In vivo degradability and biocompatibility of a rheo-formed Mg-Zn-Sr alloy for ureteral implantation
Di Ti ,Rnguo Gun ,Huinn Liu ,Minng Chn ,Svitln A.Ulsvih ,Ektrin V.Skorb ,Ptrii Holt-Torrs,Xiopng Lu,Norbrt Hort
a Engineering Research Center of Continuous Extrusion,Ministry of Education,Dalian Jiaotong University,Dalian 116028,China
b School of Materials Science and Engineering,Northeastern University,Shenyang 110819,China
c Department of Bioengineering,University of California,Riverside,CA 92521,USA
d School of Materials Science and Engineering,Tianjin University of Technology,Tianjin 300384,China
eInfochemistry Scientifi Center,ITMO University,St.Petersburg 192007,Russia
fMagnesium Innovation Center -MAGIC,Helmholtz Zentrum Geesthacht,Geesthacht D-21502,Germany
Abstract The introduction of biodegradable implant materials has significantl improved the postoperative subjective feelings of patients within the past few decades,among which magnesium alloy is widely considered a favorable choice as its appropriate biodegradability and evident antibacterial activity.Here,we reveal a semisolid rheo-formed Mg-Zn-Sr alloy ureteral implant that displayed suitable degradability and biocompatibility in a pig model.Refine non-dendritic microstructure was observed in the rheo-formed alloy,which led to ca.47% increase in ultimate tensile strength (from 195.0 MPa to 288.1 MPa) and more homogeneous degradation process compared with the untreated alloy.No post-interventional inflammatio or pathological changes of the test animals were observed during the implantation period,and the corrosion rate (0.22±0.04 mm·y−1) perfectly fitte the clinical ureteral stent indwelling time.The urine bacteria numbers decreased from 88±13 CFU·mL−1 at 7 weeks post operation to 59±8 CFU·mL−1 at 14 weeks post operation,which confirme the evident antibacterial activity of the alloy.Our study demonstrates that the Mg-Zn-Sr alloy is clinically safe for urinary system,enabling its efficaciou use as ureteral implant materials.
Keywords:In vivo;Biodegradability;Biocompatibility;Mg-Zn-Sr alloy;Ureteral implant.
1.Introduction
The human ureters are tubes which propel urine from the kidneys to the bladder,and tract stricture is one of the most frequent disease plaguing it [1,2].Endoscopic surgery is the current standard treatment for this condition[3].After urological procedures,a temporary metallic Double-J ureteral stent,usually manufactured of medical stainless steel or titanium,is necessarily placed in the ureter for suture lines to heal and allowing fluen drainage.After serving its purpose,the stents are needed to be removed by a cystoscope with anesthesia[4].Surgery for removal of the stents brings about suffering and economic burden for patients,which have been important factors in studies when evaluating the applicability of implanting stents in urological procedures [5,6].Therefore,researchers are seeking alternate implant materials either to drain the urinary system or to avoid a second removal surgery[7].Several studies have reported on biodegradable ureteral stents based on polymer and metallic materials [8-10].Polymeric materials including poly glycolic acid,poly lactic acid and poly lactic-co-glycolic acid are widely studied as potential biodegradable ureteral implant materials [11,12].However,due to their naturally intrinsic low strength,polymeric stents are easy to suffer from deformation when resisting external compression forces [13,14].Instead,metallic biodegradable stents have similar mechanical properties to traditional stents,and therefore could provide improved dilatation effects[15-17].
Zinc,iron,magnesium and their alloys are the most widely studied metallic biodegradable materials [10,16].Zinc and its alloy showed steady corrosion rate and low local toxicity in Drelich et al.’s study [18] and Champagne et al.’s study [10].Iron based coronary stents also have shown their safety and effica y in animal models [19].However,zinc and iron usually have much longer degradation period in comparison with magnesium according to Hernandez-Escobar et al.’s and Bowen et al.’s studies [20,21].Magnesium alloy has adjustable degradation rate which can exactly adapt to the regular clinical indwelling time of ureteral stents (8-12 weeks)[22,23].Furthermore,the antibacterial activity of magnesium alloys cannot be neglected either,particularly when used as ureteral implant materials [24-26].Biodegradable magnesium alloy has a constantly exfoliated surface,on which biofil can hardly form,and therefore reduces the probability of clinical infections [27-29].
