Production and characterisation of new bioresorbable radiopaque Mg-Zn-Y alloy to improve X-ray visibility of polymeric scaffolds
Alok Srivastava,Naresh Bhatnagar
Mechanical Engineering Department,Indian Institute of Technology,Delhi,India
Abstract For the medical diagnosis,radiopaque materials (RM) made from high specifi gravity elements like Pt,Au,Ta,Iodine,Bromine are either attached or blended or coated on an implant to makes it detectable under X-ray/Fluoroscopy/CT-Scan.RM facilitate the surgeon in an operation theatre to position an implant during the surgery.Mainly,RM are non-degradable,thus in case of biodegradable implants,it may detach from the body and accumulate in vital organ cause serious health issue.Therefore,a new bioresorbable radiopaque material (BRM)was produced by alloying the high specifi gravity elements Zn (35% w/w) and Y(4% w/w) with Mg metal.In this alloy,three main phases were identified alpha Mg,Mg7Zn3 and icosahedral quasicrystalline I-phase Mg3Zn6Y,which reinforce the Mg matrix.Hereafter,BRM was powdered to a size of less than 25 microns and blended in different ratios with bioresorbable poly-L-lactic acid(PLLA) for fabricating PLLA/BRM bio-composite.BRM microparticles were uniformly distributed and interfacial bonded with the matrix.The X-ray was passed through bio-composite to capture μCT radiograph for evaluating linear attenuation co-efficien (μ) and optical density(OD).Thermal analysis reveals that BRM particles act as a nucleating site and enhance the crystallinity of the polymeric chain.During the In Vitro accelerated degradation study,the alkaline nature of BRM neutralise the acidity of PLLA and balance the pH of the body flui to reduce the inflammator reactions,but this compromises the stability of the polymer as it increases the decomposition rate.
Keywords: Magnesium alloys;X-ray;Quasicrystal;Polymer matrix composites;Radiopacity.
1.Introduction
To improve the life expectancy of a human,medical and digital technologies should interact with each other to overcome challenges.One of such digital technology is the X-ray,where an implanted device is required to be observed in cardiovascular,lymphatic,neurological,integumental,skeletal,optical,nasal and oral [1-3].Therefore,to improve radiographic visibility,a sufficien amount of radiopacity is required to detect the precise location of the implant at the site of a lesion or deployment.Additionally,it can also help to monitor the healing process and degradation rate of bioresorbable implants [5].
Herein,radiopacity is a characteristic value that is directly proportional to intrinsic properties of a material like an atomic number,specifi gravity,thickness and energy of an incident beam.As it is known the bioresorbable scaffolds like PLLA,PLGA,PCL or Mg alloy are composed of low atomic number elements like H,C,O,Mg,due to their low electron density,the X-ray can easily pass through these materials without any hindrance or attenuation.Thus to render X-ray,many researchers have attached RM with polymers either by polymerising/chemical conjugation/physical blending or using some advance technologies like 3D printing system and electro-spinning [4-8].Wherein,bioinert RM is produced from heavy metals or its salts or oxides such as Au,Pt,Ta,Gd,BaCl2,ZrO2,calcium tungstate (CaWO4),ytterbium trifluorid or with limited bioresorbability like BaSO4,iodine and bromine [9-14].Recently,bio-inert or biocompatible radiopaque nanoparticles was developed from BaSO4,gold,iodixanol,Fe3O4to improve radiopacity in polymer,but research is in progress to identify their effect on the human body [15-17].However,the attachment of RM on a bioresorbable scaffold is a challenging work as it may fla e or leak out from the implant body during the degradation process.As a consequence,it can either hamper the blood fl w or deposited in the organ that can further lead to a severe medical issue.Moreover,it may initiate the galvanic corrosion due to their difference in electronegativity,hence accelerate the degradation rate and compromise the mechanical performance of implants [18].
