., municipal sewage sludge[25], cattle manure[26], shaddock peel[27]and sugarcane bagasse[28]), which could not only reduce the pollution of heavy metals in TDS but also recovered energy of TDS. As a newly developed energy source,TDS gasification not only reduced the volume and eliminated the risk of heavy metals in TDS, but also converts the sludge into valuable gas[29]. Wang et al.[30]investigated the effect of CO2flow rate on the gasification efficiency of TDS, and the slag melted at high temperature improved the fixation of heavy metals.
Moreover, TDS contains high amount of basic components, which can be used to regulate the ash fusion behavior of high AFT coal[31]. Sludge could improve the coal ash fusion characteristics (i.e., coal AFT, slag flowability), mitigate the slagging problem of high AFT coal and utilize sludge energy reasonably.Folgueras et al.[32]used three types of sewage sludge to modify lignite AFT, and the reduced AFT closed to the eutectic at low temperature, and formed amorphous silicate and/or phosphate. Schwitalla et al[33]explored the ash melting behavior, element release and slag viscosity of coal blended with sewage sludge. This showed that sewage sludge functioned as the flux during co-gasification of high AFT coal.
These investigations provide the references for the AFT regulation during coal EFB gasification by sludge addition. However, due to the differences in ash composition and complicated interaction of the minerals, the AFT modification mechanisms vary with the types of coal and sludge. To the best of our knowledge, the investigation on the ash fusion characteristics of the high AFT coal by TDS with high iron content are rare. Moreover, the production of TDS is about 21 million tons annually in China over the past five years[34,35], which urgently needs to recovery and disposal of TDS. The co-gasification of coal and sludge is of great significance in cost, oxygen consumption,energy, environment, etc[33]. In this work, the ash fusibility of the high AFT coal, TDS, and their mixed ashes in reducing atmosphere was investigated. The variation mechanism was explored through X-ray diffraction (XRD), Fourier transform infrared (FT-IR) )and theoretical calculation (FactSage software)). It might provide some references to develop the cogasification technology of high AFT coal and sludge and to recover the sludge energy.
1 Experimental
1.1 Treatment of raw materials
High AFT coal was provided by the coal chemical laboratory, Heze University, and TDS was obtained from the Hongrui printing and dyeing factory. They were firstly dried in an oven at 105 °C for 24 h ), and then crushed to a particle size less than 0.2 mm. The samples were stored in the sample bag for analyses and ash preparations. The results of proximate (GB/T 212—2008) and ultimate analyses (GB/T 31391—2015) of the coal and TDS were shown in Table 1. The ash content in the TDS was high (58.63%), and its fixed carbon was extremely low.
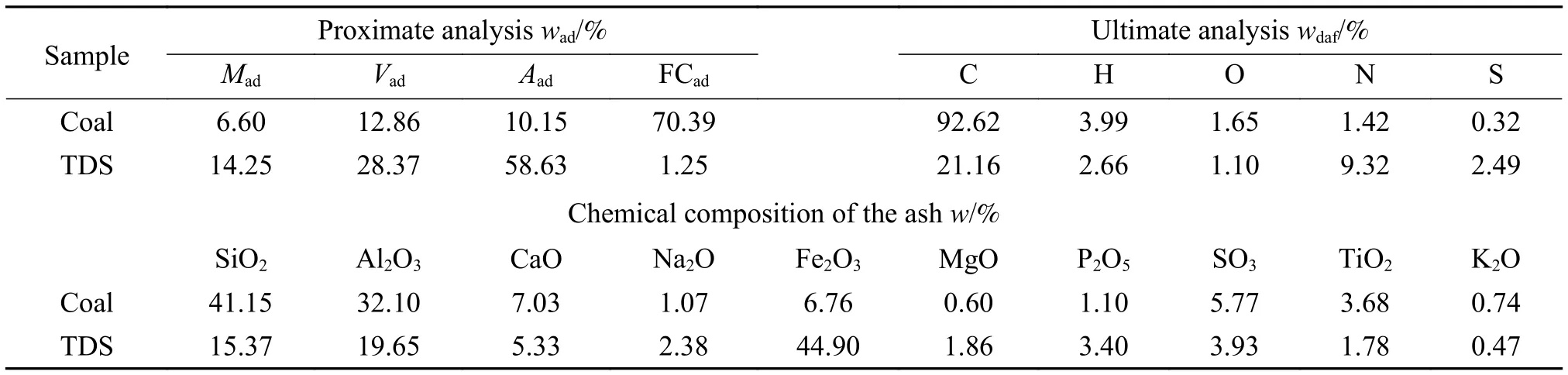
Table 1 Proximate and ultimate analyses of coal and TDS samples
1.2 Ash preparation
To simulate coal gasification of premixing fuel in industrial production, the TDS was added to the coal at mass ratios of 5%, 10%, 15%, 20% and 25% on a dried basis and the blends were named as C + 5%S, C +10%S, C + 15%S, C + 20%S and C + 25%S,respectively. Mixed ashes were prepared in a muffle furnace according to Chinese standard (GB/T 212—2008). The temperatures were heated from room temperature to 500 °C at 10 °C/min and kept at 500 °C for 30 min. Then, they were heated from 500 to 815 °C for another 30 min and kept at 815 °C for 2 h. Finally,the mixed ashes cooled to room temperature, removed,and stored for use.
