Mechanisms of electrochemical magnesium (de)alloying of Mg-Sn and Mg-Pb polymorphs
Clément Pechberty ,Antoine Klein ,Bernard Fraisse ,Lorenzo Stievano ,Romain Berthelot,∗
a ICGM,CNRS,Univ.Montpellier,ENSCM,Montpellier,France
b RS2E,CNRS,Hub de l’Energie,Amiens,France
Abstract Different polymorphs of Mg-Sn and Mg-Pb intermetallic compounds were prepared by high-energy mechanical alloying and then investigated as active material in magnesium batteries.Beside thermodynamically stable Mg2Sn and Mg2Pb crystallizing in the anti-fluorit structure,other polymorphs Mg~2Sn and Mg~2Pb were prepared by increasing the ball-milling time.The firs dealloying process is almost complete only for the cubic polymorphs,then similar capacities are observed during the subsequent alloying and dealloying sequences.Thanks to operando X-ray diffraction,the electrochemical mechanism is revealed and shows that the cubic polymorphs Mg2Sn and Mg2Pb tend to preferentially form during the alloying whatever the pristine intermetallic.Weak traces of Mg~2Sn and Mg~2Pb are observed during the alloying,suggesting that these polymorphs act as a by-product and/or an intermediate phases of the electrochemical process.Finally,the compatibility of cubic Mg2Sn and Mg2Pb with Mg(TFSI)2-based electrolyte is confirme in full cell vs. a positive electrode based on the Chevrel phase Mo6S8,although limited performance is achieved.This fundamental work provides new insights in the behavior of alloy-type negative electrodes for magnesium-ion batteries.
Keywords: Magnesium batteries;Alloy electrode;Mechanochemistry; Operando X-ray diffraction.
1.Introduction
Magnesium batteries are currently investigated as a possible alternative to the well-established lithium-ion technology[1,2].Driven by the growing market of electric vehicles,the increasing demand for batteries could indeed face some durability and sustainability issues regarding specifi raw materials,such as cobalt,nickel or lithium.It is thus important to investigate other types of batteries employing more abundant elements such as magnesium.The story of rechargeable magnesium batteries really started with the prototype systems of Aurbach,with the Chevrel phase Mo6S8at the positive electrode,a magnesium metal negative electrode and Grignardbased electrolytes [3].This pioneering work already underlined the two major challenges of the Mg-batteries:the easy surface passivation of the magnesium in contact with conventional electrolytes typically used for metal-ion battery,and the lack of positive electrode materials due to the sluggish ionic diffusion of Mg2+into classical host electrodes [4,5].Tremendous efforts have been made to identify new generations of electrolytes suitable for a magnesium metal electrode,and more practical than the Grignard-based ones(simpler,less corrosive and with a wider electrochemical stability window).At the positive electrode,sulfur-based composites seem to focus nowadays most of the attention [6].The theoretical combination of magnesium and sulfur in a same battery would offer high energy density above 3200 Wh/L,while being sustainable and affordable.However,such a promising system would also come along with the well-known drawbacks of metal/sulfur systems (insulating nature of sulfur implying limited loading in the electrode,polysulfid shuttle effect,etc.) [6].
Recently,alloy-type electrodes for magnesium-ion batteries have been investigated,thanks to the relatively high theoretical capacity and the low alloying potentials of elements from groups IIIA,IVA and VA.Bismuth,antimony were the firs alloy-type electrodes implemented in magnesium-ion batteries,followed by reports on silicon,germanium,tin,indium and more recently gallium [7].Obviously,most studies evaluated these elements in half-cell configuratio with magnesium counter electrode and Grignard-based electrolytes.However,the compatibility with more conventional and practical electrolytes has been rapidly underlined and stands now as the main advantage of these alloy-type electrodes.Consequently,the use of magnesium alloys of general formula MgxM has been proposed to investigate Mg-free positive electrode materials,such as the Chevrel phase standard Mo6S8,but also vanadium oxide V2O5or carbon/sulfur composites,in simple electrolyte generally made from Mg(TFSI)2salt dissolved in acetonitrile,tetrahydrofuran or dimethoxyethane [8-10].