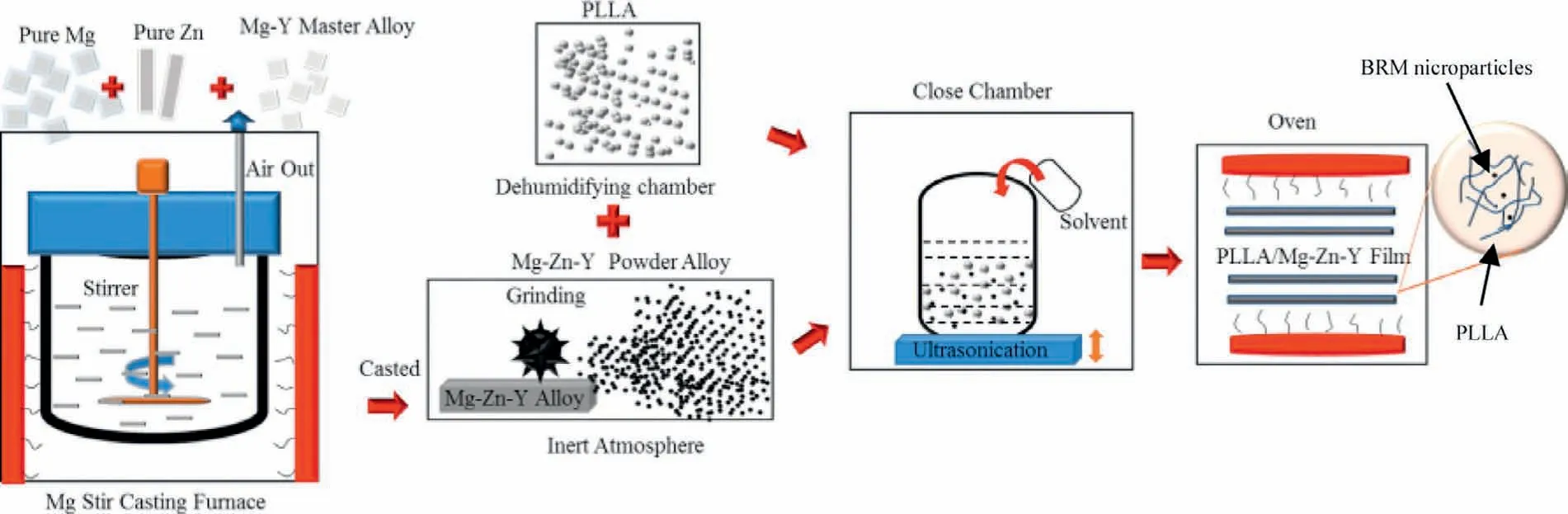
Fig.1.Schematic layout for producing PLLA/BRM films
To overcome the limitation of permanent radiopaque materials,here in this study,BRM is produced from Mg-Zn-Y alloy.In this the high specifi gravity elements like Zn and Y were chosen to impart radiopacity and act as a grain refine in Mg alloy,thereby contributing an increase in strength with improved high-temperature corrosion-resistance[19,20].In this alloy,Zn forms a binary phase Mg7Zn3,whereas Y promotes a stable intermetallic icosahedral quasicrystalline (I-phase) Mg3Zn6Y as a precipitate to reinforce the Mg matrix [21].As major elements like Mg and Zn are bioresorbable,contribute nearly 96% w/w of the alloy,whereas,Yttrium is present below the toxicity limit of 4.2 mg day−1[22-24].Similar existing composition of Mgalloy WE-43 (Yttrium=3.7-4.3%,Neodymium=2.4-4.4%,Zirconium=0.4-1%,Zinc=0-0.2% and remaining Magnesium) is already been used as a commercial biodegradable biomaterial by Biotronik Inc [22,25-28].
Furthermore,Mg alloy is non-toxic,show anti-bacterial features and increases the bioactive site.Despite their excellent biocompatibility,the rapid in vivo corrosion rate approximately 2-6 month is one of the constraints which restrict the clinical applications as it fails to match with healing rate[29-31] As Mg alloy is prone to react with Cl−ions present in the body flui to form MgCl2,this salt deposited near the site cause debonding between the interface of tissue and implant[32-34].It is further complicated as this reaction evolves hydrogen gas,which can cause embrittlement,failure of the implant and other health issues.However,it can be controlled by obstructing the direct contact of the body flui by coating or surface modification
Thus,in this work,Mg alloy was blended in different ratios with polymer PLLA to control the degradation and simultaneously introduce a radiopacity in the polymer.The polymer coating can reduce the degradation of alloy,but due to the hydrophilic nature,the body flui ions diffuse into the polymer matrix,creating a pathway from where localised pitting corrosion starts in the composite.As a result,leaching of Mg alloy can be faster in the human body but less severe as compared to permanent RM,although it can directly influ ence the amount of radiopacity in the polymer.Therefore,it is crucial to balance the degradation kinetics of the composite as it facilitates in monitoring endothelialisation rates and implant stability.