The mixed ashes with different TDS proportions were analyzed with XRD, FT-IR, and Raman were prepared. About 2 g sample was charged into the small porcelain boat and transferred into an ALHR-2 AFT analyzer. The composition of minerals and the structure of slag at high temperature, quenching slag was used[36].To simulate the gasification atmosphere, the mixed ashes were first heated to 900 °C at 15 °C/min, and then increased at a rate of 5 °C/min under a reducing atmosphere (CO/CO2=6:4, volume ratio) was introduced into the AFT analyzer[37,38]. After heating to a certain temperature, the porcelain boat was quenched into the ice water mixture immediately to avoid crystal phase transition. The reduction time was not less than 1 h based on the heating rate, preparation temperature and other factors[39,40]. The quenched ashes were dried for 36 h, and ground to less than 0.075 mm for analysis.
1.3 Characteristics analyses of ashes
The four characteristic temperatures of the original ash (deformation temperature (DT), softening temperature (ST), hemisphere temperature (HT) and FT of coal and TDS ashes were tested on an ALHR-2 AFT analyzer (Ao-lian Co. Ltd., China) according to Chinese standard (GB/T 219—2008) and presented in Table 2. The DT of coal was 1481 °C, while the ST,HT and FT of coal were over 1500 °C. Therefore, the coal belonged to high AFT coal, whereas the AFT of TDS was low, whose FT was only 1321 °C.
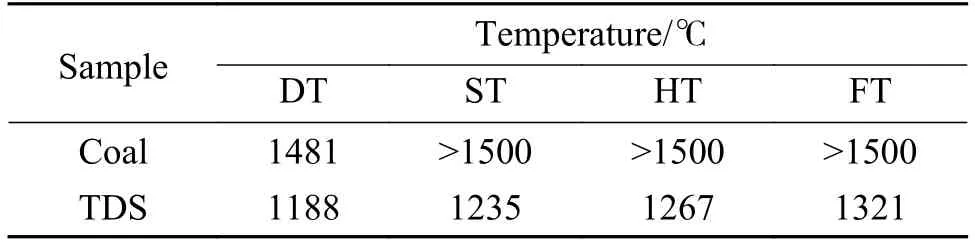
Table 2 AFTs of the coal and TDS
The ashes compositions were determined by X-ray fluorescence spectrometer (XRF, AxiosmAX, Netherlands)with less than 0.05% relative standard deviation, and presented in Table 1. Table 1 illustrated that the content of SiO2and Al2O3in coal was higher than 70%, while the content of Fe2O3in TDS reached 44.90%. The ash composition variation with the increasing TDS proportion was shown in Figure 1. Clearly, the acidic component content decreased gradually in acidic components, while that of the basic components increased.
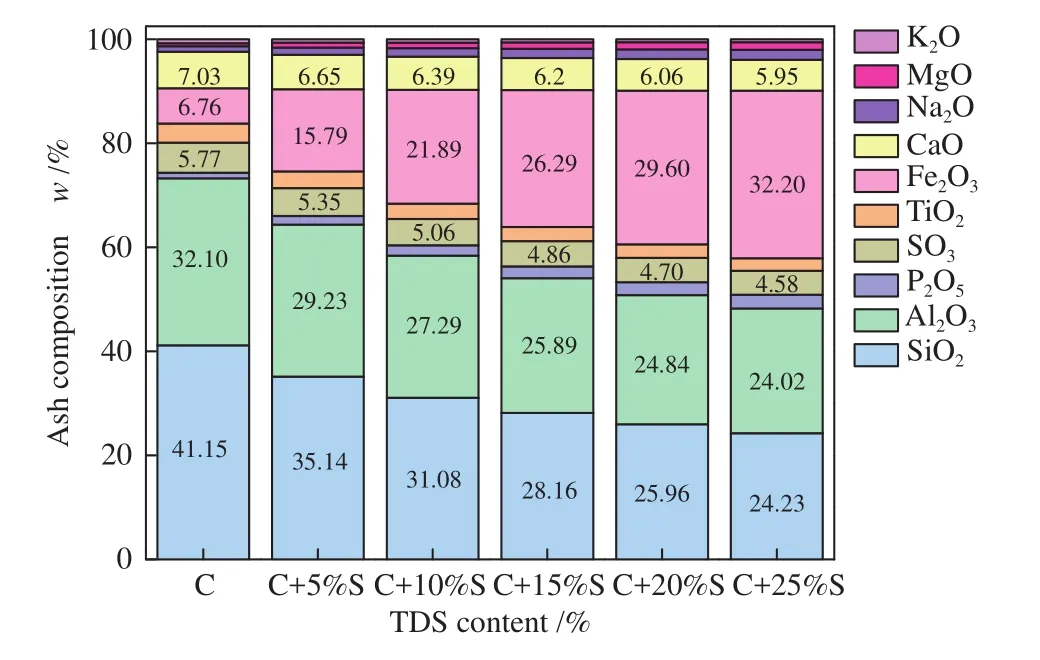
Figure 1 Main components of mixed ash with different mass ratios
1.4 Characterizations
The diffraction peaks of minerals in the ashes were detected by D/max-rB XRD (Rigaku, Japan). The analyzing condition were as follows. Working voltage was 40 kV, current 40 mA, CuKα radiation withKα1=0.15408 nm, the scanning speed 6(°)/min from 2θ=10°-70°, step size with 0.01°. MDI Jade 6.0 was used for further analysis.