Among the different MgxM alloy systems,promising results can be predicted for Sn-and Pb-based alloys based on the behavior of these metals as alloy-type negative electrodes.Concerning tin,during the firs investigation of its electrochemical behavior in magnesium batteries,Singhet al.[11]observed at C/200 an alloying plateau around 0.15 V ending with a high discharge capacity of 900 mAh/gSn,i.e.,close to the theoretical value corresponding to the formation of Mg2Sn.However,they also showed that magnesiated electrodes further deliver a fraction of this initial capacity (~300 mAh/gSn) [11].Irreversible pulverization and amorphization upon cycling could explain this limited capacity.The electrochemical behavior of bulk Mg2Sn prepared by ball-milling was then investigated by Nguyen and coworkers.After an initial capacity below 200 mAh/galloya maximum of 270 mAh/galloywas reached upon cycling.Galvanostatic intermittent titration technique,or optimized electrode formulation,enabled reaching higher values [12,13].
In the case of lead,its electrochemical behavior was investigated in 2015 by the group of Obrovac,starting from electrodes either prepared by sputtering or made from micrometric Pb powder [14].At 60 °C and current rates of C/40 or C/50,reversible capacities of 450 and 275 mAh/gPbwere obtained,respectively,with Grignard-based electrodes.The alloying process occurs at 125 mVvs.Mg.Only indiumbased electrodes exhibit a lower alloying potential [15].To bypass an initial catalytic reaction on the electrode surface,the application of a current pulse prior to the discharge was proposed.To the best of our knowledge,no investigation of the electrochemical behavior of Mg2Pb has been performed until now.
In this work,different Mg-Sn and Mg-Pb polymorphs are prepared by mechanical milling and the mechanisms of electrochemical dealloying and alloying process are investigated throughoperandoX-ray diffraction.Their compatibility with a conventional electrolyte formulation is also confirme by assembling full cellsvs.a Chevrel phase positive electrode.
2.Experimental
Magnesium,tin and lead powders (Sigma-Aldrich,99%,325 mesh,Alfa Aesar,99.5%,100 mesh and 98%,20-230 mesh,respectively) were used as received and mixed in a 3D Spex mill (Spex SamplePrep 8000 M) in argon atmosphere with stainless steel jar and balls.For 1 g of powder mixture,typically 4 grinding balls of 10 mm diameter are used.Following our experience on the preparation of magnesium-based alloys by mechanical alloying [8,16,17],a nominal excess of 10% of magnesium powder was systematically added to the stoichiometric ratio 2:1 to avoid or at least minimize traces of pristine non-alloyed tin or lead.Different batches of Mg2Sn and Mg2Pb were prepared with milling times of 5 or 10 h.
The four resulting powder materials were characterized by X-ray diffraction (XRD) on a Panalytical X’Pert Prodiffractometer operating with Cu Kαradiation,under a protective airtight polymeric fil to limit moisture reaction.Chemical analysis was performed through inductively coupled plasma atomic emission spectroscopy (ICP-AES) at FILAB(Dijon,France).Powder morphology was also analyzed by scanning electron microscopy (SEM) with a Hitachi S-4800 microscope.
Self-supported electrodes were prepared by mixing 70 wt.% of active powder (Mg2Pb or Mg2Sn),10 wt.% of carbon (C65,Timcal),10wt% of vapor grown carbon fiber(VGCF-H,Showa Denko K.K.) and 10 wt.% of polytetrafluo roethylene(PTFE,Sigma-Aldrich,>40 μm)in a mortar.The paste obtained was punched into discs to form 8 mm diameter electrodes with an average mass loading of 30 mg/cm².Such high loading was chosen to optimize the conditions ofoperandoX-ray diffraction measurements,although it is clear that an optimization of the formulation is necessary to target interesting electrochemical performance.This goes,however,beyond the scope of this fundamental study that aims at highlighting the structural changes during the (de)alloying process.
