Microstructure analyses and phase-fiel simulation of partially divorced eutectic solidificatio in hypoeutectic Mg-Al Alloys
Joo-Hee Kng ,Jiwon Prk,∗ ,Kyung Song ,Chng-Seok Oh ,Oleg Shchyglo ,Ingo Steinch
a Korea Institute of Materials Science,Changwon 51508,Republic of Korea
b Interdisciplinary Centre for Advanced Materials Simulation,Ruhr-University Bochum,44801 Bochum,Germany
Abstract In this study the partially divorced eutectic microstructure of α-Mg and β-Mg17Al12 was investigated by electron backscatter diffraction,transmission electron microscopy,and phase-fiel modeling in hypoeutectic Mg-Al alloys.The orientation relationships between the individual eutectic α grains,eutectic β phase,and primary α grains were investigated.While the amount of eutectic morphology is primarily determined by the Al content,the in-depth microstructure analyses and the phase-fiel simulation suggest non-interactive nucleation and growth of eutectic α phase in the β phase grown on the interdendritic primary α dendrites.Also,phase-fiel simulations showed a preferred nucleation sequence where the β phase nucleates firs and subsequently triggers the nucleation of eutectic α phase at the moving β phase solidificatio front,which supports the microstructural analysis results.
Keywords: Mg-Al alloy;Partially divorced eutectic;Solidification Electron backscatter diffraction;Phase-Field modeling.
1.Introduction
In the majority of cast Mg alloys containing more than 2 atomic% Al,eutectic microstructures composed ofα-Mg andβ-Mg17Al12are readily formed during solidification Their morphologies vary prominently from fully divorced eutectic through several intermediate structures to eutectic lamellar depending on the cooling rate and the content of the alloying elements [1-5].The importance of theβphase morphology on the mechanical and electrochemical properties of Mg-Al alloys has been discussed in several studies [6-8].The control of fraction,distribution and shape of the eutectic constituents and their overall morphology are crucial in optimizing the mechanical properties and the corrosion resistance of the alloys because the intermetallicβphase is brittle and electrochemically more noble than theαphase.In addition,the nucleation and growth of theβphase in an interdendritic liquid between primaryαphase dendrites occur at the last stage of solidificatio and is closely related to hot-tearing,which could be prevented by providing an open feeding path in the liquid channel.In view of its technical importance,it is necessary to have an improved understanding of how the eutectic microstructure is formed.
In Mg-Al alloys,the eutectic fraction is primarily determined by the Al content.While the maximum solubility limit of Al in theαphase is 11% [9] (hereafter,in atomic%) to form an equilibrium singleαphase during solidification the metastable eutectic microstructure can be formed in the nonequilibrium solidificatio of alloys containing less than 11%of Al.Liquid phase readily enriched with solute over the solubility limit during technical non-equilibrium alloy solidifica tion [10] which enters eutectic-forming region in phase diagram.The solidificatio sequence follows the formation of the primaryαphase (hereafter,αp),and thereafter,the eutecticβphase andαphase (hereafter,αe) in the interdendritic channel ofαp.In addition to the Al content,the cooling rate and other alloying components such as Zn also affect the specifi morphology of the eutectic region.Dahle et al.[2] reported a drastic change in the eutectic structure within a singleβgrain along the growth direction in a directionally solidifie Mg-9.1Al-0.4Zn alloy.The rapid morphological transition from fully divorcedβthrough the partially divorced eutectic structure with thickβhalo to fibrou and lamellar eutectics with thinβhalo occurred within several tens of μm.The result emphasizes the importance of temperature control in accomplishing the desired microstructure and in understanding the underlying mechanisms of divorced eutectics.To achieve quantitative comparisons of different cooling conditions and to obtain simulation results reflectin actual microstructure evolution,this study conducted direct temperature measurement of the melt during the solidificatio process.
This study compares partially divorced eutectic morphologies of equiaxed Mg-Al alloys containing 5-15% Al with different cooling rates.Thorough microstructural analyses were conducted by optical microscopy to observe the overall casting microstructure and by transmission electron microscopy(TEM) for a comprehensive investigation of the individual phases and grains.The partially divorced eutectic microstructure developed during the interdendritic solidificatio of the residual liquid pocket was simulated using the phase-fiel approach and compared with the experimental microstructure.