The functional groups in the coal ashes were analyzed by FT-IR (Nicolet iS50, Thermo Scientific,America) at 4000-400 cm-1, the scanning number and resolution was 16 scans, and 8 cm-1, respectively.
The phase transformations and mineral compositions of coal-TDS ash were calculated by the FactSage software package (version 7.2 ) under t reducing atmosphere (CO/CO2, 6∶4, volume ratio) and atmospheric pressure. Oxides SiO2, Al2O3, K2O, CaO,Na2O, MgO, Fe2O3, SO3, P2O5and TiO2were inputted into the equilibrium module and calculated from 700 to 1500 °C with a step of 20 °C.
2 Results and discussion
2.1 AFT variation with increasing TDS mass ratio
The AFTs of the mixed ashes were analyzed by an AFT analyzer under a reducing atmosphere (CO/CO2=6∶4, volume ratio). Figure 2 showed the AFTs of mixed ashes decreased gradually with increasing TDS mass ratio. When 5% TDS was added, DT decreased significantly by 100 °C; an obvious turning point of decline in the FT curve at a ratio of 10%; when TDS mass ratio reached 20%, the FT of mixed ash dropped to 1372 °C. Therefore, addition of 20%-25% when TDS could meet the requirement of slag tapping for EFB gasifier.
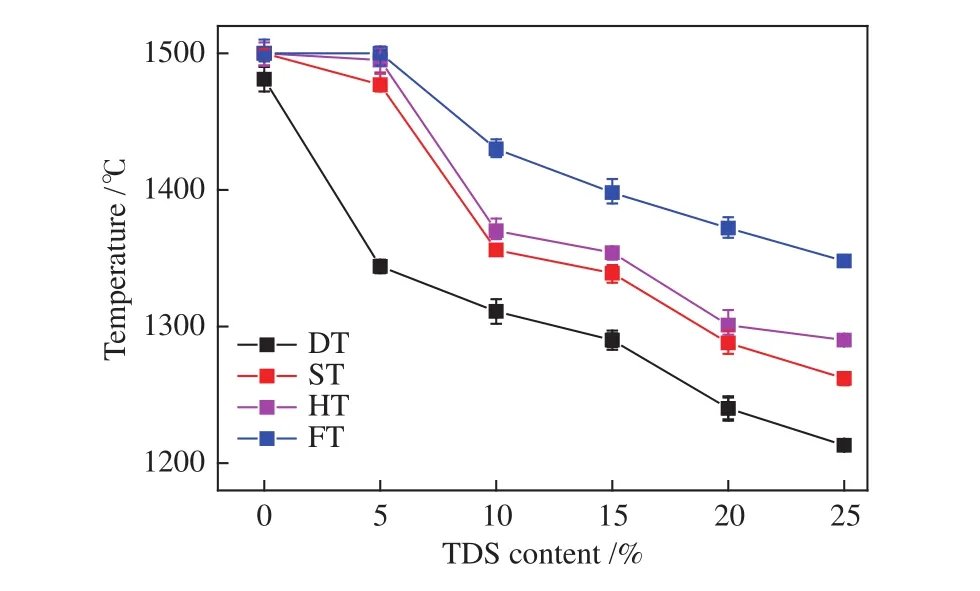
Figure 2 AFTs of mixed ashes with different mass ratios
In general, the acid oxides (SiO2, Al2O3,TiO2)components increase AFT while basic oxides (Fe2O3,MgO, CaO) decrease AFT[41]. Comparatively, the base to acid ratio B/A (B/A = (Fe2O3+ CaO + MgO + Na2O +K2O)/(SiO2+ Al2O3+ TiO2+ SO3+ P2O5)) better describes the ash fusion characteristics of coals and the mixtures[42,43]. AFT decreases as the B/A value becomes higher, and the AFT is the lowest when the B/A is about 1[6]. Figure 3 showed the B/A variation of mixed with different TDS ratios. The B/A ratio increased with the increasing TDS addition. The B/A value of blends containing 20%-25% TDS varied between 0.6 and 0.7,which predicted the increase of AFT.