Previously we developed biodegradable antibacterial Mg-Ag alloys and biodegradable Mg-Sr alloys as bone implant materials [30,31].We also investigated thein vivodegradation behavior of Mg-4Zn-1Sr(mass%,ZJ41)in urine[32,33].Based on these results,we evaluated the applicability of a novel Mg-4Zn-0.5Sr (mass%,ZJ40) alloy for fabricating ureteral stents in pig models.We investigated the microstructure,electrochemical properties,in vivodegradability,histocompatibility and urinary compatibility of the alloy.These results will reveal that ZJ40 alloy may be an ideal candidate for the manufacture of ureteral implant devices.
2.Material and methods
2.1.Materials preparation and characterization
High-purity Mg (99.999 mass%;Luxfer,Manchester,UK),pure zinc (99.99 mass%,Zhuye Group,Zhuzhou,China) and Mg-10mass%Sr master alloy (Norsk Hydro A.S.,Oslo,Norway) were used as raw materials for alloy preparation.The studied alloy was named ZJ40,which had a nominal composition of 4.00 mass% Zn and 0.50 mass% Sr in Mg matrix.The raw materials were mixed according to the nominal composition and melt at 710 °C in protective atmosphere in an electrical resistance furnace (Hengli HLJ,Henan,China).A rheological slurrying machine designed by ourselves was used to prepare the semisolid slurry [34].The melting temperature was from 690 °C to 695 °C,and the treatment time was adjusted by the melt fl w rate.The melt was poured into a continuous rheo-extrusion machine at 675 °C to prepare alloy wires with a diameter of 1.0 mm [34].The alloy wires were then made into stents by using a manual coiler.
An inductively coupled plasma optical emission spectrometer (ICP-OES;Varian,USA) was used to determine the actual composition of the ZJ40 alloy.The tension test was performed using a universal mechanical tester (Z050,Zwick,Ulm,Germany).Specimens from the cross section were mechanically polished using silicon carbide paper and grinding paste (Jingxian,Shenzhen,China) for optical microstructure observation using a metallographic microscope (LV150N,Nikon,Japan).The following equation was applied to calculate the shape factor of the primary solid phases [35]:
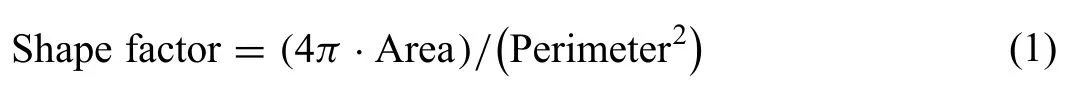
An X-ray diffractometer (XRD;D8 ADVANCE,Bruker,USA) was employed for phase identification with step time of 3.0 s and step size of 0.02 mm.The prepared specimens by beam milling were further analyzed by a High-Resolution Transmission Electron Microscopy (HR-TEM;JEM-2010,JEOL,Japan) for more accurate phase identification
2.2.Cytotoxicity and biodegradability assessment
Primary human osteoblast cells were cultured provided by China Medical University,and were cultured in Dulbecco’s Modifie Eagle Medium(DMEM;GlutaMAX,Thermo Scientific Germany) plus 15vol% fetal bovine serum (FBS;Gibco,New Zealand).The cells were cultured in the cell-culture well-plates (control group) and on the surface of ZJ40 alloy at a seeding density of 2×104ml−1.The cytotoxicity of the alloy was evaluated by live/dead staining (Yeasen,Shanghai,China)and fluorescen microscopy(EVOS,Thermo Fisher,USA).The cells on the specimens were fi ed by standard procedures,and the cellular attachment was observed using a scanning electron microscopy (SEM;JCM-5000,Nikon,Japan) [36].
Thein vitrodegradation behavior of the alloy was analyzed by linear sweep voltammetry and electrochemical impedance spectroscopy(EIS)using an electrochemical workstation(Parstat,Princeton,USA).DMEM plus 15vol% FBS was used as the corrosion media,and all measurements were performed under cell culture conditions.Open circuit potential was determined after 1800 s polarization,and single sweep voltammogram test was performed from −1.7 V to 0.0 V (vs.standard hydrogen electrode).Electrochemical impedance spectroscopy analysis was carried out between 0.01 Hz and 10 kHz at open circuit potential.