1.1.Experiments
Mg-Al alloys containing 5%,10%,and 15% Al were prepared with reagent grade metal granules and melted in an induction furnace under protective CO2and SF6atmospheres up to 973 K.Thereafter,the melt was poured into the preheated (523 K) steel mold with Type-K thermocouples placed inside the mold.The wedge-shaped mold was adopted to obtain two different cooling rates from a single heat to reduce the compositional variation as shown in Fig.1.The analyzed microstructure specimens were taken from the volume close to the thermocouples,which assures quantitative assessment of the cooling rate effect and provides reasonable input parameters for the phase-fiel simulations.
The casting microstructures were analyzed by polarized light microscopy (PM),scanning electron microscopy (SEM),electron backscatter diffraction (EBSD),and TEM in diverse scales.The PM specimens were etched with 4.2 g of picric acid,10 mL of acetic acid,700 mL of ethanol,and 10 mL of distilled water mixture etchant.The SEM and EBSD specimens were mounted,ground and polished to colloidal silica suspensions finish SEM and EBSD examinations were carried out using JEOL JSM-7001F and JSM-7900F fiel emission SEM,both equipped with Oxford EBSD (NordlysNano and Symmetry) and energy dispersive spectroscopy (EDS).The EBSD maps and EDS data were analyzed using Oxford Aztec 4.2 and TSL OIM 8.0 software to produce orientation maps,pole figures and elemental profiles TEM specimen was prepared using a focused ion beam (FIB,Carl Zeiss AURIGA Dual-Beam) from the pre-analyzed EBSD specimen to obtain the designated specimen orientation.Selected area diffraction patterns (SADP) and EDS profile were acquired in JEOL JEM-2100F fiel emission TEM equipped with Oxford EDS.
2.Phase-fiel modeling
The multi-phase-fiel approach with a locally linearized phase diagram scheme was applied to simulate the solidifi cation microstructure evolution during the eutectic condition[11-14].In this approach,each grain/phase is assigned a distinct phase-fiel parameter,φp(x,t) ∈[0,1].The evolution of the phase-fiel parameter is described by the following equations:

whereMijis the interface mobility between phaseiand j,Nis the local number of the non-vanishing phase-fieldηis the interface thickness,andΔGijis the thermodynamic driving force.The anisotropic interface energy,γα−liq,is used for the interface betweenαand the liquid phases following the approach from Böttger et al.[15].For the interface between theβand liquid,as well as theβandα,isotropic interface energy is used.The evolution of the composition,˙c,is described by the following diffusion equation:
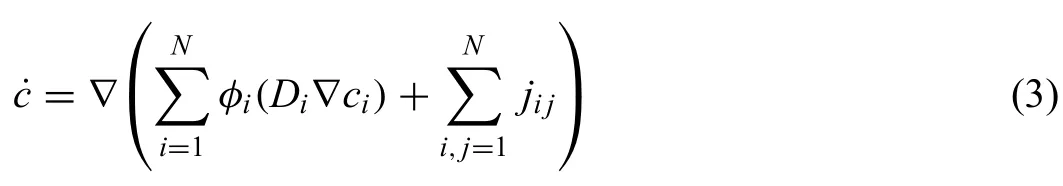
whereDiis the diffusion coefficien of the solute andjijis the anti-trapping current [14].The phase-fiel equation given above were discretized using the finit difference scheme.The heat extracted upon cooling is balanced with the heat of phase transformation as provided below:

whereρis density,cPis the heat capacity,nxyzis the discrete volume element,˙ψi jis the local transformation rate between pair-wise phasesiandjdefine byLijis the latent heat released during the phase transformation,and ˙Qis the heat extraction rate.
The nucleation model was adopted from Greer et al.[16],which determines the onset of the nucleation and growth of aseedcorresponding to the achieved undercooling.The seeds of each phase were randomly distributed over the simulation domain with their size following the normal distribution(only the positive tail of the distribution is used),

whereN0is the nucleation seed density,dis the seed diameter,μis the center of the distribution,andσis its standard deviation.The numerical parameters used in the simulation are listed in Table 1.