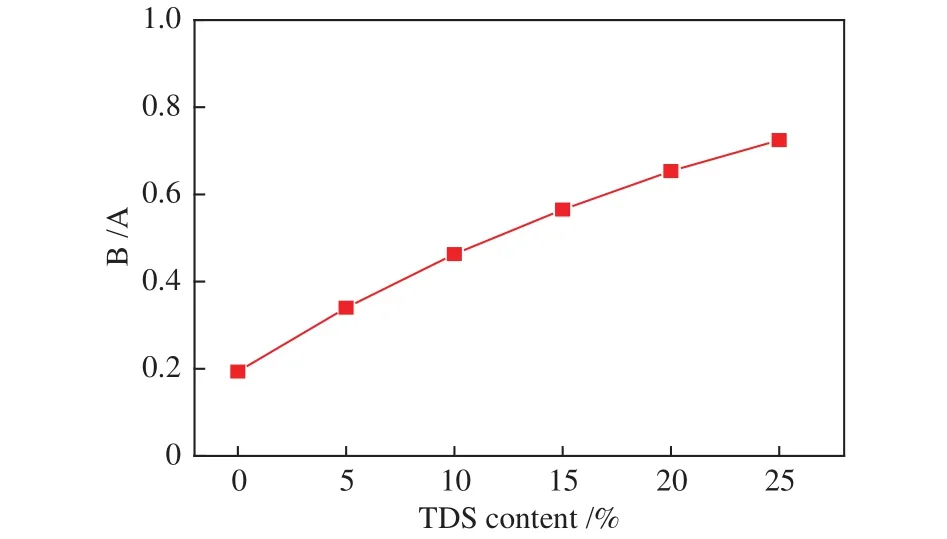
Figure 3 Acid-base ratio B/A based on different sludge ratio
2.2 Analysis of mineral evolution of mixed ash
To ensure complete collection of the hightemperature slag from the porcelain boat, the preparation temperature should be 100 °C less than FT,therefore, the ash was obtained at lower than 1300 °C[44].The quenched ashes of C + 20%S at different temperatures (from left to right: 900, 1000, 1100, 1200 and 1300 °C) were shown in Figure 4. With increasing temperatures, the volume of quenched ashes shrank gradually. Although the ash prepared at 1300 °C seemed slightly bigger in volume than that of 1100 and 1200 °C, it was melted and adhered to the porcelain boat. The colors of quenched ashes were turned gradually into the dark with increasing temperature. In addition, the quenched ash at 900 °C showed subdued red due to its high Fe2O3content (44. 9% ).

Figure 4 Picture of C+20%S with different proportions at 900-1300 °C (step size of 100 °C)
XRD facilitated the analysis of coal ash mineral evolution. Most researchers have discovered the role of low MP minerals formation (albite, anorthite, and hercynite, etc.) in regulating high AFT. Figure 5 showed the XRD diffraction patterns of coal and mixed ashes at different ratios with increasing temperatures.Figure 5(a) illustrated XRD patterns of coal ash at different temperatures, the main minerals of coal ash were quartz (SiO2) and anhydrite (CaSO4) at low temperatures (900 °C). The diffraction peak of quartz weakened gradually and even disappeared above 1000°C, but the diffraction peak of mullite (Al6Si2O13) and anorthite (CaAl2Si2O8) strengthened at 1000-1300 °C.It can be deduced that mullite (MP: 1860 °C) was formed by the combination of quartz and alumina(Al2O3). Anorthite (MP: 1550 °C) was synthesized by calcium oxide (CaO), quartz and alumina in reducing atmosphere. The main high MP dominant crystal of mullite and quartz raised the AFT of the coal.