Eight-week-oldGuangxi Bama Minipig(Northern Theater General Hospital,Shenyang,China) were divided into control group (n=3) and test group (n=3).Animal procedures were performed according to the guidelines issued by the State Scientifi and Technological Commission and approved by the local animal welfare committee (DB11/T 1463.1-2017).The stents were placed in the mid-section of animals’ right ureter.The degradation process and potential post-interventional inflammatio or pathological changes were monitored using a PET-CT scanner (Discovery VCT,GE,Boston,USA).The implants were removed after 14 weeks implantation,and an atomic force microscopy (AFM,Bruker Dimension FastScan,Billerica,USA) was employed to characterize the surface topography in semi-contact mode with scan frequency of 0.5 Hz.The surface morphology was observed by SEM,and an energy dispersive X-ray spectrometer (EDS;Ametek,New Jersey,USA) was used to analyze the surface chemical compositions.An X-ray induced photoelectron spectroscopy(XPS;Shimadzu,Manchester,UK) was employed to identify the compositions of the surface products.
2.3.Histocompatibility and urinary compatibility tests
Representative histological sections of liver,myocardium and kidney collected from the animals were fi ed,sectioned,H/E (hematoxylin and eosin) stained and characterized by an optical microscope (X-71,Olympus Tokyo,Japan) under standard procedures [37].The prepared samples were pathologically inspected in a blind randomized fashion.Serum concentrations of alanine aminotransferase (ALT),aspartate aminotransferase (AST),total bile acid (TBA) and total bilirubin levels (TBIL) were determined to assess the liver functions every 4 weeks during the implantation period.
The ureteral wall surrounding implantation site collected from the test animals were fi ed,sectioned,H/E(hematoxylin and eosin) stained and characterized by an optical microscope under standard procedures after 14 weeks implantation.Chromatography filte paper (GE-Whatman,Kent,GB) was placed under the culture cages to monitoring the urinary frequency of the test animals.The chromatography filte paper was dried and analyzed using a UV-visible-NIR microscope(MJT Tech,Beijing,China) every 2 h.The distribution and morphology of the void spots were analyzed by ImageJ software (V1.5,Bharti Airtel Ltd.,New Delhi,India) to calculate the urine output and the urinary frequency.
Midstream urine from the test animals was microbiologically investigated every two weeks to evaluate the impact of implantation on urine and potential peri-implant infections.The urinalysis was performed by using an automatic urine analyzer (iRicell,Beckman Coulter,Atlanta,USA),in which pH value,urinary white cell count and urinary red cell count were recorded.Bacteriuria was tested via a standard quantitative urine culture method.Nonselective chromogenic agar plates (Forthright Biotech,Shanghai,China) were used as quantitative reference while selective colistin-nalidixic acid agar plates (Forthright Biotech,Shanghai,China) enabled identificatio of Gram positive bacteria.The bacterial results were presented as colony forming unit per milliliter(CFU·mL−1).
2.4.Statistical analysis
Experimental results were presented as mean value±standard deviation (SD).One-way analysis of variance (ANOVA)tests were carried out for multi-group comparisons of the means using SPSS V19.0 software (SPSS Inc.,Chicago,USA).∗P <0.05,∗∗P <0.01 and∗∗∗P <0.001 indicate confidenc intervals of 95%,99% and 99.9%,respectively.NS stands for no significan difference.
3.Results and discussion
3.1.Microstructure and mechanical properties
The actual chemical composition of ZJ40 alloy was:4.21±0.04 mass% of Zn,0.53±0.02 mass% of Sr and balanced Mg.The representative optical micrographs of the alloy with different rheo-treatment time are shown in Fig.1a.The semisolid melt treatment device led to a dynamical nonequilibrium solidificatio of the slurry.Globular morphology of the primary phases has been fully formed after 0.5 s treatment.To avoid segregation of primary phases dispersed in the secondary phase matrix,the slurry was further treated for longer time.The mean shape factor was found to increase from 0.49 after 0.5 s treatment to 0.74 after 2.0 s treatment.This improved spheroidization of the slurry was caused by enhanced nuclei formation ofα-Mg grains which decreased the melt surface tension [38].During the semisolid rheo-solidificatio process,primary solid phases were firs solidifie in theα-Mg matrix,and the residual melt was nonequilibrium solidifie by eutectic reaction [34].Therefore,grains were significantl spheroidized and refine during this rapid solidificatio process.