Fig.1.Experimental set-up.

Table.1List of simulation parameters.
3.Results and discussion
In the as-cast microstructures of specimens with various Al contents and cooling rates shown in Fig.2,equiaxed grain refinemen is evident with an increase in the Al content and cooling rate.The addition of Al promotes the formation of the eutectic structure consisting ofβand eutecticαephases in the interdendritic channel between primaryαpdendrites.The fractions of the eutectic region in Fig.2(a) and 3 in fast cooling condition are in reasonable agreement with the calculated solid fractions of 7,18,and 31 mol% for 5%,10%,and 15%Al specimens from the Scheil-Gulliver solidificatio model using TCMG2 [17] database in Thermo-Calc [18].The actual eutectic fraction also depends on the cooling rate[5]since the phase transformation is controlled by the solute diffusion in diffusion-controlled eutectic solidificatio scheme [10].Previous works [12,13] by two of the current authors examined the effect of the cooling rate on the eutectic volume fraction using phase-fiel modeling.This study focuses on the Al content which has a significan impact on the morphology changes from the (partially) divorced eutectic to the coupled eutectic microstructure as shown in Fig.3.In the SEM micrographs,the bright region indicates theβphase and the dark phase indicates theαphase.The light hue around theβphase inside the darkαphase indicates the Al enrichment in the interdendritic region which often transforms into continuous and discontinuous precipitations.
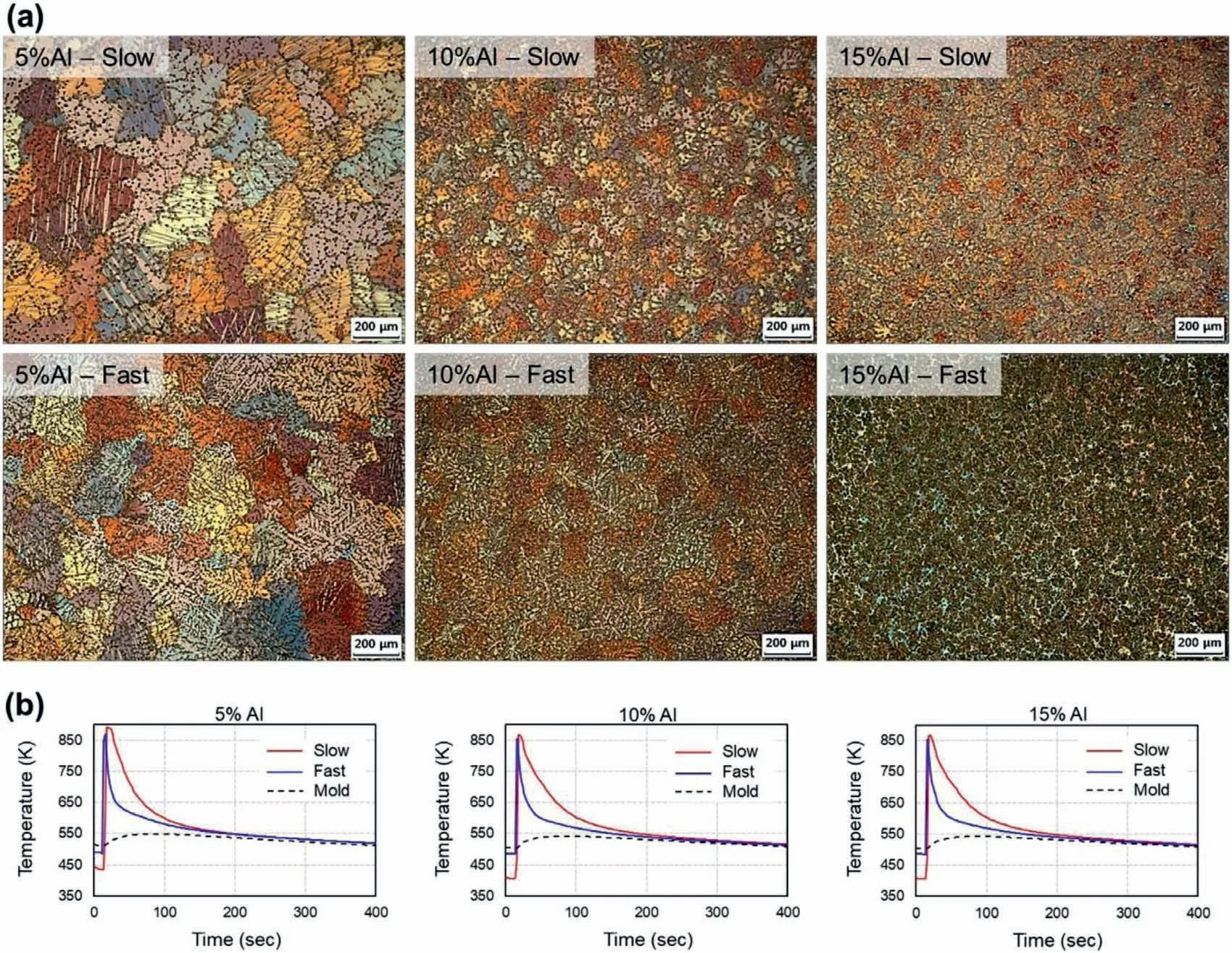
Fig.2.(a) PM micrographs showing as-cast grain morphologies corresponding to different Al contents and cooling rates and (b) cooling curves of the specimens directly measured in the melts.(For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.)

Fig.3.SEM micrographs of the primary and eutectic microstructures of the fast-cooled specimens containing (a) 5% Al (b) 10% Al (c) 15% Al.
As the Al content increases,the eutectic morphology changes from a fully divorced (5% Al) structure to a partially divorced (10-15% Al) structure [1-3].Microsegregation of Al in the liquid pocket isolated between theαpdendrites allows the formation of theβphase even if the initial composition of the alloy (5% Al) is far below the maximum solubility limit of Al in