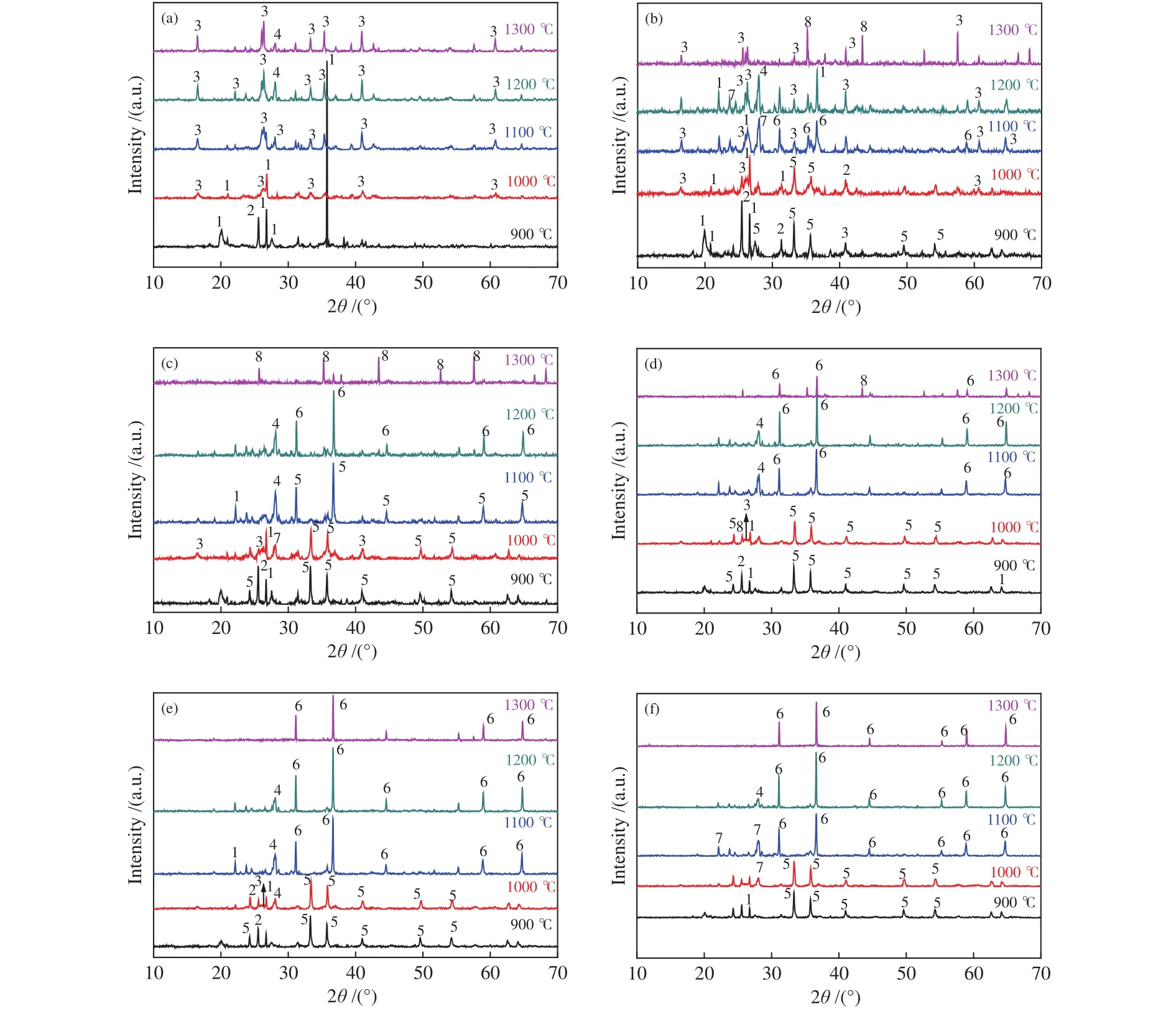
Figure 5 XRD patterns of coal ash and mixture ashes with different TDS ash mass ratios at different temperatures
As shown in Figure 5(b) to 5(f), the diffraction peak of hematite (Fe2O3) appeared at low temperatures with an increasing TDS addition. The diffraction peak of hercynite (FeAl2O4) was stronger than those of other minerals with increasing temperature. Because Fe3+was reduced to Fe2+in a reducing atmosphere, Fe2+combined with alumina to form hercynite, thus preventing the formation of mullite and weakening its diffraction peak. In addition, with the increasing TDS addition, Fe2+broke the Si-O bonds and decomposed the network structure, leading to the decrease of AFT for the mixed ash[39]. Iron promoted the ash/slag melting as reported in literature[18]. The strength of alumina diffraction peak increased obviously seen from Figure 5(b) and 5(c) at 1300 °C, which resulted from the low mullite content and the low alkaline contents of ashes from C + 5%S and C + 10%S. Therefore, the relative high MP alumina precipitated from the mixed ash, thus, the decrease of FT was not obvious when the TDS from 0 to 5%.
Compared to Figure 5(b) and 5(c), the diffraction peak of albite (NaAlSi3O8) was the stronger in Figure 5(f).The low MP albite (MP: 1100 °C) was formed by the reaction of quartz, sodium oxide and alumina, thus leading to the decrease in FT. In addition, there were diffraction peaks of one or two minerals in Figure 5(a)to 5(f) at 1300 °C. Hence, it could be deduced that the crystalline minerals might be transformed into amorphous minerals with the increasing temperature.