The Chevrel phase Mo6S8was synthetized by adapting a widely recognized 2-step protocol [18].Firstly,the ternary Cu2.5Mo6S8Chevrel phase was obtained by heating a stoichiometric mixture of Cu (Sigma-Aldrich,99.9%,325 mesh),Mo (Alfa-Aesar,99.95%,APS 3.7 μm) and MoS2(Sigma-Aldrich,99%,<2 μm) during 24 h at 1000 °C under constant argon fl w.Secondly,Cu was removed by acidic leaching with an HCl aqueous solution (6 M) under O2bubbling.Finally,the solid was filtered washed with distilled water and ethanol and lastly dried at 60 °C.The fina product was characterized by XRD to check the purity of the obtained Chevrel phase.Mo6S8electrodes fil were formulated by mixing 80 wt.% of Mo6S8,5 wt.% of C65,5 wt.% of VGCF-H,10% of polyvinylidene fluorid (PVDF,SOLEF 21,216/1001)and NMP as the solvent.The slurry was then tape-casted on 10 μm carbon-coated aluminum foil and dried following the above-mentioned procedure,leading to electrodes with an average mass loading around 1.5 mg/cm².
All-ethyl-complex magnesium electrolyte (AEC) was obtained by mixing ethylmagnesium chloride (EtMgCl,2 M in THF,Sigma -Aldrich) and diethylaluminium chloride(Et2AlCl,97% Sigma-Aldrich) in THF.The fina concentration of the EtMgCl-Et2AlCl-THF electrolyte was around 0.35 M Conventional magnesium electrolyte was prepared by dissolving magnesium bis(trifluoromethanesulfo yl)imide salt(Mg(TFSI)2,99.5%,Solvionic)in dimethoxethane(DME,Sigma-Aldrich purity>99%)) with a 0.5 M concentration.Prior to use,Mg(TFSI)2was dried at 250 °C in a vacuum flask while DME was dried firs by argon bubbling,then by addition of 4 °A molecular sieves for 3 days,next reflu ed with K/Na (3/1 wt.%) for 1 day and finall distilled.
2-electrode coin-cells (2032,316 L stainless steel) were assembled in an argon-fille glove-box,with Mg disks(Goodfellow,99.9%,250 μm,polished before use) as both negative and reference electrode.Two Whatman GF/A borosilicate glass fiber were used as separator and wetted with 100 μL of electrolyte.OperandoXRD measurements were carried out with a dedicatedin situelectrochemical cell with a specifi Be window to probe the structural evolution of the electrode material [19].For this early-stage and fundamental investigation,there is no need to balance the battery electrodes.When associated to a magnesium counter electrode,the alloyelectrode has the lowest capacity.In full cell configuratiovs.the Chevrel phase positive electrode,the latter has the lowest capacity.All Galvanostatic tests was perform on an MPG potentiostats (Biologic).
Operations including the mechanochemical synthesis of the intermetallic compounds,the electrode formulation,the electrolyte preparation and the battery assembly were carried out in an argon-fille glove box (MBraun LABSTAR) with an oxygen level<0.5 ppm.
3.Results and discussion
The XRD patterns obtained after mechanical alloying of Mg-Sn and Mg-Pb mixtures are gathered in Fig.1.The patterns obtained after 5 h of milling exhibit well-define diffraction patterns that can be all indexed with the cubic space groupFm-3 m.Profil matching refinemen confirm the formation of the cubic phases Mg2Sn and Mg2Pb,with cell parameters in good agreement with the literature(a=6.762(2) °A for Mg2Sn,a=6.820(1) °A for Mg2Pb)and no visible traces of Sn and Pb,respectively (Fig.S1).The chemical analysis (Table S1) supports this observation as magnesium contents of 28.7 and 19.0 wt.% are,respectively determined (in line with the above chemical formula).
For milling times of 10 h,the patterns significantl change(Fig.1b and d).Preliminary phase searching did not provide a unique solution but identify weak traces of cubic Mg2Sn and Pb,respectively.No further information could be obtained from the chemical analysis as the magnesium content does not significantl evolve (Table S1).Consequently,it was important to reexamine the phase diagrams of the Mg-Sn and Mg-Pb systems and the existing literature related to Mg-Sn and Mg-Pb polymorphs.Indeed,cubic Mg2Sn and Mg2Pb crystallizing in the antifluorite-typ structure are well known and widely reported as the thermodynamically stable allotropes at ambient conditions [20,21].Nevertheless,other polymorphs have been also observed in a narrow region around Mg2Sn and Mg2Pb compositions.