2.Materials and method
Commercially available pure Mg (99.95%),pure Zn(99.99%) and Mg-30wt.% Y master alloy were melted in a stir casting furnace under an inert atmosphere.The melt was stirred and held at 750 °C for 30 min,then poured into a steel mould.Casted BRM was powdered to a size less than 25 μm in an inert atmosphere to avoid oxidation,after that,the microparticles were blended from 0%,5%,15%,25%,50% and 100% w/w with PLLA (Ingeo 4032D,Naturework LLC,USA).Further,PLLA/BRM slurry was produced by solvent casting method using chloroform as a solvent and kept under vigorous mixing for 2 h of ultrasonication at a frequency of 25 kHz.The resulting slurry was used for fabricating thin bio-composite sheets of size 150-200 micron,followed by drying in a vacuum oven at 50 °C for 24 h.The schematic layout of the process adopted is shown in Fig.1,where BRM microparticles as a fille were properly entrapped and uniformly dispersed within the PLLA matrix.
2.1.Characterisation
The morphologies of BRM were observed by using scanning electron microscopy (Zeiss EVO 50,Germany) at 15 kV,whereas elemental analysis was performed by energy dispersive spectrometer (EDS).The phase volume fraction was estimated by quantitative metallographic techniques using randomly selected areas.Identificatio of phases was performed by an X-ray diffractometer (XRD,Rigaku DMAX 2400),using Cu Kαradiation.Diffraction patterns were generated between 2θvalues range from 10 to 80°,at a step increment of 0.02° and a scanning speed of 5°/min.
Thermogravimetric analyzer (DTA 6000,M/s.Perkin Elmer,USA) was used to evaluate the thermal properties of BRM.Powdered sample<10 mg was heated in a nitrogen atmosphere from 30 °C to 700 °C,followed by a dwell of 2 min.Similarly,from the same DTA instrument,the thermal decomposition and crystallinity of the bio-composites were evaluated.The samples of weight 5-10 mg were scanned from 25 °C to 400 °C with the heating rate of 10 °C/min in a nitrogen atmosphere purged at 20 ml/min.DSC analysis demonstrates the effect of Mg alloy on the crystallinity of the polymer.Likewise,from TGA thermograph curves,T10and T50were measured correspond to the temperature after the mass loss of 10% and 50% respectively.The residual mass of the samples (ωr) and the slope of the curves (Dr) in the temperature range (Tr) were recorded.
The surface morphology and distribution state of the BRM in the composite specimens were examined using SEM.Before this samples were cut and sputtered with gold to improve the conductivity of polymer for better image quality.The radiopacity of bio-composite was demonstrated by using digital radiograph captured by μCT scan (Rigaku model CT Lab HX) at 55 kV,current of 44 μA at a scan speed of 300 ms.The linear attenuation coefficien of the specimens was calculated from the software Materialize-mimics medical-19.0.Optical density was evaluated by ImageJ-1.8 software where the X-ray radiographs were compared with a Kodak No.3 calibrated 21 step tablets scanned from Epson Expression 1680 Professional scanner.
In Vitroaccelerated degradation tests were performed according to the ISO 13,781:1997.The fil sheets (n=3) were completely dipped in the phosphate-buffered saline (PBS) solution of pH 7.35.The ratios of the PBS solution(ml) to surface area(cm2) and sample weight(g) were greater than 50:1 and 30:1,respectively.Julabo shakerR○(Germany) was used to maintain constant incubation temperature of 70± 0.1 °C above Tgof PLLA and agitate the body flui at 45 rpm.After each degradation periods 1,3,5,and 7 days,samples removed from PBS were thoroughly washed in DI water before drying in an oven at 50 °C for 24 h.The pH of PBS solutions and the mass loss of bio-composite after each degradation intervals were reported.