With the increasing TDS mass ratio, the basic components of mixed ashes increased. With the rising temperature, metal ions (i.e. Fe2+, Ca2+and Na+)destroyed the Si-O-Si bond or Si-O-Al, the stable structure of silicon tetrahedron or silicon-aluminum tetrahedron depolymerized and relaxed. Thus, the diffraction peak of mullite weakened and disappeared.With increasing TDS mass ratio, lots of hercynite diffraction peaks appeared, which could explain that the fluxing effect of Fe2+on coal AFTs was enhanced further. The alkaline components in the sludge reacted with SiO2and Al2O3and generated a large number of crystalline substances (e.g., hercynite, anorthite, and albite). This could promote the formation of lowtemperature eutectic and the transformation into an amorphous phase with the rising temperature. The mixed ashes with different TDS ratios with higher temperature, the amorphous or low MP crystals minerals increased, which decreased its AFT. The following reactions possibly occurred during the heating of ash samples.
Figure 6 illustrated the XRD patterns of the three mixed ashes with different mass ratios of TDS ash at 900, 1000, 1100, 1200 and 1300 °C. As shown in Figure 6(a) to 6(e), the content of minerals reached the lowest value in the mixed ash with rising temperatures.For instances, comparing Figure 6(a) and 6(e), at 900 °C,the C + 25%S was mainly composed of quartz,anhydrite, and hematite, while only the diffraction peaks of hercynite were found when the temperature was 1300 °C. The diffraction peak of hematite increased gradually with the addition of TDS as shown in Figure 6(a) and 6(b). As displayed in Figure 6(b),6(c) and 6(d), under the background of strong diffraction peaks of hematite and hercynite, the weak diffraction peaks of feldspar minerals (anorthite, albite,etc.) could also be observed. In addition, the diffraction peak of anorthite was stronger than that of albite as shown in Figure 6(c) and 6(d) and low MP feldspar minerals disappeared at 1300 °C, resulting in the AFT decrease. This might explain the decreased FT to less than 1380 °C when TDS ash content was 20%-25%.
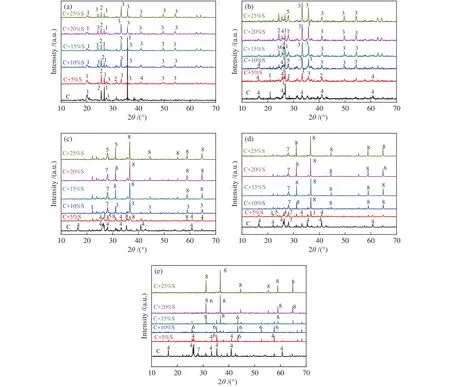
Figure 6 XRD patterns of mixed ashes with different TDS ash mass ratios at 900 to 1300 °C
2.3 Structure analysis of silicate melt
The structure of silicate was usually expressed by Qn,nwas the number of bridging oxygen (BO,Si-O-Si,n= 0, 1, 2, 3, 4). The tetrahedral structure of Q0-Q4was also presented in Figure 7. The tetrahedron skeleton of SiO2was represented by Q4; The planar layered structure of [Si2O5]2-was represented as Q3;[SiO3]2-, [Si2O7]6-, [SiO4]4-was represented as Q2, Q1and Q0[45,46], respectively. In addition, the oxygen bond of Si-O-M(Al, Ca, Fe and Na) was classified as nonbridging oxygen (NBO). An increasing number of the stable network structure of silicate with the increase of BO, which led to higher AFT. The decrease in the AFT with TDS addition can be deduced in this way. Firstly,with increasing TDS ratio, the basic components in the mixed ashes increased. Then metal ions of basic components destroyed the BO bond in the polymers, and more NBO bonds formed, which led to the decrease in Q3and Q4, but the increase in Q0, Q1,and Q2. Finally,these network structures of the minerals gradually depolymerized, and formed of new minerals with loose structures. In other words, the high MP mineral crystals were transformed gradually into low MP minerals or amorphous minerals and reduced AFT of the mixture.
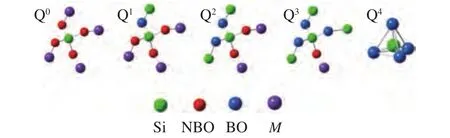
Figure 7 Five kinds of Si-O tetrahedral structure diagrams
2.4 Analysis of the mixture coal ashes using FT-IR spectroscopy and structure of silicate melts
FT-IR analyze the minerals in coal ash, revealing anions in inorganic salts, and coordination compounds[47,48]. In addition, FT-IR could be used to analyze both crystals and amorphous minerals[49].