theαphase,which is 11% at the eutectic temperature.As the eutectic fraction increases with the Al content,its morphology undergoes a prominent transition from isolated globular grains to interconnected networks.The convex curvature of theβgrains in Fig.3(a) changes to the mixture of convex and concave in (b) and finall to the fully concave structure in (c),which suggests that the solidificatio of theβgrains with convex shape is completed before theαpdendrite reaches them.It is also noticeable that the halo ofβis formed along the perimeter of the eutectic region.

Fig.4.EBSD orientation maps (a-c) and phase maps (d-f,red: α phase,blue: β phase) of (a,d) 5% Al (b,e) 10% Al (c,f) 15% Al.(For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.)
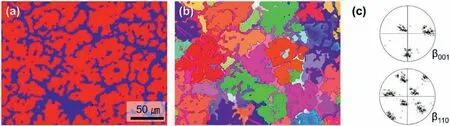
Fig.5.Large-area EBSD maps of 15% Al specimen (a) phase map (red: α phase,blue: β phase),(b) orientation map (c),and (001) and (110) pole figure of β from (b).(For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.)
The size of theβgrain can be estimated from the comparison between the orientation and the phase maps in the EBSD analyses shown in Fig.4.The isolated and partially connectedβgrains in Fig.4(a,b) grow up to approximately 20 μm as single grains.Conversely,the eutectic network which spans over several tens ofμm consists of multipleβgrains in Fig.4(c).Due to the effective resolution limit of the EBSD analysis,theαegrains inside theβgrains are not visible in the low-magnificatio EBSD analysis.However,the large-area EBSD maps of the 15% Al specimen and the corresponding(001) and (110) pole figure of theβgrains in Fig.5 confir that the orientation of theβgrains does not significantl differ.Such a similar but discrete distribution of theβorientations could be due to the independent nucleation events in a locally confine thermal condition.Theβhalo stretched over multiple grains in the eutectic region can be explained by the preferential growth of theβphase along theαpperimeter where the Al rejected from the growingαpfront is piled-up.Theβphase grows by consuming Al in the liquid,and reduces the Al composition of the melt allowing the formation ofαein a eutectic manner.The EDS composition profil of a partially divorced grownβandαeof the 10% Al specimen in Fig.6 shows a smooth transition of the Al profil across theαp/βboundary.