The precipitates distributed along grain boundaries were identifie by XRD analysis (Fig.1b),which were mainly composed of MgZn2and Mg17Sr2.Fig.1c presents the HRTEM micrograph of the interface between the primary phase and the secondary phase,and the corresponding diffraction pattern of the precipitates in primary phases is presented in Fig.1d.Mg17Sr2predominantly dispersed inside primary solid phases with an average diameter of ca.4.0 nm,whose diffraction patterns were indexed as base-centered orthorhombic structured metastable phases(Fig.1d)[31,39].Fig.2 summarizes the mechanical properties and the average grain size of the alloy rheo-treated with different time.The average grain size decreased significantl with increasing treatment time(significanc levelP <0.05).As a result,the tensile strength as well as the elongation showed pronounced increases.The alloy after 2.0 s treatment gained ca.47% increase of the ultimate tensile strength compared to untreated alloy,from 195.0 MPa to 288.1 MPa.Interesting to note is that the prolonged treatment time (4.0 s) did not provide any improvement in tensile strength,which was due to the grain growth by prolonged solidificatio process.Due to the fully refine microstructure and the optimized mechanical properties,the alloy after 2.0 s rheo-treatment was chosen as the material to fabricate ureteral stents.During rheo-solidificatio process,the majority of precipitates concentrated in secondary phases and bore more shear stress.This sharp strength difference between primary phases and secondary phases further improved the effectiveness of semisolid slurring.As a result,refine non-dendritic microstructure was observed in the rheo-formed alloy,which led to the improved mechanical properties.

Fig.1.(a) Representative optical microstructure of the semisolid rheo-formed ZJ40 alloy with different rheo-treatment time.(b) The XRD pattern for phase identificatio of ZJ40 alloy.(c) HR-TEM micrograph of the alloy showing the boundary between primary solid phase and secondary grain phase.(d) The electron diffraction patterns of the precipitates in the primary solid phase.

Fig.2.The mechanical properties and average grain size of the alloy with different rheo-treatment time.The measurements were performed in fi eduplicates and mean value ± SD is presented.
3.2.Cytotoxicity, in vitro and in vivo degradation process
The fluorescenc analysis of human osteoblast cells in the control group and the ZJ40 group both showed homogenous distribution and normal morphology in fusiform shape(Fig.3a,d).The cellular membrane,the nucleus and the mitochondria maintained their regular morphology,indicating the normal colonization and proliferation of the cells on the alloy.After 7 days culture,very few dead cells were observed on the surface of ZJ40 samples (Fig.3e).Cell viability results showed a mean percent viability of 97.9±1.6%and 98.2±1.9% for the cells grown on the alloy and on the well-plate,respectively.The cell adhesion results indicated that ZJ40 has no significan difference in cytocompatibity with control.SEM images of the specimens after 7 days culture of osteoblasts showed that dense cell layer formed on the surface ZJ40 alloy (Fig.3f).The fluorescenc analysis and the attachment observation demonstrated the nontoxicity of ZJ40 alloy on human primary cells.It is also worth noting that the proliferation rate in ZJ40 group was higher than that in control group,which is due to the cell growth promoting activity brought by the Mg2+and Sr2+ion release [31].
According to the EDS maps (Fig.4),it is evident that the small-sized zinc and strontium rich precipitates formed during the semi-solid solidificatio process and distributed in secondary phases along grain boundaries.Due to the more positive potential of these secondary solid phases,they formed a network-like cathode in galvanic corrosion.The cathodic network could effectively prevent corrosion products from spreading to other anodic areas,which led to a homogenous degradation process [40].From the anodic polarization behavior of ZJ40 alloy (Fig.5a),it can be observed that the open circuit potential was −1.41 V (vs.SHE) and the shift of break down potential at −0.45 V (vs.SHE) in the anodic direction was responsible for the oxidation status change.Alloying of zinc and strontium shifted the anodic curve to a lower slope compared with pure magnesium,and changed the pitting corrosion potential towards the cathodic direction,indicating that both homogeneous corrosion and pitting corrosion were decreased [41].Electrochemical impedance spectroscopy results are presented by a Nyquist diagram(Fig.5b).The inductive loop at the initial stage was attributed to the ion release,while the change of the capacitive arc diameter was due to the generation of product fil [42].The equivalent circuit was fitte by using Orazem et al.’s model as applied for the characterization of the interfacial processes,in which the solution resistance=18.2Ω·cm−2,the fil resistance pore resistance=8.4 kΩ·cm−2,the charge transfer resistance=201.0Ω·cm−2and the double layer capacitance=9.1Ω−1·cm−2·sn[43].