For the Mg-Sn system,an hexagonal phase was reported firstl at high-pressure and high-temperature [22,23],but then also through long-time mechanical alloying [24].A slight Mg-deficien composition,Mg9Sn5(or Mg1.8Sn)was later proposed for this dense form with cell parametersa=13.222 °A andc==13.15 °A [25].Additionally,an orthorhombic metastable phase was firstl detected on thin fil by metal evaporation [26],and then obtainedviaball-milling and briefl characterized with cell parametersa==15.23 °A,b==8.90 °A andc==13.10 °A [27].In both cases,no further details are provided on the crystallographic structure.Nevertheless,profil matching was attempted on XRD of Fig.1b with both“models”.Owing to the quite broad diffraction peaks commonly observed for materials obtained by mechanochemical alloying,however,the refinement were not satisfactory enough to unequivocally identify the crystal structure.To avoid confusion,this polymorph will hereafter be named“non-cubic”with a chemical formula Mg~2Sn.
Regarding the Mg-Pb system,in 1965 Eldrigeet al.showed that cubic Mg2Pb melts incongruently and forms aβ’ phase at a different composition slightly deficien in Mg[28].This polymorph was not included in the Mg-Pb equilibrium phase diagram proposed in 1985 by Nayeb-Hashemi and Clark,which is now widely spread and used in reference books [21].More recently,additional studies specially focused on the composition region around Mg2Pb reinstated the existence of theβ’ phase [29,30].However,the crystallographic structure of the latter has not yet been determined with certainty.An orthorhombic PbCl2-type structure (space groupPnma,a==7.50 °A,b==4.45 °A andc==8.82 °A)was proposed initially,but has not been confirme afterwards[28].More recently,ab initioevolutionary methods were performed and concluded on tetragonal symmetry (space group P4/nmm,a==4.5396 °A,b==7.5278 °A) [31].Here again,profil matching attempts with the above-mentioned models were not satisfactory,it is not possible to unambiguously assign a particular symmetry.To avoid any further confusion,this polymorph will hereafter be named“non-cubic”with a chemical formula Mg~2Pb.
The particle morphology of these intermetallics is shown in insets in Fig.1.Unsurprisingly,the ball-milled powders consists on large aggregates of sub-micrometric particles,as already reported for alloys prepared in similar conditions[8,15].

Fig.1.XRD patterns and SEM pictures of cubic Mg2Sn (a),non-cubic Mg~2Sn (b),cubic Mg2Pb (c) and non-cubic Mg~2Pb (d).Scale bar is 100 μm.For the cubic polymorphs,profil matching refinemen is provided in supplementary section.
The electrochemical behavior of the as-prepared Mg-Sn and Mg-Pb polymorphs is evaluated in half-cell at C/30.Here it is worth mentioning that this current rate is higher than in previous studies dealing with alloy-type electrode materials[7],but still very low considering practical applications.The firs two cycles are presented in Fig.2 with specifi capacities to ease the comparison between polymorphs.For all,the dealloying process starts with a stable plateau slightly increasing from 0.25 to 0.3 V before a progressive increase to reach the cut-off value of 0.7 V.With the cubic polymorphs,the corresponding capacities of 590 and 460 mAh/galloyshow that almost all the magnesium seems to be extracted from Mg2Sn and Mg2Pb,respectively,during this initial charge (theoretical values of 640 and 420 mAh/galloy).The slight extra capacity in case of Mg2Pb could be assigned to weak parasitic reactions already observed with Pb-based electrodes [14].The subsequent alloying occurs at a low voltage (below 0.2 and 0.1 V for tin-and lead-based electrodes,respectively),with a rather limited capacity around 200 mAh/galloyin both cases that is maintained upon cycling.Regarding the two other polymorphs,Mg~2Sn and Mg~2Pb,the initial electrochemical charge starts similarly to the cubic polymorph with the same plateau potentials;with however a cut-off value reached more rapidly and a corresponding capacity significantl reduced (Fig.2b and d).