3.Results and discussion
3.1.Scanning electron microscope (SEM)
FE-SEM with EDS analysis was conducted on BRM to demonstrate the elemental distribution of Zn and Y metal inside the Mg matrix,as shown in Fig.2a.In the as-cast alloy,three main phases were recognised,i) primaryα-Mg(Fig.2b),ii) Mg-Zn binary phase,and iii) Mg-Zn-Y ternary phase.The binary phase is shown in Fig.2c) corresponds to Mg7Zn3as its Zn/Mg (at%) stoichiometric ratio 2.8,which was found close to the EDS value 2.4 [35].For ternary phase(Fig.2d),the Zn/Y found to be 7.6 from EDS this matches to the combined ratio of Mg3Zn6Y (I-phase)+α-Mg lies in the range of 6-8 [36-39].
Thus,Zn and Y in the Mg forms intermetallic eutectic phases,which strengthen the matrix.It is observed that quasicrystal I-phase was precipitated in the inter-dendritic region of anα-Mg matrix to reinforce the metal matrix [40-42].The volume fraction of alloy was measured from Image-J software,where I-phase was found to be in the range of 10-15%,binary phase Mg3Zn7contributes 22-24%,the rest wasα-Mg phase.From the annealing process,the fraction of quasicrystal I-phase in the matrix can be raised this directly improve the mechanical properties and corrosion resistance [43].
3.2.X-ray diffraction (XRD) analysis
The XRD analysis (Fig.3a) of as-cast Mg35Zn4Y confir the presence of three phases distributed in the alloy,α-Mg,Mg7Zn3and icosahedral I-phases [44].It was noted thatα-Mg phase crystal structure was hexagonal closed packed whereas,Mg7Zn3phase is orthorhombic with space group Immm [45-47].
Indexing of I-phase of space group Fm53 was determined by using Elser’s method where the quasi-lattice parameter was calculated to be 0.734 nm from the diffraction peak of (211,111) [48,49].According to Bae and Singh,I-phase/α-Mg have excellent interfacial bonding,this result in better mechanical properties,low coefficien of friction,low interface energy,better creep and corrosion-resistant at high temperature [24,30].
3.3.Differential thermal analysis (DTA)
During continuous heating of BRM particles in differential thermal analysis (DTA),the firs (i) endothermic valley appeared at 340 °C,which can be recognised as the start of melting eutectic pockets of Mg7Zn3phase as indicated in Fig.3b) [51].The binary phase show low thermal stability and ageing temperature,whereas after the addition of Yttrium eutectic point was further improved.However,the solubility and diffusivity of Y were limited due to larger atomic size more than 12% of Mg,leads to the precipitation of I-phase[38,52,53].At 450 °C assign with point (ii),melting of eutectic I-phase pockets initiate from the interdendritic region of the alpha Mg phase [54,55].It can be associated with the dissolution and redistribution of Yttrium in the Mg matrix [56].
When the temperature reaches 540 °C,an exothermal peak was observed at (iii) from where the alloy powder starts oxidising and end at point (iv).Simultaneously it can be correlated with a peak at (v) in the TGA curve,where the weight of BRM powder suddenly increases to 125% at 695 °C.Although the alloy was heated in high purity of N2atmosphere,there is an unavoidable presence of oxygen and water vapour resulting in the oxidation of BRM powder.