Fig.3.The fluorescenc analysis and SEM images of human osteoblast cells on the surface of the materials:(a) live cells on the well-plate (control);(b)dead cells on the well-plate (control);(c) SEM micrograph of cell attachment on the well-plate (control);(d) live cells on the ZJ40 alloy;(e) dead cells on the ZJ40 alloy (f) SEM micrograph of cell attachment on the ZJ40 alloy.

Fig.4.EDS maps of ZJ40 alloy where red areas are magnesium matrix (a);blue spots are zinc containing phases (b);green spots are strontium containing phases (c);the schematic demonstration of the corrosion mechanism of ZJ40 alloy,where red arrows represent the degradation directions (d).
The changes of the average degradation rate of the stents calculated by urine magnesium content are drawn in Fig.5c.The highest degradation rate was observed when the immersion time was 2 days,which was 2.21±0.12 mm·y−1.With increasing indwelling time,ZJ40 alloy exhibited a constantly decreasing degradation rate (0.22±0.04 mm·y−1at the 14th week).Owing to the gradually thickening corrosion layer,electron transportation was blocked and therefore the degradation rate significantl decreased.Previously,it has been reported that fl w-induced shear stress could accelerate the corrosion process due to the increased mass transfer in blood environment [44].Our results were in agreement with the previous finding that urine fl w could alter the degradation behavior of the stent,particularly when the stent directly contacted with urine in the initial stage.Although the test stent showed an appropriatein vivodegradation period in comparison to clinical ureteral stent indwelling time (8-16 weeks),further in situ studies on its degradation behavior together with urodynamics analysis are still important in the future to optimize its degradation properties [22].
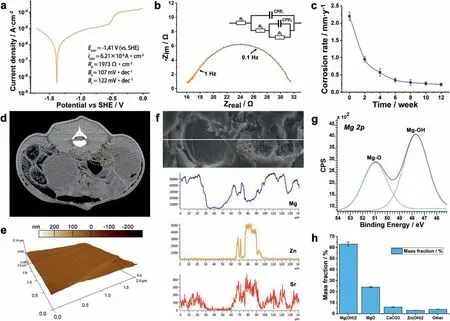
Fig.5.(a) The polarization curve of ZJ40 alloy in logarithmic scale.(b) Nyquist diagram of ZJ40 alloy with the equivalent circuit,where CPE1 stands for the constant phase element of product film CPE2 stands for double layer capacitance,R1 stands for the solution resistance,R2 stands for the fil resistance pore resistance,R3 stands for the charge transfer resistance.(c) The changes of the average degradation rate of the stents calculated by urine magnesium content.(d) The PET-CT image at 7 weeks post-implantation,where the framed area indicates the implantation site.(e) The 3-dimensional surface morphology of the stent at 14 week post-implantation using AFM characterization.(f) The SEM image and the according EDS line-scan results of the corroded surface.(g) Mg 2p XPS spectra for the surface of ZJ40 alloy stent.(h) The mass fraction of the surface corrosion products of the ZJ40 stents after implantation calculated by XPS spectra.
We used PET-CT to determine the integrity of the ZJ40 implants as well as inflammator changes at the implantation site (Fig.5d).Neither post-interventional inflammatio nor pathological changes in urinary system was observed by PETCT examination.The stent still maintained its shape integrity after 14 weeks of implantation.The 3-dimensional surface morphology of the stent at 14 week post-implantation was obtained using AFM characterization (Fig.5e).The peak and valley were at 33.2 nm and −19.7 nm separately,demonstrating that no severe localized corrosion took place during the implantation period.The secondary solid phases enriched with zinc and strontium had more positive potential than the primary solid phases,and therefore formed a network-like cathode in galvanic corrosion [40].The cathodic rheo-solidifie secondary phases prevented corrosion products from spreading to other anodic areas,and therefore the alloy degraded in form of general corrosion rather than localized corrosion(Figs.4 and 5e) [40].
