Do people with low back pain walk differently?A systematic review and meta-analysis
Jo Armour Smith,Heidi Stert,Jennifer J.Bgwell,Hsing-Ling Teng,Vernie Wde,Szu-Ping Lee
a Department of Physical Therapy,Chapman University,Irvine,CA 92618,USA
b Department of Physical Therapy,California State University,Long Beach,CA 90840,USA
c Department of Physical Therapy,University of Nevada,Las Vegas,NV 89154,USA
Abstract Background: The biomechanics of the trunk and lower limbs during walking and running gait are frequently assessed in individuals with low back pain(LBP).Despite substantial research,it is still unclear whether consistent and generalizable changes in walking or running gait occur in association with LBP.The purpose of this systematic review was to identify whether there are differences in biomechanics during walking and running gait in individuals with acute and persistent LBP compared with back-healthy controls.Methods:A search was conducted in PubMed,CINAHL,SPORTDiscus,and PsycINFO in June 2019 and was repeated in December 2020.Studies were included if they reported biomechanical characteristics of individuals with and without LBP during steady-state or perturbed walking and running.Biomechanical data included spatiotemporal,kinematic,kinetic,and electromyography variables.The reporting quality and potential for bias of each study was assessed. Data were pooled where possible to compare the standardized mean differences (SMD) between back pain and back-healthy control groups.Results: Ninety-seven studies were included and reviewed. Two studies investigated acute pain and the rest investigated persistent pain. Nine studies investigated running gait.Of the studies,20%had high reporting quality/low risk of bias.In comparison with back-healthy controls,individuals with persistent LBP walked slower (SMD=-0.59, 95% confidence interval (95%CI): -0.77 to -0.42)) and with shorter stride length(SMD=-0.38,95%CI:-0.60 to-0.16).There were no differences in the amplitude of motion in the thoracic or lumbar spine,pelvis,or hips in individuals with LBP.During walking,coordination of motion between the thorax and the lumbar spine/pelvis was significantly more in-phase in the persistent LBP groups(SMD=-0.60,95%CI:-0.90 to-0.30),and individuals with persistent LBP exhibited greater amplitude of activation in the paraspinal muscles(SMD=0.52,95%CI:0.23-0.80).There were no consistent differences in running biomechanics between groups.Conclusion: There is moderate-to-strong evidence that individuals with persistent LBP demonstrate differences in walking gait compared to back-healthy controls.
Keywords: Biomechanics;Low back pain;Running;Walking
1. Introduction
The experience of acute low back pain(LBP)is almost universal,with up to 80%of people experiencing an acute episode of LBP in their lifetimes.1However, the greatest burden to individuals and society comes from the pain and disability associated with persistent LBP.2,3Persistent LBP is characterized by symptoms lasting or recurring over months and years.4Recently, researchers have differentiated between persistent LBP that is experienced almost every day (chronic LBP) and persistent LBP that follows a more episodic pattern (recurrent LBP).5,6Although there are attempts to standardize definitions for recurrent and chronic patterns of persistent LBP,5,7,8these definitions have not yet been widely adopted.
Walking and running gaits are frequently assessed in individuals with acute and persistent LBP during clinical evaluations and as part of observational and interventional research.Adaptations in gait biomechanics in individuals with LBP may include changes in spatiotemporal characteristics like speed or step length, kinematic characteristics like joint/segmental motion or coordination between joints/segments, kinetic characteristics like joint forces and torques,and electromyography(EMG) characteristics like amplitude and timing of muscle activation. The magnitude of trunk motion and joint loading during gait is relatively low.9-11Despite this,due to the repetitive, cyclical nature of walking and running, adverse loading over time in response to changes in gait mechanics in the trunk or lower limbs may contribute to the onset,recurrence,or persistence of LBP symptoms.12Conversely, changes in gait mechanics in individuals with LBP may represent adaptive strategies used to mitigate the loading associated with locomotion and to protect pain-producing tissues. Recent work has highlighted the inconsistent evidence for biomechanical differences during tasks such as gait in individuals with persistent LBP compared to pain-free controls.13In part, this is due to small sample sizes in individual studies.It is also potentially a result of heterogeneity in clinical back pain presentations.13There is currently little consensus on how to effectively subgroup individuals with back pain based on their clinical presentation or movement characteristics. Therefore, it is critical to determine whether there are biomechanical traits that generalize across individuals with LBP during important functional activities such as walking and running. This will facilitate development of appropriate rehabilitation strategies for back pain management.Of the 2 recent reviews investigating gait in individuals with LBP,14,15neither performed a quantitative synthesis of the results,and only one included EMG data.15
The aims of this systematic review, therefore, were to review and quantitatively synthesize evidence for differences in walking and running gait biomechanics in individuals with acute and persistent LBP compared to back-healthy controls.
2. Methods
This review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA). The protocol was registered in the International Prospective Register of Systematic Reviews(PROSPERO:CRD42018078746).
2.1. Search strategy
The search was conducted in the PubMed, CINAHL,SPORTDiscus, and PsycINFO databases without date restriction.The search terms combined keywords or MeSH terms for gait AND low back pain and were tailored for specific databases. The search strategy is shown in full in the supplementary materials.After removal of duplicates,2 authors(JAS and VW) double-screened titles and abstracts based on the inclusion criteria (described in detail below). Full text manuscripts of remaining articles were then retrieved and additionally screened.Reference lists from retrieved articles,previous systematic reviews, and NCBI citation alerts were also checked.The search was initially conducted in June 2019.In September 2020, the search was repeated using identical search terms in the same databases to identify studies published since the original search.
2.2. Inclusion criteria
Included studies were peer reviewed, original research works that were available in English. Eligible studies compared gait variables between a group of individuals with acute or persistent LBP and a group of back-healthy controls.Study types included case-control, cross-sectional, and prospective cohort studies. Studies had to include objectively quantified gait data in 1 or more of the following categories: (a) spatiotemporal data(speed,distance,step and stride characteristics);(b)kinematic data(peak excursion or total range of motion in the thoracic or lumbar spine, pelvis, or lower extremities or coordination in kinematics between 2 or more joints/segments); (c) kinetic data (net joint moments, joint impulse,work,power); (d)ground reaction force data(vertical or horizontal ground reaction forces); (e) EMG (amplitude or timing of activation in the trunk or lower extremity musculature).Gait paradigms included overground and treadmill steady-state walking and running as well as walking under dual-task conditions involving additional mechanical or cognitive tasks. In studies that included pre- and post-intervention data, only the pre-intervention outcomes were included in this review. Studies were excluded if they were conference abstracts, case reports, dissertations, or review articles, if they did not report comparisons between individuals with and without LBP, or if LBP was experimentally induced in previously asymptomatic participants.