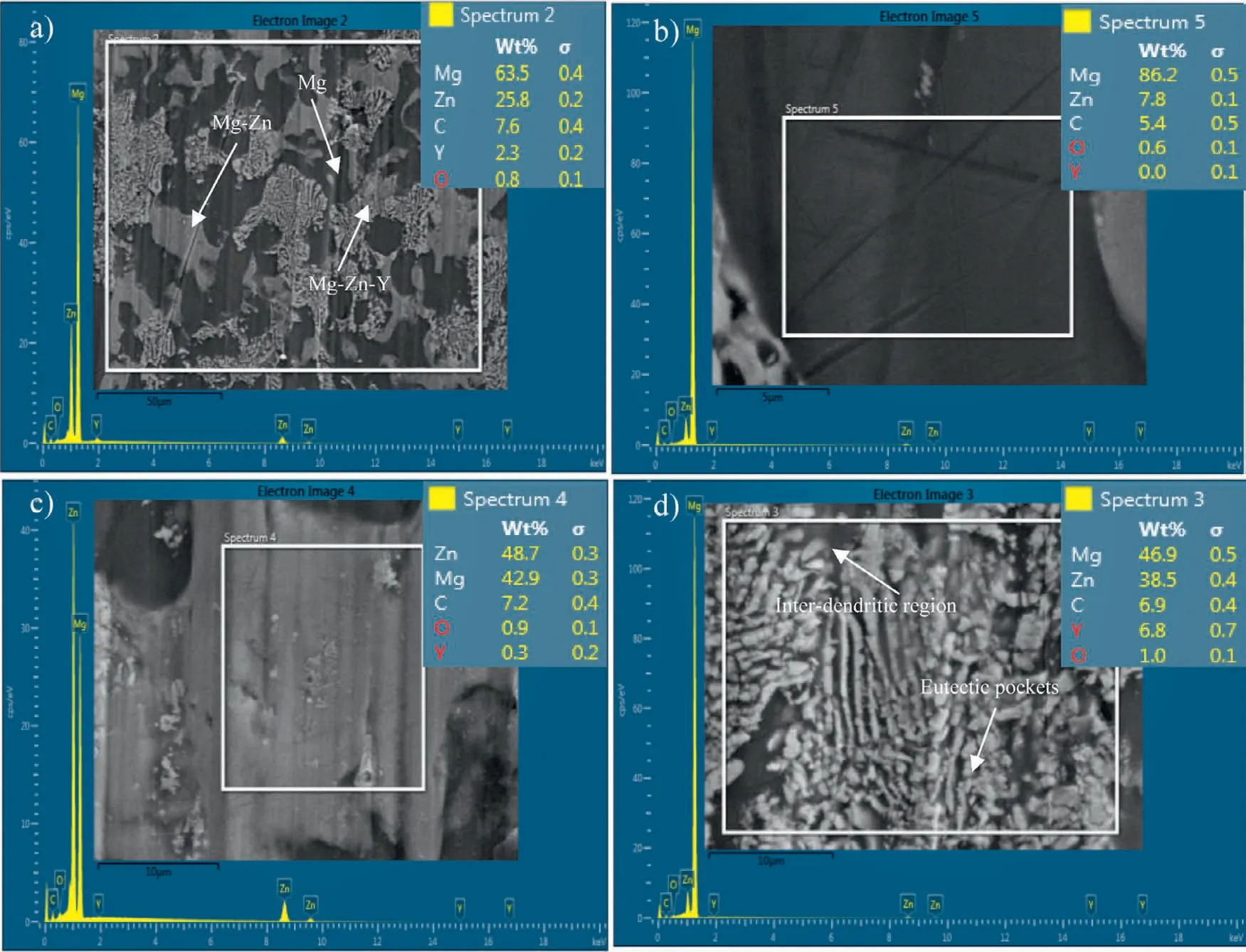
Fig.2.SEM show microstuture and EDS analysis was used for elemental distribution-a) phases distribution in Mg-Zn-Y,b) represent Mg primary phase,c)Mg-Zn binary phase and d) Mg-Zn-Y ternary phase precipitated in interdentritic region.

Fig.3.a) XRD patterns of as-cast Mg-Zn-Y alloy and b) DSC and TGA analysis of as-cast Mg-Zn-Y alloy powder.
3.4.Surface morphology
The distribution state of the metallic particles in the polymeric matrix was observed in the micrographs Fig.4(a-e)obtained from SEM.They reveal a homogeneous distribution of Mg particles indicating that the blending time was enough to disperse the filler in the matrix with no aggregation.The size of particles was approximately 25 μm calculated from the SEM images.At higher magnificatio (Fig.4f) show the irregular shape particles were attached to the matrix due to the micro-anchoring action [57].Fig.4g,show the mapping of Mg alloy microparticles in the matrix,which is quite well embedded as well as interfacially bonded with the matrix without any noticeable defects,such as cracks or delamination.Thus,the alloy coating with the polymer protects them from the direct exposure to the corrosive body flui environment this ultimately controls the corrosion behaviour.Furthermore,the EDX analysis (Fig.4h),confirm the Mg-Zn-Y alloy was blended with the bio-polymer PLLA.