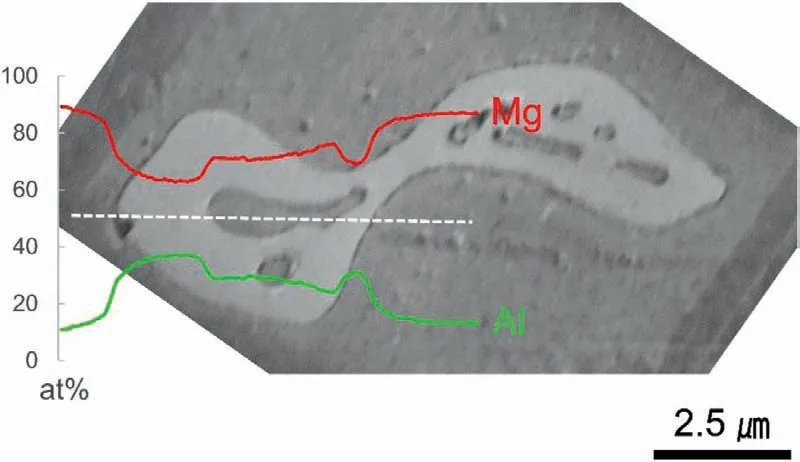
Fig.6.EDS composition profil of a β grain in 10% Al specimen.
In technical Mg alloys,increasing the cooling rate,decreasing the Al content,and increasing the Zn content are known to promote divorced eutectic morphology by reducing the amount of eutectic region developed at the end of the solidificatio sequence.Meanwhile only weak evidence of the(partially) divorced eutectics was presented,which can distinguish between divorced eutectics and isolated coupled growth,mainly due to the limitation of the technical resolution.In addition to the regular eutectic morphology,the isolated secondary phase (αein this case) can be grown in a eutectic manner with rod/irregular lamellar shapes[19-21].In addition to the detached growth,divorced eutectics rarely incorporate the interactions between two eutectic phases such as solutal exchange or orientation relationship (OR).A thorough literature review reveals the occurrence of highly non-sphericalβgrain at theαgrain boundary or in the bulk of theαwith six preferential ORs in the solid-solid phase transformation[22-25] and one relationship in directional solidificatio [26].While these crystallographic relationships are not reported in the equiaxed solidificatio structure,they could indicate a preferred orientation of a nucleus at the interface to the opposite phase.Table 2 shows the list of previously reported ORs between theαandβphases.To examine possible ORs between the eutecticβandαegrains,seven reported ORs are checked in a partially divorced eutectic region of the 10% Al specimen using EBSD.In Fig.7(b-h),the red lines in each map indicate the matching boundaries with OR and the gray lines indicate the phase boundaries without OR.When these ORs are checked in EBSD within fi e degrees of tolerance for both planes and directions,the results reveal that no reported OR occurs between the eutecticβandαephases.Whereas theαpandβphases show some matching ORs as indicated with the white arrows in Fig.7,which corresponds to the precipitation in the solid state.

Fig.7.Seven reported ORs between the α and β phases (a) EBSD phase map of a partially divorced eutectic region in 10% Al specimen (b) Pitsch-Schrader OR (c) Burgers OR (d) Crawley OR (e) Porter OR (f) Potter OR (g) Gjönnes-Östmoe OR (h) Ryum OR.

Table.2List of ORs between the α and β phases.
Further analyses were conducted for 40αegrains on the surface of the specimen in Fig.7 and for three grains beneath the surface with respect to the adjacentαpandβgrains.The vertical sectioning using FIB confirm that theαegrains are not part of the fibrou eutectic morphology as shown in Fig.8(a).The spatial arrangement of the grains and their relative orientations were determined by the stepwise angle records from the EBSD orientation maps,the FIB sectioning in the designated cutting angle close to the [0001] zone-axis of theαpgrain in the target region,and the specimen tilting in the TEM analysis.The SADP micrographs of theαegrains buried under the surface in Fig.8(a) show that grain 1 and 2 are close to the [0001] zone-axis but rotated in the plane by 2.18° with respect to each other.Conversely,the c-axis of the grain 3 is slightly misaligned with respect to the beam incident direction and that of grain 1 and 2.The zone axis patterns inside theαegrains in Fig.8(a) also indicate that these grains are of a different orientation.Fig.8(d) shows the SADP micrographs of theαpandβgrains adjacent to theαein Fig.8(a).