2.3. Study quality assessment
The quality of the reporting of the included studies was assessed along with risk of bias using a 16-criteria checklist16-19(Table 1). A positive score was given for each criterion met by the study.A total quality score was calculated as the sum of all positive scores from Criteria 3 through 16 relevant to the study type (8, 12, and 9 for cross-sectional, casecontrol, and prospective cohort studies, respectively), and a percentage of the possible maximum score was calculated.Each study was independently scored by 2 authors(JAS scored all studies,and SPL,JB,and HLT each scored one third of the studies).Where there was a difference in scores,the 2 scoring authors discussed the criteria for which the scoring discrepancy occurred and reached a consensus on a final score.Studies were designated as having high reporting quality if they scored 50%or more.18
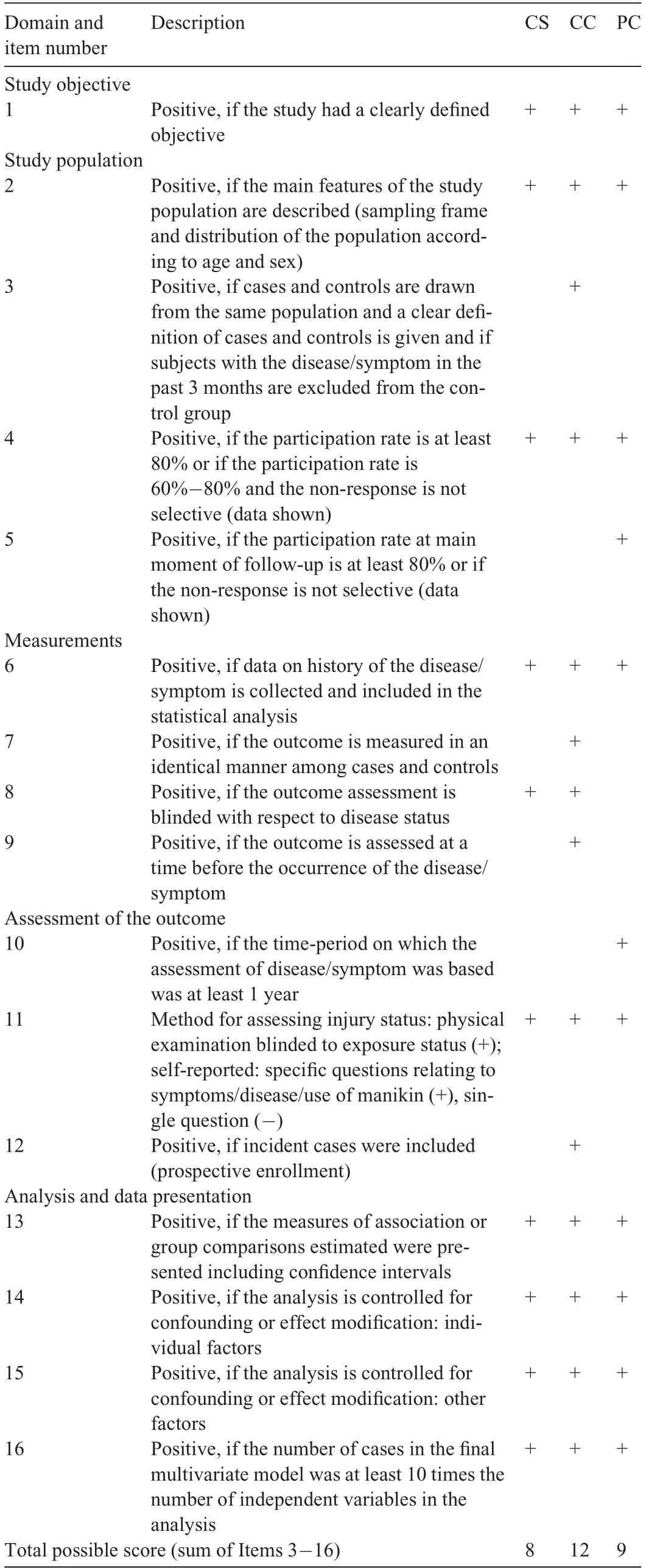
Table 1 Checklist for assessment methodological quality for cross sectional,case-control,and prospective cohort study designs.
2.4. Data extraction
The following data were extracted from all eligible studies:study design, sample size, study inclusion/exclusion criteria,study population demographic characteristics, any additional metrics characterizing the LBP cohort, and the biomechanical outcomes. Data were extracted and double-checked by 3 authors(HS,VW,JAS).
Data were synthesized qualitatively if there were at least 2 articles with different populations that reported equivalent outcomes. Data were pooled for meta-analysis for outcomes in which there were equivalent data available from more than 3studies with different cohorts. Where there were multiple articles from the same author groups,the demographic characteristics of the study populations were checked to ensure that outcomes from the same population were not double-counted in the meta-analysis. Where necessary, authors of studies that did not report group means/standard deviations (SDs) were contacted to provide these data. Group averages/SDs were calculated from confidence intervals, standard errors, effect sizes, and median and interquartile ranges as needed using standard methods. For the pooled analyses, group averages/SDs from LBP or male/female sub-groups reported in some studies were combined.20A random effects model was used to calculate standardized mean differences (SMD) and 95%confidence intervals (95%CIs) for the SMD between the LBP and back-healthy groups (Review Manager Version 5.4.1;Cochrane Training,London,UK).21Effect sizes of ≥0.8 were considered large,and those from 0.5 to <0.80 were considered moderate.Group differences were significant if the p value for the test of overall effect was <0.05. The mean difference between groups in the original measurement units was calculated for those significant group comparisons where all studies used the same units of measurement, or it was calculated as a percentage of the control group value for outcomes with varying units of measurement. The heterogeneity in the results within the pooled analyses was evaluated for each outcome using the χ2test to detect significant heterogeneity and the I2statistic to quantify the heterogeneity,with I2greater than 0.75 indicative of substantial heterogeneity.22Studies were excluded from pooled data analyses if the 95%CI for the group effect in that study did not overlap with the confidence interval for the SMD effect and if the removal of the outlier study did not affect the direction or significance of the pooled effect.22
The level of evidence for the pooled analyses was defined using the following criteria:23,24
1) Strong evidence—homogenous data (χ2p ≥0.05) pooled from studies of which at least 2 were high quality;
2) Moderate evidence—either heterogeneous data (χ2p <0.05) pooled from studies of which one was high quality,or homogenous data (χ2p ≥0.05) from lower quality studies;
3) Limited evidence—heterogeneous data(χ2p <0.05)from lower quality studies.23,24
3. Results
The initial search identified 3272 articles (Fig. 1). Following the removal of duplicates,2202 articles were available for further evaluation. An additional 7 articles were identified manually and during the repeat search in 2020. The abstracts and titles of 2209 articles were screened. Lastly, 124 full-text articles were retrieved and assessed for inclusion.A total of 97 articles were included.Attempts were made to contact authors of 22 studies that did not present group data for one or more variables of interest,and responses were received for 7 studies.Median score for reporting quality/risk of bias was 33%(range 13%-89%,Supplementary materials). Only 19 out of 97 studies received high scores for quality (scoring >50%,Table 2). The range of scores is similar to those reported in other systematic reviews investigating factors associated with musculoskeletal disorders that used the same quality assessment tool.18,19Most studies did not report the participation rate; therefore, the potential influence of non-response was unclear. Very few studies reported blinding of researchers or presented confidence intervals in their analyses. Fifty-five articles were included in the quantitative meta-analysis.