Fig.4.(a-e) show surface morphologies of PLLA with (0,5,15,25 and 50wt.%) of Mg alloy f) SEM at a magnificatio of 5000X indicate that microparticle was embedded and bonded with the matrix without any defects or agglomoration,g) Mapping of Mg particle shown in red colour indicate distribution of alloy inside the PLLA matrix and h) EDX analysis showing Mg alloy blended with bio-polymer PLLA.
As expected,the surface of biocomposites becomes rougher after adding irregular microparticles.This microrough surface can improve the cellular response as it provides a platform for the host cell to anchor on an implant [58-60].Moreover,the Mg particles significantl enhance the bioactivity of scaffolds,which might further improve the healing process.
3.5.Radiopacity
An implanted device should have sufficien attenuation of electromagnetic waves produced by an X-ray/fluorosco y/CT scan for better diagnosis.RM are usually elements with large atomic numbers that have k-/l-edges of higher energies than the tissue to facilitate the absorption of X-ray photons.As per Stinson,linear attenuation coefficient (μ) for any RM should be higher than 5.46 cm−1at 50 KeV and thickness must be more than 20 μm depending upon the application[61].Additionally,to make bioresorbable scaffold radiopaque,it should have at least one alloying element of atomic number range from 22 to 83 [62].
As per the above criteria,BRM was produced by alloying Mg with Zn(35% w/w) and Y(4% w/w) of atomic number 30 and 39,respectively.The objective was to achieve the overall effective atomic number (Zeff) greater than 22,which was calculated by the Markowicz-Van Grieken expression given in Eq.(1),

Where‘i’is the number of elements in the alloy,Wi,AiandZiis the weight fraction,the atomic fraction and the atomic number of theith element,respectively.From the above equation,Zeffof the BRM was estimated to be 23.92,which is more than the desired value required to make it radiopaque.
Digital radiographic images obtained from μCT-scan were used to compare the μ and OD of PLLA/BRM bio-composite,where BRM content was varied from 0%,5%,15%,25%,50%and 100%.In Fig.5a,neat PLLA is shown in dark grey with μ value of 0.312 cm−1,which recognise as a radiolucent material.On the other hand,100% of BRM (Fig.5f) is visible in white colour and μ was measured to be 8 cm−1,which is above the acceptable limit makes required for medical diagnosis.As shown in Fig.5b-e,as BRM quantity increases in PLLA,X-ray visibility also gets gradually enhanced.It can be seen from the μ-CT radiograph,due to the strong radiopacity of BRM particles it was easily distinguished with a clear boundary from PLLA.

Fig.5.μCT-Scan of different materials where white colour indicate BRM particles a) Pure PLLA,b) PL LA/BRM(5%),c) PLLA/BRM(15%),d)PLLA/BRM(25%),e) PLLA/BRM(50%),f) Pure BRM and g) Comparision between the optical density of PLLA with different ratios of BRM addition.
It was further calculated as per ASTM standard F640-12,where“Radiopacity”is determined by measuring the optical density (OD) or pixel intensity of test specimen and the image of a user-define standard,with or without the use of a body mimic.Here,the optical density (OD) refers to the degree of blackening of the radiographic fil as given in Eq.(2) [63-65].

Where Iorepresents incident X-ray intensity on the sample,Isis the intensity of transmitted X-ray from the specimen to the radiographic fil or a digital system,whereas ‘t’ and μ is the thickness and linear attenuation coefficien of the sample,respectively.
By using the above equation,the optical density (OD) was evaluated from the scanned radiograph images and compared with the calibrated step tablet.As per the industrial codes and standards,the typical range of OD should be between 2 and 4 for acceptable X-ray viewing.OD value of 2.0 and 3.0 are the results of only 1% and 0.1% of the incident X-ray pass through the sample,respectively.
As shown in Fig.5g,OD reading of pure PLLA is less than 0.5,whereas,after the addition of BRM in PLLA,OD of bio-composite increases.It was found that minimum 5wt.%of BRM content in PLLA show OD>2 had achieved enough radiopacity to makes it detectable on radiographic film
3.6.Thermal stability
In Fig.6a,the presence of Mg alloy in the PLLA matrix does not affect the glass transition temperature (Tg) and melting point (Tm) whereas,the crystalline temperature (Tc) was found to be absent [38,39].Thus,the enthalpy of crystallisation ‘ΔHc’ corresponding to the area under the curve at Tccan be neglected.The enthalpy of melting ‘ΔHm’ at Tm,was estimated from the heating curve of the polymeric sample.