The SEM image and the according EDS line-scan results of the corroded surface at 14 week post-implantation are depicted in Fig.5f.It can be seen that the amount of zinc and strontium corrosion products highly depended on the amount of magnesium products.The abrupt decrease in magnesium content indicates urinary calculus layer formed and attached on the surface of the stent during the degradation process.The broadening of the Mg 2p peak (Fig.5g) could be deconvoluted with Mg(OH)2and MgO sub-peaks [45].Fig.5h summarizes the species and their amount of the corrosion products in the surface layer of the stent after 14 weeks implantation.The results demonstrated that Mg(OH)2,MgO and small amount of CaCO3,Zn(OH)2are the primary corrosion products in the surface layer.Compared to other biodegradable metallic materials,magnesium has an evident advantage in reduction of urinary calculus because of its relatively short and adjustable indwelling time [20,21].As we discussed in previous section,the degradation period of ZJ40 alloy is compatible with the clinical treatment cycle,so the incidence of urinary calculus could be farthest decreased.
3.3.Histocompatibility,urodynamics and urinary compatibility
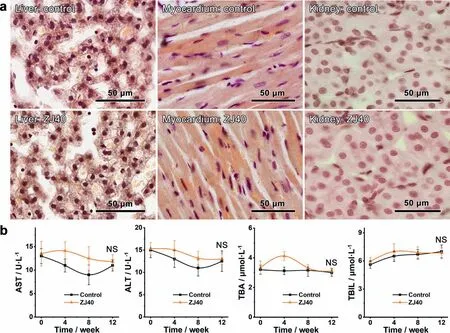
Fig.6.(a) Representative H&E-stained liver,myocardium and kidney sections of animals at 14 week post-implantation.(b) Main hepatic function indexes of the test animals at 0,4,8 and 12 weeks post-implantation.Measurements were performed in six-duplicates and the results are presented as mean value ± SD.
To assess the comprehensive biocompatibility of ZJ40 alloyin vivo,we evaluated the histocompatibility on the representative samples of liver,myocardium and kidney.As shown in Fig.6a,absence of necrotic tissue was found in both control and test groups.No inflammator infiltrate were observed in liver tissue,and hepatocytes showed normal structure.No morphological changes and hydropic degeneration in the endomysium and nuclei were found in the myocardium.Clear and integrated proximal convoluted tubules were observed in the kidney samples,illustrating that no inflam mation or tissue damage occurred in the both groups.Increases in serum concentrations of AST,TBA and TBIL were found in the test group up to approximately 4 weeks of implantation,and recovered to normal levels after 8-12 weeks implantation (Fig.6b).Specificall,the highest TBA level (4.12±0.30 μmol·L−1) was measured at 4 weeks postimplantation,which hinted a temporary hepatocellular disorder due to peri-implant infections.
Given the ZJ40 stents had similar degradation period with clinical indwelling time,the main hepatic functions and inflammatio indices of the test animals could return to normal levels immediately after the degradation period.Due to its biodegradability,a ZJ40 stent is immune to a second operation to remove it.Apart from suffering and economic burden brought about by second operation,adverse effects due to extra indwelling time should also be paid attention to.Lim et al.[46] reported a study on patients underwent ureteral stent implantation for more than three years.In this case,the storage symptoms and pain of the patients were both aggravated with increasing implantation time.Moreover,studies on short-term ureteral stenting reported a reduction of ureteral stent symptoms with decreased indwelling time [47].From this point of view,ZJ40 stents’ advantage is not just being immune to a second operation but being free of adverse effects brought about by extra indwelling time too.
Similar to other urinary track operations,stent implantation negatively impacted bladder function.ZJ40 stents significantl decreased the single urine output whilst increased the frequency of urination during the firs few weeks postimplantation (Fig.7a).In addition,peri-implant infections might increase the number of bacteria in the bladder and reduce the bladder capacity [48].In our study,the decreased bladder capacity led to frequent small urination events of the test animals,suggesting the test animals’ feeling of urgency.Along with postoperative recovery,the urine output as well as the urinary frequency of the test animals returned to normal level after 6 weeks implantation.Due to its decreasing volume and antibacterial activity as we previously reported[32],ZJ40 group did not exhibit significan influenc on urine output and urination (93.6±9.7 mL and 10.7±1.2 times)compared to control group (99.1±8.2 mL and 10.2±1.0 times) after 14 weeks implantation.

Fig.7.(a) The changes of urine output and urinary frequency of the test animals during the implantation period.(b) Representative ureteral wall sample of the animals after 14 weeks implantation.(c) Urinalysis results of the control and the test animals at 0,7 and 14 week post-implantation.Measurements were performed in six-duplicates and the results are presented as mean value ± SD.