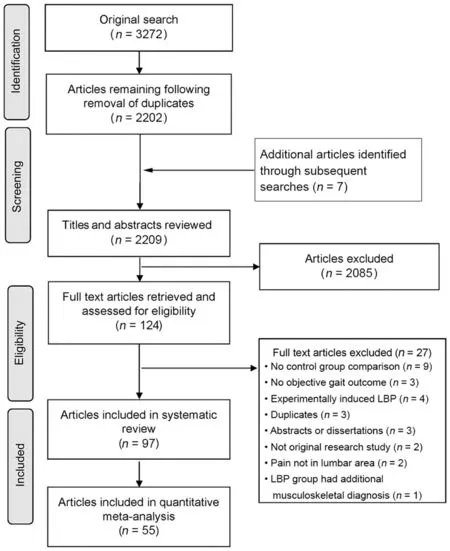
Fig.1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines(PRISMA)flow diagram summarizing study selection processes.
3.1. Participant numbers and demographics
Of the included articles,93 were case-control,3 were crosssectional,25-27and 1 was a prospective cohort study.28Nine studies investigated running gait.27,29-36Of the 97 included articles, there were 83 different study cohorts resulting in a total of 3364 individuals with ongoing LBP and 2315 backhealthy controls.Of the studies that reported participants’sex,the LBP groups included 767 males and 2075 females,and the control groups included 653 males and 961 females.The range of mean age was 21.4-73.6 years for the LBP groups and 18.7-73.5 years for the control groups.Participants with LBP were described as having back pain, nonspecific or idiopathic LBP,chronic LBP,or recurrent LBP.However,as the criteria for these categories varied between studies, we did not subgroup participants based on these descriptors. Persistent LBP was commonly defined based on duration of symptoms, with minimum duration varying from 6 weeks to 1 year and the most frequent criterion being 3 months(Table 2).Two studies included participants described as having acute LBP, defined as symptom duration of less than seven days, who were retested once symptoms had resolved.37,38Four studies sharing the same full or partial cohort included a separate group with a history of resolved LBP.29,30,33,34Nineteen studies25,39-41,46,48,49,51,68,78,81,92,95,97,100,121,123,124,126quantified minimum or maximum pain severity as part of their inclusion criteria (Table 2). Seven studies required LBP to be severe enough to impact function.32,36,39-43Fourteen studies used pain frequency as a measure to define chronicity or recurrence of episodes.32,36,39-41,44-52Location of LBP was defined in 16 studies, with location predominantly described as occurring below the costal margin and above the gluteal folds(Table 2).