Based on Eq.(3),the degree of crystallinity was estimated,where,is the latent heat of fusion of 100% crystalline PLLA polymer=93.7 J/g andη={(1-(ωr/100)} is the weight fraction of the PLLA present in the composite.

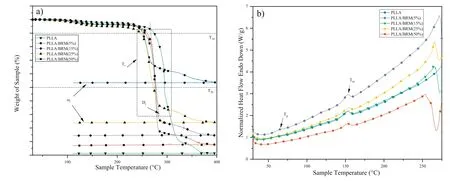
Fig.6.Comparision between polymer and composite-a)DSC and b)TGA thermograph.
The results shown in Table 1,validate that the crystalline structure of the PLLA got improved as inorganic microparticles acted as a nucleating agent and aligned the polymeric chains.It was also noted that crystallinity increased with the addition of Mg alloy.From the literature,bulk crystallinity improve the barrier properties and stability[66,67].But at high temperature due to an auto oxidant nature of Mg,it can initiate free radical reactions and scissor the ester bond of PLLA,thereby compromises the thermal stability [68].

Table 1 DSC and TGA data of PLLA with different addition ratio of Mg alloy.
Thermogravimetric (TGA) curves of the composites were evaluated to explore the thermal stability.Presence of BRM particles significantl decreased the thermal decomposition temperature (T10) and (T50),as shown in Fig.6b.It is due to the metal particles directly influence the bulk heat conductivity of the composites,which increases heat transfer in the samples result in high thermal degradation [69,70].However,the slope of cure (Dr) in the temperature range (Tr) decreases with BRM content signifie the rate of decomposition was slower at higher temperature as compared to pure polymer.
At the end of the heating curve,the residual weight (ωr)in the composites validate the amount of BRM present in the blends.A similar study was carried out on PLLA-Mg composite,where particles enhance the crystallinity of the polymer as well as improve an interface bonding [66].These results indicate that the oxidising nature of alloy instigates a random breakdown of the ester bond and compromises the thermal stability.
3.7. In Vitro accelerated degradation study
Controlled degradation rate is essential criteria for the success of bioresorbable medical implants any mismatch with the tissue healing process can lead to failure.If the degradation rate is slow,it acts as a permanent scaffold and hinders or reduce the tissue recovery rate.On the other side,the higher degradation rate can cause structural failure of implants before tissue remodelling.As per the study,depending upon the implant site,the bioresorbable scaffolds should stay approximately 9-12 month in the human body for proper replacement or recovery with the tissue.Therefore it becomes essential to balance the degradation rate with tissue recovery rate,which mainly depends on the selection of material and location of the implant [71].
In the case of PLLA,the degradation takes 2-3 years for total resorption depends on the site of implant,thickness,molecular weight and crystallinity [72].On the other hand,the Mg alloy under the corrosive physiological environment fails within 6-month due to the H2gas evolution causing embrittlement to the implants and accumulation of Mg salts at the interface weaken the bond with host tissue.
Therefore,the coating of alloy with PLLA can slow down the corrosion rate of Mg but increase the overall degradation rate of composite.It can be confirme from theIn Vitrodegradation study carried out at a temperature above the glass transition temperature (Tg) of the polymer.For a better understanding of degradation kinetic,BRM with varying wt.% in PLLA was dipped in PBS solution to measure their mass loss and pH variation for a comparative assessment and usefulness of alloy composition.

Fig.7.a) In Vitro accelerated degradation process for PLLA/BRM biocomposite,b) pH variation for 0%,5%,15%,25% and 50% of BRM addition in PLLA with immersion time,c) weight loss vs the amount of BRM content in PLLA and d) graph showing the relationship between first-orde rate constant and Mg alloy content in the polymer.(∗p <0.05).