There was neither loss of transitional epithelium nor von Brunn’s nest in test groups,hinting at no severe epithelium lesion after 14 weeks implantation (Fig.7b).On the cellular level,implantation induced neither degenerative changes nor enlargement of the cellular size.Boundaries between cytoplasm and nucleus were clear,and the chromatin was more compact in transitional epithelium.With local Mg2+ion supply available in the ZJ40 group,we found a decrease in widening of the lamina propria which is commonly found after stenting.It is suspected that inhibitions to peri-implant infection prevented these changes in ureter [49].A signifi cant increase in pH value of the urine collected from the test group corresponded to the degradation of the alloy (Fig.7c).Interestingly,regardless of many previous reports on pH value change inin vitrostudies,an increase of pH at implantation sites is scarcely reported.Notable urinalysis data also included elevated leukocyte counts (2.00 in ZJ40 and 0.33 in control at 7 weeks;0.67 in ZJ40 and 0.00 in control at 14 weeks),and elevated bacteria numbers (88±13 CFU·mL−1in ZJ40 and 54±6 CFU·mL−1in control at 7 weeks;68±10 CFU·mL−1in ZJ40 and 59±8 CFU·mL−1at 14 weeks).Both urinalysis and urine bacterial culture results proved that peri-implant infections occurred and recovered during the 14 weeks implantation,whereas no evidence of urinary tract obstruction was noted on urination monitoring (Fig.7a).The urinalysis results confirme the ZJ40 stent’s evident antibacterial activity.
Different from permanent implant materials,biodegradable magnesium alloy has a constantly exfoliated surface,on which biofil can hardly form [27].The increase of pH due to presence of Mg2+was found to significantl decrease the number of CFUs in Robinson et al.’s study [28].Previously,we also observed the evident antibacterial activity of pure magnesium and several magnesium alloys,and clarifie the correlation between magnesium ion release and bacterial growth [27].Although our results have proved that ZJ40 alloy has evident antibacterial activityin vivo(Fig.7),it is still essential to fully reveal the mechanisms of biodegradable magnesium alloys in the inhibition of biofil formation and bacterial growth when translating them to clinical applications.
4.Conclusions
We developed a biodegradable magnesium alloy ureteral stent and evaluated itsin vivoperformance in pig ureter models.The following essential conclusions regarding the applicability of the tested alloy were drawn:
(1) Refine non-dendritic microstructure was observed in the rheo-formed alloy,and the average grain size decreased significantl with increasing treatment time.The alloy after 2.0 s treatment gained ca.47% increase in ultimate tensile strength compared with the untreated alloy,from 195.0 MPa to 288.1 MPa.
(2) Alloying of zinc and strontium led to a more cathodic pitting corrosion potential,which demonstrated that homogeneous and pitting corrosion both decreased.The biocompatible results demonstrated that the ZJ40 stents did not cause tissue damage or show obvious adverse effects on physiological indicators after 14 weeks implantation.
(3) Due to its decreasing volume and antibacterial activity,ZJ40 alloy did not exhibit significan influenc on urine output and urination(93.6±9.7 mL and 10.7±1.2 times) compared to control group (99.1±8.2 mL and 10.2±1.0 times) after 14 weeks implantation.Inhibitions to peri-implant infection practically prevented the potential damage in ureter structure.Ourin vivoresults demonstrated the good biocompatibility as well as the evident antibacterial activity of ZJ40 alloy.
Declaration of Competing Interest
None.
Acknowledgments
Authors acknowledge National Natural Science Foundation of China (grant numbers 51771045 and U1764254) and the Fundamental Research Funds for the Central Universities(grant number N2002016) for the financia supports.
Special thanks are due to the instrumental analysis from Analytical and Testing Center,Northeastern University.All the staff in Animal Experimental Center of China Medical University are grateful acknowledged for the animal experiments.
 Journal of Magnesium and Alloys2022年6期
Journal of Magnesium and Alloys2022年6期
- Journal of Magnesium and Alloys的其它文章
- EDITORIAL BOARD
- Aims and Scope
- Surface oxidation study of molten Mg-Al alloys by oxide/metal/oxide sandwich method
- Production and characterisation of new bioresorbable radiopaque Mg-Zn-Y alloy to improve X-ray visibility of polymeric scaffolds
- Quantitative study on the tension-compression yield asymmetry of a Mg-3Al-1Zn alloy with bimodal texture components
- Microstructure analyses and phase-fiel simulation of partially divorced eutectic solidificatio in hypoeutectic Mg-Al Alloys