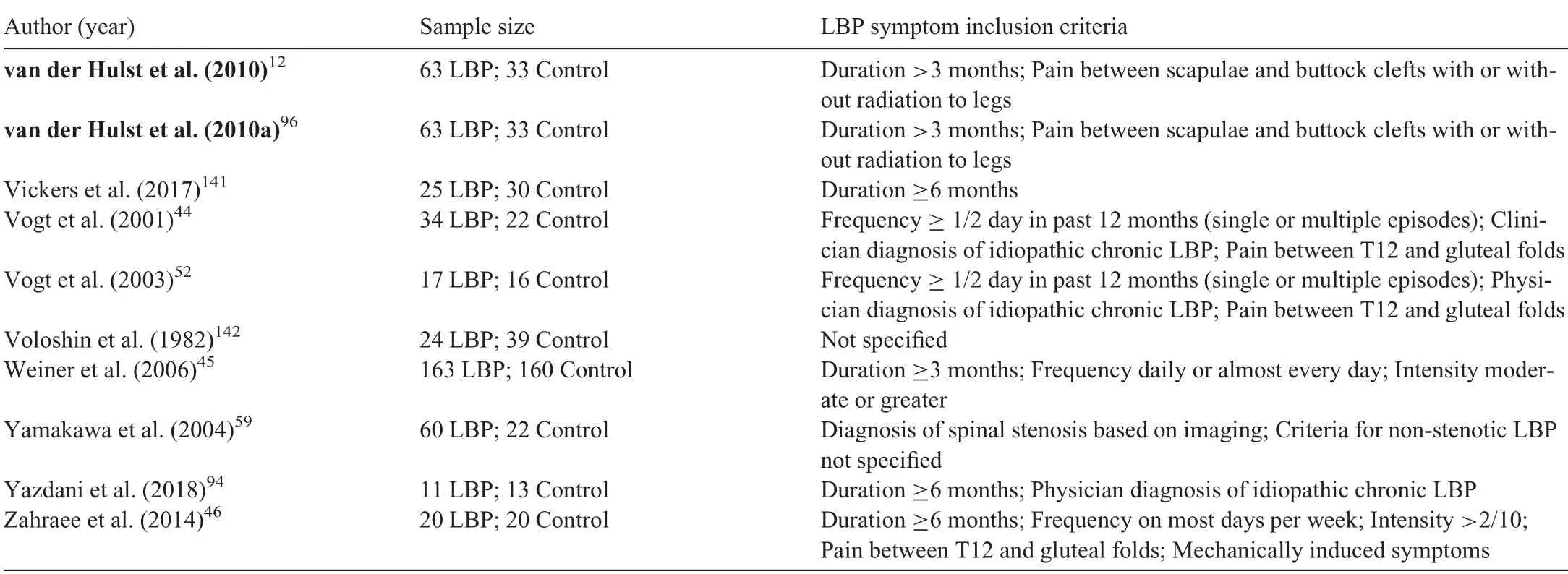
Table 2(Continued)
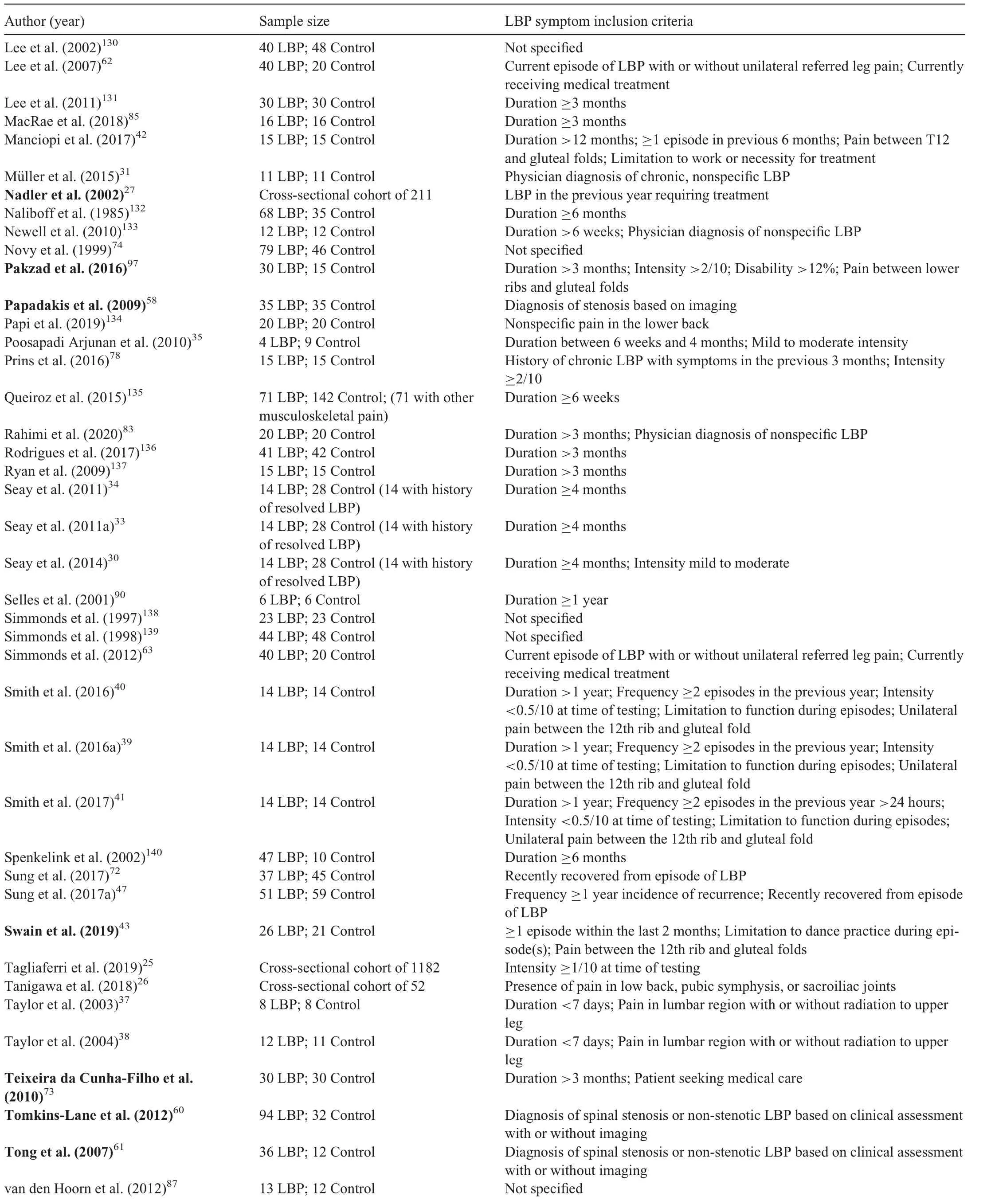
Table 2(Continued)
Fourteen of the studies48,49,53-64sub-grouped individuals with LBP based on pathoanatomical diagnoses. Among thesearticles, 32 participants were diagnosed with lumbar disc herniation.53-57One hundred and eight participants were described as having spinal stenosis.58-61Forty participants had LBP with pain referred to the lower limbs.62,63Thirty-nine participants were described as having radiculopathy.48,49Lastly,1 article recruited a pain group with LBP diagnosed as stenosis, degenerative instability, or disc herniation.64For the control groups, most studies required controls to be healthy,pain-free individuals, and they were frequently matched by sex and age to the experimental group. Exclusion criteria varied widely across studies. Eighty studies explicitly excluded participants whose LBP was associated with known pathoanatomical diagnoses such as radiculopathy and participants who had a history of spinal surgery.
3.2. Walking gait
Due to limited available evidence, data were not synthesized or pooled from the 2 articles reporting walking gait in individuals with acute LBP. All of the following analyses are for study cohorts with persistent LBP.Findings from the metaanalysis for persistent LBP, including metrics of strength of evidence,are summarized in Table 3.
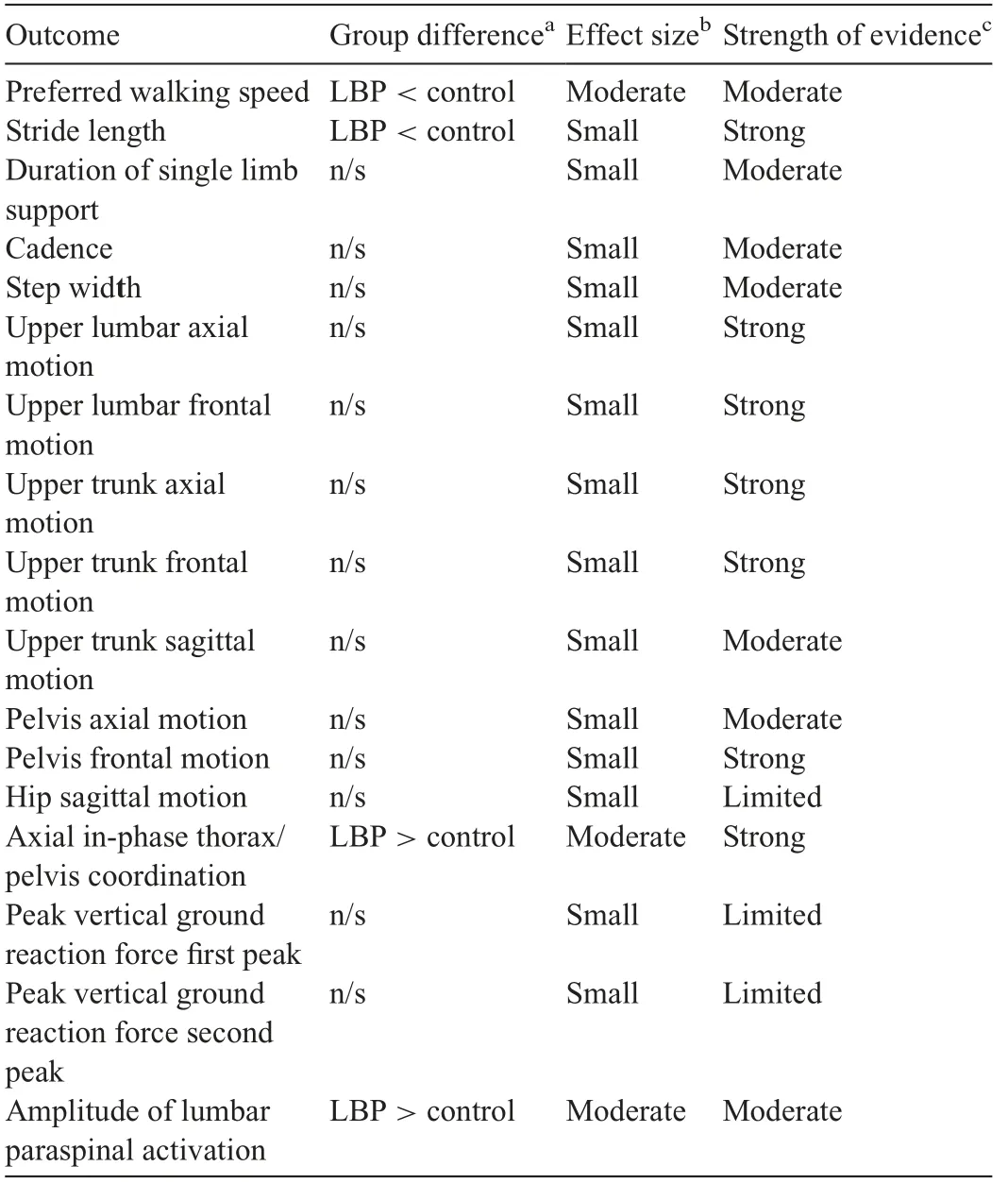
Table 3 Summary of meta-analysis results for walking gait.The direction of the difference between groups is indicated for comparisons with a significant standardized mean difference.Effect sizes and the strength of the evidence are shown for all comparisons.