When the orientation of theαegrains on the surface is compared to the adjacentαpgrain marked as point 9 in Fig.8(b),eachαegrain shows a high misorientation angle with a maximum of 65° as presented in the (0001) pole fig ure in Fig.8(f).In Fig.8(e),relative orientations of theαegrains determined in the TEM and EBSD analyses are represented with hexagonal unit cells.Such a weak relationship between theαegrains suggests non-interactive nucleation and growth of theβandαegrains,which are the crucial factors in divorced eutectic morphology.While each of theαegrains has different orientations,some neighboring grains exhibit associated arrangements within a few degrees which might be a result of the similar thermal conditions analogous to theβgrains in Fig.5(c).
The absence of the solutal exchange in the divorced eutectic determines the characteristic morphology of theαegrains in its chemistry and shape.Fig.9 compares the Al distribution between theαeandαpgrains,which display a fla Al concentration profil within theαegrain that is lower than the eutectic composition (Fig.9(a)).Conversely,Fig.9(b) shows that Al is enriched in the last solidifie region owing to the solutal rejection from the growingαpfront.During eutectic solidificatio the nucleation of theαephase can be promoted if sufficien Al is consumed by the growingβphase and the Al concentration in the liquid drops below the eutectic composition.When theαephase grow cooperatively with theβphase,gradual concentration changes in the adjacentαeandβgrains are observed with the highest Al concentration at theα/βboundary as shown in Figs.6 and Fig.9(b).However,sharp concentration changes on theαe/βinterface and the fla distribution of Al in Fig.9(a) indicate that theαeandβare grown without a solutal exchange in the divorced eutectic scheme.

Fig.8.The orientation analysis of the αe grains (points 1 to 8) with respect to the αp grain (point 9):(a) TEM micrograph taken from beneath the surface and the schematic illustration of the FIB sampling and (b) EBSD orientation map.The dot-dashed red line indicates the FIB sectioning plane and R indicates a reference point for the FIB sampling (c) SADP of for three αe grains with α-tilt:−1.8° and β -tit:−2.8°,(d) SADP of the αp and β grains around the αe,(e) relative orientations represented in the unit cell of the α phase,and (f) (0001) pole f gure for αe grains from (b).(For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.)
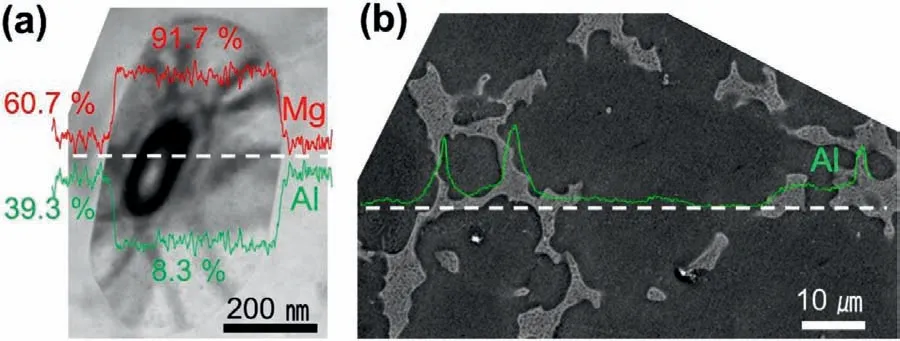
Fig.9.Al and Mg distribution along the white dashed line measured using EDS (a) αe and β grains having fla concentration profiles and (b) αp and β grains showing smooth Al enrichment toward the dendrite growing direction.(For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.)