This sharp contrast in the electrochemical signature of the firs dealloying between cubic and non-cubic polymorphs is appealing.This contrast cannot by attributed to a different morphology,since the SEM pictures in Fig.1 do not reveal any distinct grain size and aspect.Without any detail on the crystallographic structure of the non-cubic Mg~2Sn and Mg~2Pb,it is also not possible to assign this capacity difference to distinct surface energies.More investigations going beyond the scope of this work are thus necessary to fully understand this difference in the dealloying.
To grasp further details of the electrochemical processes,anoperandoXRD follow-up of the firs galvanostatic cycles was carried out.Previousex situXRD patterns collected after magnesiation of tin-based or lead-based electrodes confirme the formation of the cubic Mg2Sn and Mg2Pb,respectively [11,32].No details,however,were reported about possible intermediates formed along the electrochemical discharge.In Fig.3,it is firstl important to note the presence of weak diffraction peaks of tin or lead in the pristine electrode whatever the polymorph.This result is in contradiction with the XRD collected after the mechanical alloying.The only explanation is a partial spontaneous dealloying occurring chemically between the cell assembly in the glovebox and the beginning of the electrochemical test,never less than 15 min.Then,the dealloying sequences of all the intermetallic compounds are characterized by the disappearance of the peak of the pristine polymorph,while the peaks of tin (β) on one side,or lead on the other side,regularly increase in intensity.This observation is in line with the steady-fla voltage plateau characterizing a biphasic alloying reaction.

Fig.2.Galvanostatic behavior of Mg/Mg2Sn and Mg/Mg2Pb half-cells at C/30 current rate with EtMgCl-Et2AlCl/THF electrolyte (firs cycle in blue,second cycle in red):cubic Mg2Sn (a),non-cubic Mg~2Sn (b),cubic Mg2Pb (c) and non-cubic Mg~2Pb (d) (For interpretation of the references to color in this figur legend,the reader is referred to the web version of this article).

Fig.3.Operando XRD investigation of the electrochemical behavior of cubic Mg2Sn (a),non-cubic Mg~2Sn (b),cubic Mg2Pb (cubic) and non-cubic Mg~2Pb(d).For each case,peaks indexation or at least Bragg peaks are provided when possible.To enhance the magnesiation process during discharge,a float ng was applied once the cell reaches 0 V.Symbols (∗) and (#) stand for the beryllium window and unidentifie inactive impurities on its surface.
In case of cubic Mg2Sn pristine electrode,the subsequent discharge shows the reformation of the cubic Mg2Sn,the latter exhibiting better crystallinity (narrower diffraction peak at 26.5 ° in Figs.3a and S2).The magnesiation appears more complex for pristine Mg~2Sn with the concomitant apparition of cubic and non-cubic polymorphs (Figs.3b and S3).As long as the alloying proceeds,the proportion of the cubic Mg2Sn slightly increases.In both cases,a significan amount of tin remain at the end of the discharge,in agreement with the limited alloying capacity observed in Fig.2.The explanation could lie on an important alteration of the grains during the firs dealloying process leading to isolated tin particles no longer in electrical contact.
For lead-based electrodes,the early stages of the electrochemical magnesiation are characterized by the appearance of weak reflection assigned to non-cubic Mg~2Pb,whatever the pristine polymorph (Figs.3c,d and S4,5).This is somehow logical considering that these polymorph is slightly poorer in magnesium than the cubic one.As the alloying process continues,these peaks slowly disappear in favor of cubic Mg2Pb.
A more detailed analysis of theoperandoXRD datasets using a chemometric approach,provided in the SI section,confirme the above-mentioned observations and also supplied complementary information on the evolution of the crystallinity between the pristine and electrochemically formed phases (Figs.S6-16).In summary,the most important observation is the preferred formation of cubic polymorphs Mg2Sn and Mg2Pb,even if the other polymorphs are considered for the pristine electrode.After that,the weak and/or short apparition of Mg~2Sn or Mg~2Pb polymorphs during the alloying process is actually not surprising as such phenomena have already been observed during the electrochemical alloying of otherp-block elements,such as antimony,with alkali ions[33,34].