3.2.1. Spatiotemporal characteristics
Thirty studies and a total of 1570 participants were included in the pooled analysis of preferred walking speed in individuals with persistent LBP. Individuals with persistent LBP walked more slowly than back-healthy individuals(SMD=-0.59,95%CI:-0.77 to-0.42),test for overall effect p <0.001; heterogeneity I2=58%, χ2p <0.001) (Fig. 2A).The mean difference in speed between groups was 0.12 m/s.Two studies that quantified gait biomechanics across a range of controlled treadmill speeds noted that individuals with LBP were not able to maintain gait at controlled speeds of greater than approximately 1.4 m/s.65,66Pooled data with 687 participants indicated that individuals with LBP had shorter stride length when walking at preferred speed (SMD = -0.38,95%CI: -0.60 to -0.16, effect p <0.001; I2=45%, χ2p=0.05) (Fig. 2B). The mean difference in stride length between groups was 0.05 m.Pooled evidence from 510 participants demonstrated no significant difference between groups in cadence (SMD = -0.19, 95%CI: -0.46 to 0.09, effect p=0.18; I2=53%, χ2p=0.03) (Fig. 2C). Duration of single limb support did not differ between groups (SMD = -0.17,95%CI: -0.56 to 0.23, effect p=0.41; I2=72%, χ2p=0.001).47,67-71Five studies with a total of 385 participants reported step width,and pooled analysis also showed no difference between groups(SMD=0.34,95%CI:-0.06 to 0.74,effect p=0.10;I2=72%,χ2p=0.006).42,47,51,70,72In studies investigating distance walked in 5 min, individuals with LBP walked significantly shorter distances than did healthy controls.73,74
3.2.2. Kinematic characteristics—single segment/joint
The peak-to-peak amplitude of lumbar motion was modeled with markers fixated around the spinal levels of T12,44L1,43,50L2,66and L3,43,75,76referenced to the global coordinate system, to L5, or to the pelvis. Pooled analyses of 5 studies with 193 participants examining the magnitude of lumbar motion during walking gait demonstrated that there was no significant difference in amplitude of motion in the axial plane in individuals with persistent LBP(SMD=0.07,95%CI:-0.26 to 0.39,effect p=0.69;I2=21%,χ2p=0.28).43,44,50,66,75The peak-topeak frontal plane lumbar motion was pooled from 6 studies and also demonstrated that there was no difference between LBP and control groups (SMD=-0.13, 95%CI: -0.39 to 0.13, effect p=0.32; I2=0%, χ2p=0.95).43,44,50,66,75,76As few studies investigated sagittal plane lumbar motion, these data were not pooled, but no studies reported a significant difference between individuals with and without LBP.44,50,76
Upper trunk motion was modeled with markers fixated on the sternum,42,77acromioclavicular joints,31and/or the spinal levels of C7,31,42T1,43,75T3,66and T6.50,78Motion was referenced to the global coordinate system,or to the lower thoracic,lumbar,or pelvic segments.Nine studies with 307 participants reported data for peak-to-peak axial plane motion in the thorax during walking that could be pooled.31,42,43,50,66,75,77-79There was no difference between groups (SMD=-0.10, 95%CI:-0.33 to 0.13,effect p=0.40;I2=0%,χ2p=0.56)(Fig.3A).Evidence pooled from 6 studies demonstrated that the amount of frontal plane motion also did not differ between groups(SMD=-0.16, 95%CI: -0.45 to 0.12, effect p=0.26;I2=13%, χ2p=0.33).42,43,50,66,75,79Of the studies investigating sagittal plane motion that could be pooled, there was no significant difference between individuals with and without LBP (SMD=-0.54, 95%CI: -1.30 to 0.22, effect p=0.17;I2=0%, χ2p=0.40).31,42,50,79Intra-subject stride-to-stride variability of lumbar or thoracic kinematic motion was reported in several studies,but without consistent methodological approach or findings.44,66,80
Data from 5 studies with 179 participants investigating pelvis kinematics in the axial plane during walking were pooled.31,44,50,66,78Amplitude of peak-to-peak pelvis motion was modeled with markers on the sacrum44,50,66,78and greater trochanters31and was usually referenced to a global coordinate system. There was no significant difference between groups(SMD=-0.12, 95%CI: -0.42 to 0.18, effect p=0.43;I2=50%, χ2p=0.09). Similarly, in the frontal plane, the amplitude of pelvic motion did not differ between groups(SMD=-0.09, 95%CI: -0.75 to 0.58, effect p=0.80;I2=43%, χ2p=0.15).44,50,66,81Sagittal plane pelvis kinematics were only available in 2 studies,and neither reported a significant group difference.44,50
Eight studies reported hip kinematics during steady-state gait.50,52,56,82-86Pooled data available from 4 of these studies with 128 participants indicated no difference in total sagittal plane hip motion in individuals with LBP (SMD=-0.08,95%CI:-0.43 to 0.27,effect p=0.65;I2=0%,χ2p=0.94).In the frontal plane,two studies reported reduced motion,83,84with a large effect size occurring in a study of obese adults,84and 2 reported no difference.56,82In the axial plane, 2 studies reported no difference,56,82and one reported decreased motion in individuals with LBP.83Knee flexion during late stance or swing phase was reduced in 3 out of 5 studies that reported knee kinematics,31,82-84,86but there were no consistent trends evident for frontal or axial plane knee motion or for ankle motion.