Figure 8 compared the spectra of C + 25%S at 900-1300 °C. To avoid the interference of -OH, the range in 2000-400 cm-1was analyzed[50]. The presence of the Si-O-Si stretching vibrations in these spectra occurred at 700-800 cm-1and around 1040 cm-1. And Si-O-Si has an obvious absorption peak at 1100-1200 °C,Combined with the XRD analysis results, which had other silicates in coal ash. In addition, the absorption peak at 700-800 cm-1was designated to the Si-O-Si bending vibration in alkali feldspar. The aluminosilicate band at about 915 cm-1could be associated with vibrations of Al-O or Al-OH; the bands in the region 460 cm-1were commonly assigned to bending vibrations O-Si-O presented in silicate tetrahedra, and the Si-O-Al vibrations were around 430 and 667 cm-1. The band at about 560 cm-1could be associated with vibrations of Fe-O for octahedral coordination of iron, the sharp peak around band 560 cm-1is attributed to Fe2O3. As the temperature increased, the fine structures of aluminosilicate during 1000-400 cm-1weakened, while the hercynite(FeAlO4) appeared. With content of hercynite increased, the bray center of the band would shifted to longer wavelength. The Fe-O bond shifted and split into peaks with the increasing temperature, which lengthened the Si-O bond and destroyed the stability of the network structure, thus leading to the decrease in AFT. These results obtained from FT-IR were consistent with the XRD results that with the increasing temperature, the intensity of all peak intensities was weakened.
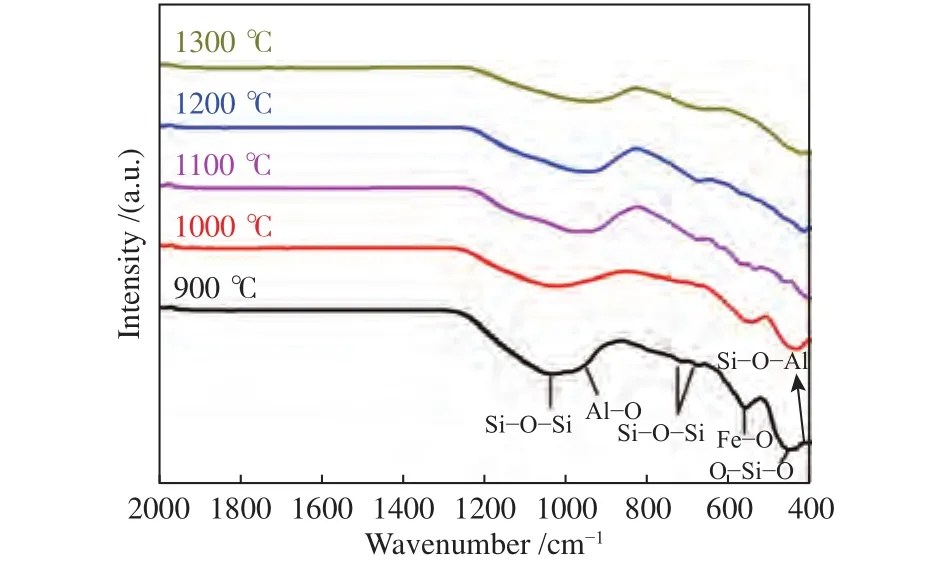
Figure 8 FT-IR spectra of C+25% S at 900-1300 °C
2.5 Thermodynamic equilibrium calculations and analysis by FactSage software
FactSage was used to explain AFT variation mechanisms through simulating the mineral evolution[51,52]. Figure 9 illustrated phase assemblagetemperature curves of coal ashes and their mixtures. A large number of minerals melted gradually into the liquid phase with the increasing temperature. A large amount of quartz, titania, mullite, feldspar (albite,anorthite, and potassium feldspar), and cordierite was formed in Figure 9(a). However, the liquid phase began to increase substantially at 1150 °C, and all minerals except for mullite melted at 1350 °C. The temperature at which all minerals have been changed into the liquid phase (tliq) of ash was higher than 1500 °C[53], mullite and anorthite contents were higher at 1300 °C, which were consistent with XRD results and increased the AFT.