Finally,the possible use of these intermetallic compounds as negative electrodes in Mg-ion batteries with simple electrolyte formulations,such as Mg(TFSI)2/DME,was evaluated in full cellsvs.Mo6S8Chevrel phase(CP)positive electrodes.As we previously demonstrated that the cubic forms of Mg2Sn and Mg2Pb(i)exhibit the best firs cycle capacity during the de-alloying process and(ii)are mainly formed during electrochemical alloying of both tin and lead forms,only those polymorphs were considered for full cell tests (Fig.4).
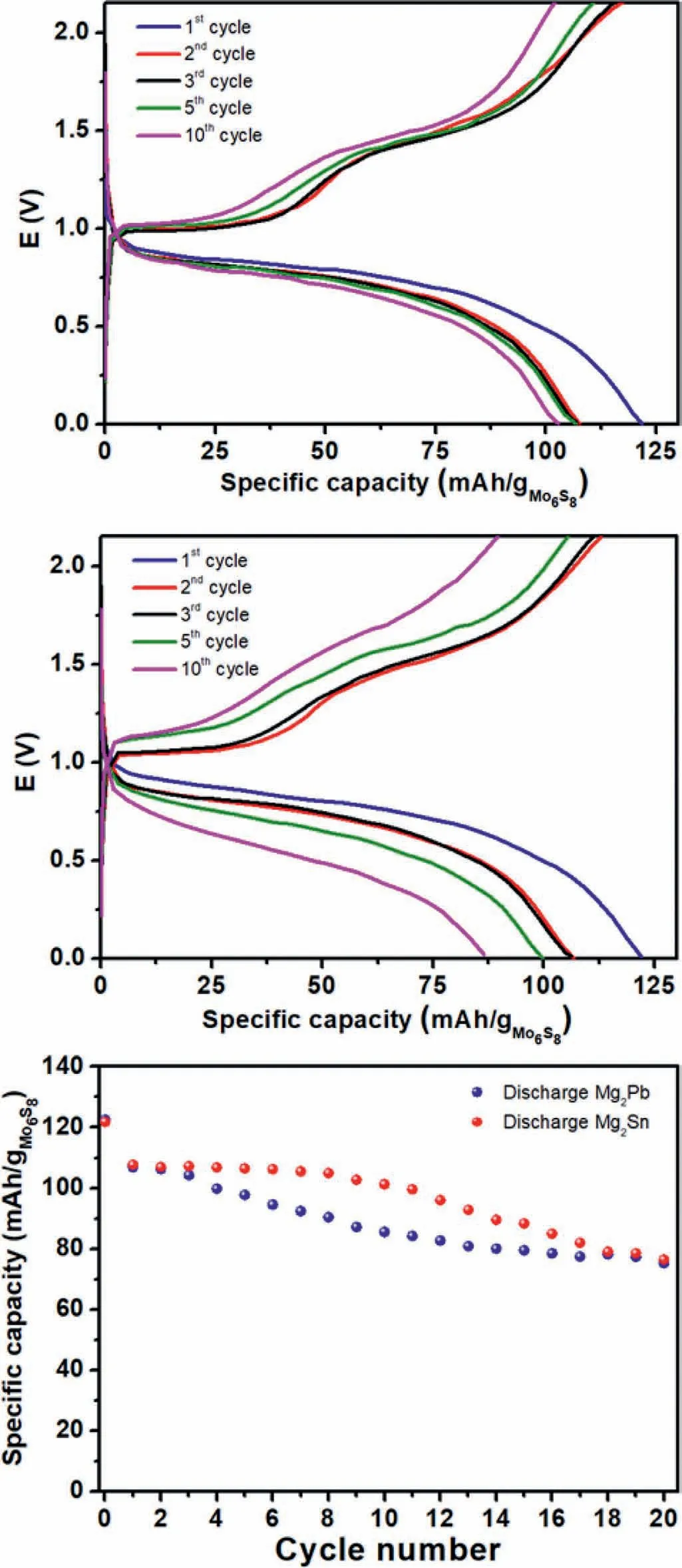
Fig.4.Galvanostatic cycling of full cells Mg2Sn-cubic/Mo6S8 (top) and Mg2Pb-cubic/Mo6S8 (middle) at C/10 current rate with Mg(TFSI)2/DME electrolyte,and capacity evolution upon cycling (bottom).