3.2.3. Kinematic characteristics—inter-segmental coordination
Multiple studies investigated coordination of kinematic motion between spinal segments during steady-state gait.33,34,50,66,77,78,87-91Most examined coordination in motion between the thorax and pelvis across a variety of controlled walking speeds. Time- and frequency-domain techniques were used to quantify phase relations between segments.In the axial plane, pooled analyses of 185 participants demonstrated that motion between the thoracic spine and the lumbar spine/pelvis was significantly more in-phase in individuals with LBP than in controls (SMD=-0.60, 95%CI: -0.90 to -0.30, effect p <0.001;I2=0%,χ2p=0.96)(Fig.3B).The mean difference in relative phase between groups was 25%. This finding was supported by three out of four additional studies from which data could not be pooled.33,87,88,90Multiple studies also investigated the stride-to-stride variability of inter-segmental coordination in the axial plane during steady-state gait,33,66,87,89-91with 3 reporting less variability in individuals with LBP.87,89,90
Fewer studies reported frontal or sagittal plane intersegmental coordination, and there was insufficient data available to pool the findings.In the frontal plane,coordination between the thorax and lumbar spine/pelvis was reported as being more in-phase in individuals with LBP33,34,50or the same.66In the sagittal plane, one study reported more in-phase coordination in individuals with LBP,92and 2 others sharing the same cohort reported no difference.33,34Stride-to-stride variability in sagittal thorax-pelvis coordination was reported as being less in individuals with LBP92or the same.33
3.2.4. Kinetic characteristics
Five studies reported kinetic measures in the lower extremities during walking.82,84,85,93,94In 3 studies examining sagittal plane total net joint moments at the hip,there were no differences between individuals with and without LBP.82,85,93
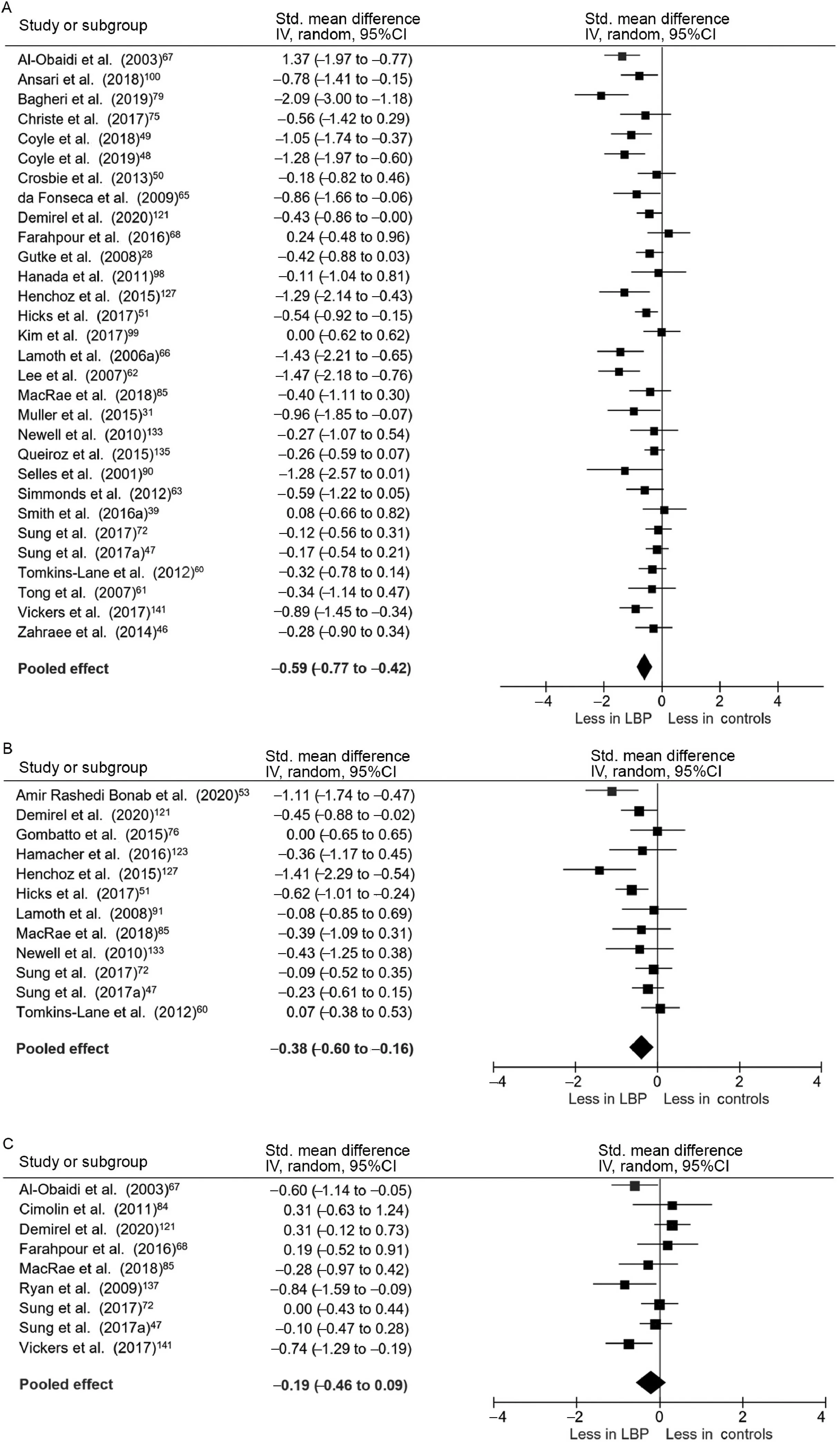
Fig.2. Meta-analysis of spatiotemporal gait variables.(A)Preferred walking speed,(B)Stride length,and(C)Cadence.95%CI=95%confidence interval;IV=inverse variance;LBP=low back pain;Std.=standardized.
3.2.5. Ground reaction forces
Five studies investigated ground reaction forces during walking gait,46,62,65,68,94of which 4 studies with 138 participants had data that could be pooled. There was no difference between groups when walking at preferred speed in peak vertical ground reaction forces during either the first(SMD=0.29,95%CI:-0.54 to 1.11,effect p=0.49;I2=82%,χ2p <0.001)or second vertical force peaks (SMD=-0.21, 95%CI: -0.86 to 0.46,effect p=0.56;I2=72%,χ2p=0.01).
3.2.6. Muscle activation characteristics
Fifteen studies included EMG measures of the paraspinal and abdominal musculature during walking, using preferred and controlled walking speeds.12,35,39,42,52,64,66,82,89,95-100Of these,14 used surface EMG electrodes,and one used intramuscular EMG. Most studies investigated amplitude of muscle activation, with two investigating timing of activation.39,52During steady-state gait,pooled analyses of data from 210 participants for surface EMG of the low lumbar paraspinal musculature across the entire stride cycle or within the stance phase indicated that individuals with LBP had greater amplitude of activation(SMD=0.52,95%CI:0.23-0.80,effect p <0.001;I2=1%, χ2p=0.40) (Fig. 3C). On average, individuals with LBP exhibited 31%greater activation than back-healthy controls.