To understand the competition between cooperative growth and the nucleation-dominated formation of the divorced eutectic structure,phase-fiel simulations were performed.The detailed simulation scheme was provided in previous works[12,13].This study concentrates on simulating the remaining liquid pockets in theαpinterdendritic region near eutectic temperature.The simulation started from a residual liquid pocket with the eutectic composition surrounded by theαpphase.The system was cooled starting from the eutectic temperature with the experimentally determined cooling rate of 25 K/s for the 10% Al-Fast specimen in Fig.2(b).Theαpwas still growing as the system was cooling.Following the experimental observations,the nucleation density of theβphase was set to be 10 times lower than the nucleation density of theαephase.and all nuclei were assigned random and uncorrelated orientation.Both theαeandβphases were allowed to nucleate at the same time in the entire volume of the liquid pocket if the thermodynamic driving force is sufficien to overcome their nucleation barriers set by the corresponding critical nuclei diameter.Once the undercooling is sufficient the nucleation of theβphase occurs first and a small number of grains begins to grow in the residual liquid as shown in Fig.10.A strong tendency for theβphase to grow on the perimeter of theαp/liquid interface can be observed in Fig.10(b),which is due to the strong segregation of Al rejected by the growingαp.Since the solubility of Al in theαphase is lower than in the liquid,enriched Al concentration in front of theαpdendrite results in the preferential growth of theβphase along theαp/liquid interface.During the growth of theβphase along theαpboundary,the cooling stops,and the temperature even increases toward the eutectic temperature due to the significan latent heat release.At the same time,the nucleation of theαephase is also activated,but actual nucleation does not occur due to insufficien thermodynamic driving force.Before the onset ofαephase nucleation,theβphase fully covers theαp/liquid interface as shown in Fig.10(c) in a manner analogous to the experimental microstructure in Fig.8(b).Thus,the simulation supports the cooperative growth of theαpandβphases involving solutal exchange as the firs stage of the eutectic solidificatio process.Subsequently,when the surface ofαpphase is fully covered by theβphase,further growth ofαpphase is restricted,and the remaining Al in the melt is consumed to form theβphase,reducing the Al concentration in the liquid.This brings the system into the state where theαephase nucleation that completes the eutectic solidificatio process of the remaining liquid pockets presented in Fig.10(d-f).

Fig.10.Nucleation and growth of the β phase in the liquid pocket near the eutectic temperature followed by the nucleation and growth of the αe phase in the residual liquid.(a-c) The β phase nucleates randomly in the liquid but preferentially grows along the perimeter of the αp/liquid interface and covers it entirely.(d-f) Nucleation and growth of the αe phase leading to complete solidification The remaining liquid phase was colored opaque yellow.(For interpretation of the references to colour in this figur legend,the reader is referred to the web version of this article.)
4.Conclusion
The solidificatio morphology of the Mg-Al alloys exhibiting a (partially) divorced eutectic structure was investigated with respect to the alloy composition and the cooling rate.The results show that during the interdendritic solidification eutecticβphase formed a closed shell around theαpdendrite by following the Al enrichment along theαp/liquid interface.In contrast to the prominent solutal exchange betweenαpandβphases,the divorced eutectic structure followed the formation ofαephase after rapid coverage of theαp/liquid interface by theβphase and consequent inhibition of further growth of theαpdendrites.The weak OR and the sharp solute concentration change between theβandαegrains in the eutectic region suggest non-interactive nucleation and growth of a partially divorced eutectic structure in the Mg-10%Al alloy.The experimental finding were supported by the phase-fiel simulations which are in good agreement.The phase-fiel simulation successfully depicts the formation of theβphase halo on theαpinterdendritic perimeter and partially divorced eutectic microstructure consistent with the experimental observations.
Acknowledgement
This research is supported by the Fundamental Research Program of Korea Institute of Materials Science (PNK7760 and PNK7770) and the National Research Foundation of Korea (2020R1A2C2008416 and 2021M3H4A6A01049712).
 Journal of Magnesium and Alloys2022年6期
Journal of Magnesium and Alloys2022年6期
- Journal of Magnesium and Alloys的其它文章
- EDITORIAL BOARD
- Aims and Scope
- Surface oxidation study of molten Mg-Al alloys by oxide/metal/oxide sandwich method
- Production and characterisation of new bioresorbable radiopaque Mg-Zn-Y alloy to improve X-ray visibility of polymeric scaffolds
- Quantitative study on the tension-compression yield asymmetry of a Mg-3Al-1Zn alloy with bimodal texture components
- Promoting wetting of Mg on the SiC surfaces by addition of Al,Zn and Zr elements:A study via first-principl calculations