It was assumed thatIn Vitrodegradation of bio-composite occurs in four phases as shown in Fig.7a,this starts with a faster diffusion of water molecules and chloride ions in the polymeric matrix due to hydrophilic nature of BRM.As a consequence,corrosion of Mg-Zn-Y alloy in body flui produces Mg2+,Zn2+ions this increase the alkalinity of the surrounding tissue.Due to the formation of metal hydroxides(Me(OH)2)pocket near the particle/matrix interface the cracks were initiated and propagate inside the matrix [15,50-52].Simultaneously,metal ions act as a catalyst for PLLA that led to faster hydrolysis of ester bonds into the carboxylic end-groups,cause a rise in the acidity of PBS [53].At last,the metal ions interact with two moles of anionic PLLA to neutralise the charge by forming [Mg2+/Zn2+]·2[lactic acid-OH−] this stabilises the pH of body flui but increases the rate of degradation due to acid-base reaction [54-57].
It is improved from Fig.7b,where the pH of the medium was measured as a function of immersion time.During the degradation of pure PLLA pH of PBS continuously decreases as the degradation continues due to the formation of -COOH end group.However,in the bio-composite,with an increase in BRM content faster is the rate of reaction between their acid-base by-product.As a result,better is the stability in pH,but it also increases weight loss and degradation rate,as shown in Fig.7c.Thus,it becomes essential to optimise the BRM content in PLLA to balance pH and degradation.
The degradation of the polyester was used as a failure criterion for PLLA/BRM scaffolds and it was calculated by the first-orde kinetic mechanism as per Eq.(4) [73,74].

In this,k,C0,and C was the first-orde rate constant,the initial weight of specimen and weight remaining after time ‘t’of degradation,respectively.
The graph represents in Fig.7d)indicates that the rate constant (k) was increased as BRM content increase from 5% to 25% w/w and then decreases again.Initially,the degradation increase due to acid-base reaction between PLLA and Mg alloy,which give rise to the localised pitting corrosion[75].On the other hand,due to the equal ratio 50:50 of polymer and BRM encourage the uniform and even degradation as compare unequal pitting corrosion.Another reason may be a high crystallinity which acts as a corrosion barrier for the hydrolysis process and inhibit the acid-base reaction [76].
As BRM reduces the stability of PLLA approximately by 45%,still it gains sufficien healing time more than 12 months to replace an implant with the host tissue.
However,in the actual case,theIn Vivodegradation of polymers could be much faster thanIn Vitrobecause of additional cellular and enzymatic activities found in the body.
Conclusion
•Mg-Zn-Y alloy was cast as a bioresorbable radiopaque material(BRM).The radiolucent Mg was made radiopaque by alloying with high specifi gravity elements Zn (35%w/w) and Y(4% w/w).In Mg-Zn-Y alloy,two eutectic phases were identifie Mg7Zn3and highly ordered quasicrystalline I-Phase Mg3Zn6Y,apart from alpha-Mg.
•The intermetallic particles (I-phase) are thermally stable and precipitated in the inter-dendritic region of theα-Mg matrix.Additionally,the I-phase/α-Mg act as a particlereinforced metal matrix composite.
•PLLA/BRM bio-composites were prepared by using a solvent-casting method.Microparticles were uniformly distributed and bonded with the matrix.It was found that with the addition of 5%w/w of BRM produce enough radiopacity in the polymer that is required for medical diagnosis purposes.
•In Vitrodegradation show with an increase in the BRM quantity stabilises the pH of the body fluid but also increases the degradation rate and compromises the thermal stability of bio-composites.
•In future,to limit the role of permanent radiopaque materials,the Mg-Zn-Y alloys can be incorporated with any bioresorbable polymeric scaffolds.
Declaration of Competing Interest
The authors declare that they have no known competing financia interests or personal relationships that could have appeared to influenc the work reported in this paper.
 Journal of Magnesium and Alloys2022年6期
Journal of Magnesium and Alloys2022年6期
- Journal of Magnesium and Alloys的其它文章
- EDITORIAL BOARD
- Aims and Scope
- Surface oxidation study of molten Mg-Al alloys by oxide/metal/oxide sandwich method
- Quantitative study on the tension-compression yield asymmetry of a Mg-3Al-1Zn alloy with bimodal texture components
- Microstructure analyses and phase-fiel simulation of partially divorced eutectic solidificatio in hypoeutectic Mg-Al Alloys
- Promoting wetting of Mg on the SiC surfaces by addition of Al,Zn and Zr elements:A study via first-principl calculations