For the abdominal musculature, 2 high-quality studies reported increased rectus abdominis activity during some or all phases of gait,12,971 reported decreased activity,98and 2 studies,including 1 high-quality study,reported no difference in activity between groups.82,99Three studies reported no difference in amplitude of external oblique activation,12,82,99and 1 high-quality study reported increased activation during some subphases of gait.97One study reported increased peak activation in internal oblique compared to controls,821 reported variable results depending on subphases of gait,98and another reported decreased activity during several gait subphases.99
3.3. Running gait
Although there were nine articles that reported the biomechanics of running gait,27,29-366 of these studies appeared to report data from the same 2 populations.29,30,32-34,36As a result,running data were only available for 5 different population cohorts, and there were insufficient comparable data for meta-analyses. One study investigated Division 1 collegiate athletes.27Four articles shared the same cohort of recreational runners that were required to run at least 20 km per week in order to participate in the study.29,30,33,34Two articles shared the same cohort of participants, who were described as being recreationally active.32,36Two articles did not report any requirement for running distance/frequency or activity level in its participants.31,35Most of the articles reported the preferred running speed of the participants, but running biomechanics were quantified during running at a controlled speed in 5 of the articles.29,30,33-35
3.3.1. Spatiotemporal characteristics
Preferred running speed did not differ between groups.31,33,36
3.3.2. Kinematic characteristics—single segment/joint
During running,the extent of upper trunk motion in the axial plane was reported as being less in individuals with LBP31or the same,32,34and there was no difference in sagittal31,32,34or frontal plane motion.32,34One study utilizing a controlled running speed found that axial plane pelvic motion was reduced in individuals with persistent LBP,31and another using participant preferred running speed reported that it was greater in individuals with LBP.34Two studies reported sagittal plane hip kinematics during running and found no difference in motion between individuals with and without LBP.29,36Similarly for running,sagittal plane knee motion either did not differ31,32or was reduced29in individuals with LBP, and sagittal plane ankle motion was not significantly different.29,31
3.3.3. Kinetic characteristics
During running, sagittal plane hip moments did not differ between groups.29,36One running study reported increased external flexion moment at the knee in individuals with LBP,36but there was no difference in another study.29
4. Discussion
To our knowledge, this is the first comprehensive systematic review and meta-analysis of walking and running gait in individuals with LBP. Individuals with persistent LBP walk differently than back-healthy controls. These differences are most evident in spatiotemporal characteristics, in patterns of inter-segmental coordination,and in paraspinal muscle activation.Current evidence does not indicate that LBP is associated with a difference in the amplitude of motion in the trunk or lower extremities during walking. There is insufficient evidence to determine whether running biomechanics are altered in individuals with LBP.
Pooled data demonstrated that individuals with persistent LBP choose to walk more slowly than individuals without back pain.The mean difference between groups exceeded previously reported values for the minimal clinically important difference in preferred walking speed across a range of studies in adults.101In 2 studies that reported that LBP patients were unable to walk at controlled fast speeds,65,66it was unclear whether this inability was due to pain,fear of pain,or deconditioning. Al Obaidi et al.67examined influences on preferred walking speed and found that fear avoidance and pain anticipation significantly predicted reduced walking speed in individuals with persistent LBP. Stride length was also reduced in individuals with LBP,but the mean difference for this comparison did not exceed reported values for minimal detectable change.102,103It is possible that individuals with LBP use a strategy of slower walking velocity and slightly reduced stride length to minimize the kinematic and kinetic demands of walking.37,62
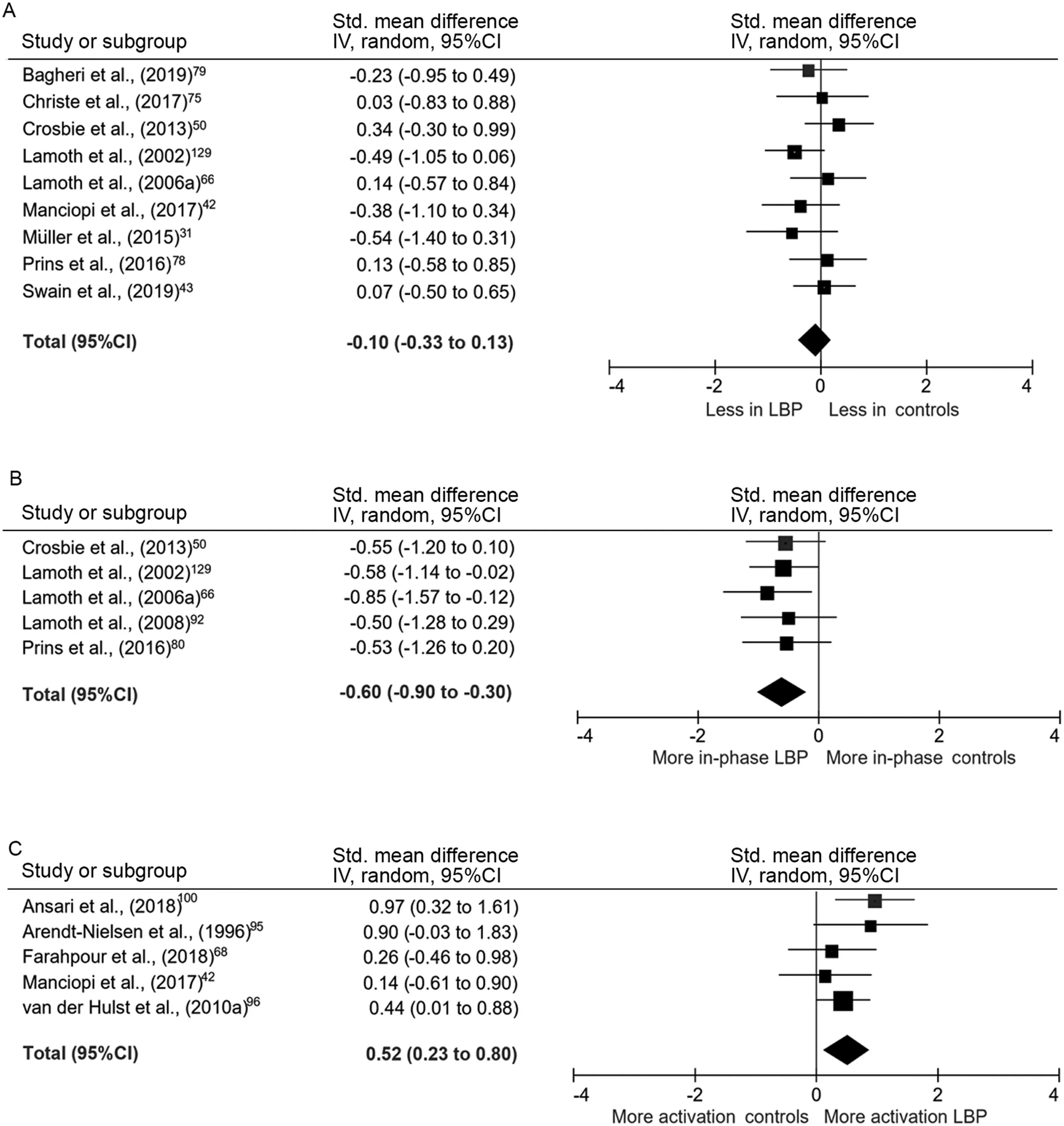
Fig.3. Meta-analysis of kinematic and EMG gait variables.(A)Axial plane thoracic motion,(B)Axial plane inter-segmental coordination,and(C)Amplitude of paraspinal activation.95%CI=95%confidence interval;EMG=electromyography;IV=inverse variance;LBP=low back pain;Std.=standardized.
This study found strong evidence for altered phase relations between motion in the thorax and the pelvis during walking in individuals with persistent LBP. In back-healthy controls, the pattern of coordination, or relative motion, between the upper trunk and pelvis in the axial plane is speed dependent,becoming more anti-phase as speed increases.66Even when walking at controlled speeds, individuals with LBP exhibited greater in-phase movement patterns. This may be due to a reduced ability to dissociate movement between the trunk and pelvis in these individuals.Anti-phase coordination during fast walking helps to generate elastic recoil between the thorax and the pelvis and may also contribute to minimizing total body angular momentum in the axial plane.104Therefore, the reduction in anti-phase coordination in individuals with LBP may help to explain their decreased gait speed and reduced stride length.