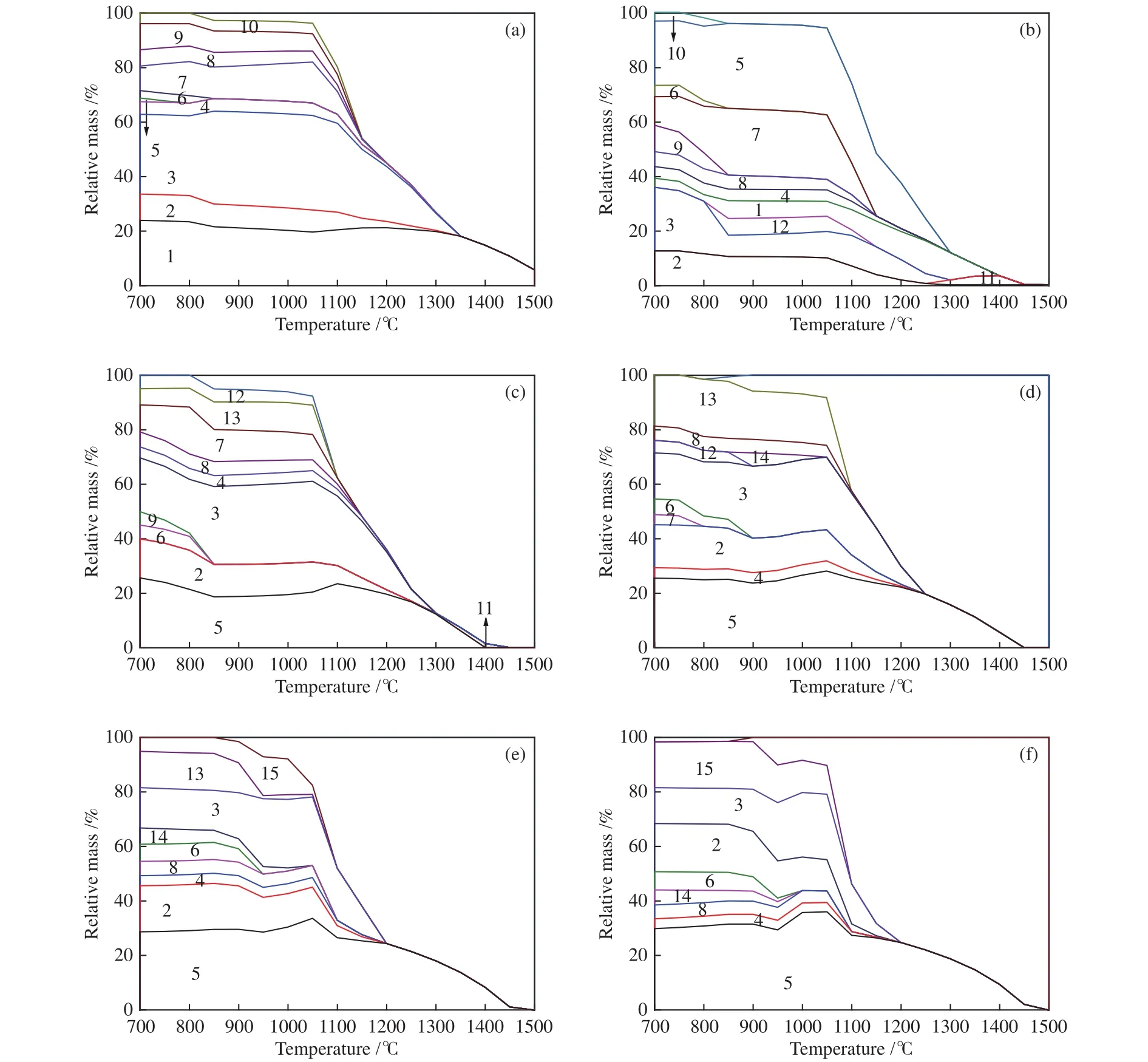
Figure 9 Phase assemblage-temperature curves of coal ashes and mixture ash
With the increasing TDS addition, the mineral content of mixed ash changed obviously at high temperatures. For example, Figure 9(b) showed phase assemblage-temperature curves of C + 5%S. Compared with Figure 9(a), the content of mullite, quartz and titania decreased, massive hercynite formed, and alumina crystal appeared. In addition,tliqshowed declining trend, and cordierite increased at 900 °C and decreased the AFT.
As shown from Figure 9(b) to 9(f), the content of feldspar minerals and hercynite increased, mullite and cordierite decreased gradually with increasing TDS addition. The minerals calculated by simulation were almost consistent with XRD test results at 1300 °C.However, the ilmenite (MP: 1540 °C) generated at 850 °C when TDS was at 5%, resulting from the reaction of titania (MP: 1840 °C) and hematite. When TDS was 15%, some ilmenite reacted with hematite to form titanium spinel (MP: 797 °C), which completely melted at 1050 °C. This contributed to the decrease of AFT. Cordierite, could transform from protopyroxene to fayalite. These changes prove that the alkaline components in TDS could effectively prevent the formation of high MP minerals and promoted the formation of minerals with low MP. These changes could not be shown in XRD, because the thermodynamic calculation with FactSage was performed at ideal conditions and other factors (e.g., kinetic limitations,mass transport, equilibrium temperature, and reaction time) of the ash-fusion process were not considered,and caused the result deviation[54,55]of AFT.
3 Conclusions
In this work, the effect of TDS on coal AFT was investigated. The results can be briefly summarized:
With the content of TDS increasing, the AFTs of coal mixtures decreased. When the TDS content increased up to 20%-25%, the FT decreased below 1380 °C,which met liquid slag removal requirements for EFB gasification.
The high AFT coal was mainly caused by quartz,mullite, cordierite. The TDS with high iron content easily change the proportion of mixture components.With the increasing TDS, a large number of low MP minerals (e.g., hercynite, anorthite, and albite) formed and decreased the AFTs of the mixtures.
With increasing TDS mass ratio, the vibration of hercynite Fe-O and alkali feldspar Si-O bonds was detected from FT-IR, and the peak strength of Si-O-Si and Si-O-Al bonds decreased gradually, which led to the relaxation of stable silicate network structures,which led to decreases in the mixtures AFTs as well astliq.