The initial discharge shows a slopy plateau occurring between 0.8 and 0.5 V for Mg2Sn,but slightly higher from 0.9 to 0.5 V for Mg2Pb due to the lower potential of Mg2Pb compared to that of Mg2Sn (calculated values of 121 and 197 mVvs.Mg2+/Mg for Pb and Sn,respectively) [35].During the corresponding charge processes,both Mg2Sn and Mg2Pb show two plateaus at 1 and 1.4 V that can be attributed to the sequential insertion of Mg2+in the two insertion sites of Mo6S8[36].The differentiation between the two plateaus is not observed on the discharge due to intrinsic kinetic limitations.In both cases,there is a progressive but limited capacity decay upon cycling.The initial capacity loss of 20 mAh/gCPcan be attributed to the partial irreversible magnesium trapping in Mo6S8[37].After that,the progressive loss of capacity may have different origins:first the degradation of the electrolyte in contact with Mg at low potential can progressively form a blocking layer on the negative electrode [38].Another possible reason could be the dissolution of the alloying element in the electrolyte and its migration to the positive side,as previously observed in the case of Mg3Bi2/S batteries [10].This shuttle phenomenon,which is not yet well understood and could have a negative effect on the cycle life,will be the object of further detailed investigations.Finally,the Mg that is non-reversibly alloyed can possibly plate on the negative electrode/electrolyte interface at low potential resulting in the formation of passive Mg and loss of active material.
4.Conclusions
Different Mg-Sn and Mg-Pb polymorphs can be prepared by mechanochemical synthesis:beside the cubic forms,thermodynamically stable at ambient conditions,this synthetic method allows also obtaining other intermetallics with increasing milling time.The electrochemical evaluation of these compounds shows that the firs de-magnesiation is almost complete with cubic Mg2Sn and Mg2Pb,but only partial with the other polymorphs.OperandoXRD reveals that,during the following magnesiation,the cubic polymorphs are obtained independent of the pristine polymorph,even though a transient metastable phase is formed at the very beginning of the process in the case of Pb.The compatibility of cubic Mg2Sn and Mg2Pb with Mg(TFSI)2/DME electrolyte when used as negative electrode materials in Mg-ion batteries was confirme in Mg2Sn/Mo6S8and Mg2Pb/Mo6S8systems.The observed cycle life shows that the behavior of Mg2Sn and Mg2Pb as negative electrodes in Mg-ion batteries with a simple electrolyte formulation is promising compared to previous studies [7].A benchmark in the same electrochemical conditions appears thus necessary to clearly determine the most relevant alloy to be used in magnesium-ion battery systems.
Acknowledgments
The authors gratefully acknowledge financia support from the French National Research Agency (project MISTRALE,ANR-19-CE05-0013,and Labex STORE-EX,ANR-10-LABX-76-01).Access to the Analysis and Characterization platform of“Pôle Chimie Balard”(PAC-Balard) is gratefully acknowledged.Calypso Baril (MEA platform,Univ Montpellier) is acknowledged for the SEM pictures.CIDETEC is acknowledged for providing the C-coated Al foil,with the support of E-Magic FET-PROACTIVE and VIDICAT FET-OPEN projects in the frame of Horizon 2020 Program(Grant agreement 824066 and 829145,respectively).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.11.013.
 Journal of Magnesium and Alloys2022年6期
Journal of Magnesium and Alloys2022年6期
- Journal of Magnesium and Alloys的其它文章
- EDITORIAL BOARD
- Aims and Scope
- Surface oxidation study of molten Mg-Al alloys by oxide/metal/oxide sandwich method
- Production and characterisation of new bioresorbable radiopaque Mg-Zn-Y alloy to improve X-ray visibility of polymeric scaffolds
- Quantitative study on the tension-compression yield asymmetry of a Mg-3Al-1Zn alloy with bimodal texture components
- Microstructure analyses and phase-fiel simulation of partially divorced eutectic solidificatio in hypoeutectic Mg-Al Alloys