Our meta-analysis demonstrates that individuals with LBP have greater lumbar paraspinal activation during walking.Phasic muscle activity in the paraspinals occurs bilaterally at initial contact and during the double support phases of the gait cycle.105,106This activation controls sagittal and frontal plane motion between the trunk and the pelvis.107The amplitude of this activity is low, typically less than 20% of maximum voluntary activation for walking,39,108although this increases to up to 100% of maximum for fast running.108Acutely,increased activation during gait may be adaptive if it serves to reduce motion and protect pain-sensitive tissues. After the acute phase, it may also be a compensation for the muscle weakness related to atrophy and fatty infiltration that occurs in multifidus in response to back pain109or for proprioceptive dysfunction.110,111However, over time this increased activation in individuals with LBP may contribute to recurrence due to increased compressive spinal loading.112Increased paraspinal activation may also be the cause of the reduced anti-phase coordination described above as a result of increased axial stiffness limiting dissociation of motion between the upper trunk and the pelvis.87,104
In comparison with the paraspinals, abdominal muscle activity during locomotion is much more variable between individuals and more dependent upon locomotor speed.105,107,108This variability within healthy individuals is perhaps due to the redundancy of the abdominal muscle system and likely accounts for the lack of consistent differences in abdominal activation in individuals with LBP in the present review.It should be noted that all but one of the studies in this review used surface EMG. Surface EMG cannot selectively quantify activation in the transversus abdominis and multifidus muscles.113,114Isolated postural impairment of these deep muscles has been a focus of LBP research and treatment for some years.However,our findings are consistent with a recent systematic review of anticipatory postural adjustments indicating that the postural function of the superficial muscles in the abdominal and paraspinal systems are also affected by LBP.115
Current evidence does not consistently demonstrate a significant difference in joint or segmental excursion in the trunk or lower extremities during walking or running in individuals with LBP. This may be because the joint range of motion utilized in the thoracic and lumbar spines during walking and slow running is a small proportion of the available range.108This is in contrast with other activities, such as standing forward flexion, where significant reductions in lumbar range of motion in individuals with LBP have been observed.116The amplitude of hip and knee motion during walking and running is a greater proportion of available range, but the current evidence does not consistently support interdependency between back pain and lower limb gait kinematics.
This review identified methodological challenges that limit the current understanding of associations between gait biomechanics and LBP. As noted earlier, many studies quantified gait biomechanics at participants’ preferred walking speeds.This makes it difficult to determine whether the observed differences in characteristics like stride length in individuals with LBP are due to these individuals walking more slowly, or whether they are still evident when walking speed is controlled.Unfortunately,as very few studies quantified gait characteristics at prescribed gait velocities for all participants or adjusted for gait velocity in their analyses,51,62,66,76there is insufficient evidence to separate the influence of slower gait velocity from the independent effects of LBP on these characteristics. Future research should focus on assessment of walking and running gait at a range of speeds in individuals with LBP. This will demonstrate how these individuals modulate gait biomechanics in response to increasing mechanical demands as speed increases. It will also allow researchers to clearly differentiate between an inability to maintain faster walking speed and a preference to walk more slowly in LBP populations. Importantly, all but one of the studies in this review used a case-control or cross-sectional design. As there are so few prospective studies tracking changes in gait biomechanics and LBP status over time,it is still not known whether walking or running biomechanics affect the development of or persistence of symptoms.It is also not clear how observed biomechanical differences in individuals with LBP influence joint or tissue loading. As a result of these limitations in existing research,it cannot currently be determined whether the biomechanics of gait associated with LBP are adaptive or maladaptive. To answer this important question, future studies should probe longitudinal relationships between gait biomechanics and symptoms.
The limited number of available studies that investigate running precluded meta-analyses of the running biomechanics in individuals with LBP.Additionally, in this review we were unable to probe differences in gait between sub-groups of individuals with LBP.The inconsistent sub-grouping or classification of individuals with persistent LBP remains problematic.Multiple classification systems based on biomechanical or kinesiopathological factors have been proposed, but none are fully supported by available evidence.117Some studies investigated patient sub-groupings based on age, sex, weight, pain severity, or psychosocial factors. Other studies in this review recruited participants based on pathoanatomical diagnoses,such as herniated lumbar discs,degenerative instability,or spinal stenosis.Studies varied in how these pathoanatomical diagnoses were made, and the inconsistent relationship between pathoanatomical findings and clinical presentation or outcome is now well known.118However, the heterogeneity of the participants included in this review results in greater generalizability of the findings to the broader clinical population.Finally,the quality of the reporting in the included studies was low overall, with only 19 studies receiving a high-quality assessment score.
5. Conclusion
We found that individuals with a history of persistent LBP exhibit different biomechanical characteristics during walking gait than back-healthy controls. Differences are most evident in spatiotemporal characteristics, coordination between the thorax and pelvis coordination, and paraspinal muscle activation. However, it is not known whether the strategies evident in individuals with LBP during gait are adaptive or maladaptive. Prospective research following the transition from acute to persistent pain or symptom resolution will provide insight into the effect of these altered gait mechanics on the trajectory of back pain symptoms over time.
Acknowledgments
The authors thank Ivan Portillo,MLIS,AHIP,for his assistance in refining the search strategy and search terms. Jo Armour Smith is supported by grant K01HD092612, awarded by the Eugene Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. Dr. Lee reports grants from the NIH (NICHD), and personal fees from the Department of Defense outside the submitted work; in addition, Dr. Lee has a patent issued for his Method and Apparatus for Performing Timed Up-And-Go Test. All the support had no involvement in the study design and writing of the manuscript or the decision to submit it for publication.
Authors’contributions
JAS designed the study, supervised the literature search,conducted quality scoring,extracted the data,performed some of the analyses, and drafted the manuscript; HS extracted the data, performed some of the analyses, and drafted the manuscript; JJB assisted with study design, conducted quality scoring, and edited the manuscript; HLT assisted with study design, conducted the quality scoring, and edited the manuscript;VW conducted the literature search,extracted the data,and edited the manuscript; SPL assisted with study design,conducted the quality scoring, and edited the manuscript. All authors have read and approved the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Supplementary materials
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.02.001.
 Journal of Sport and Health Science2022年4期
Journal of Sport and Health Science2022年4期
- Journal of Sport and Health Science的其它文章
- The Journal of Sport and Health Science:Commemorating a decade of publishing milestones and impact
- Prevalence of meeting 24-Hour Movement Guidelines from pre-school to adolescence:A systematic review and meta-analysis including 387,437 participants and 23 countries
- Which psychosocial factors are associated with return to sport following concussion?A systematic review
- Lymphangiogenesis contributes to exercise-induced physiological cardiac growth
- Influence of biological sex and exercise on murine cardiac metabolism
- Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p
