Recent progress in Mg-based alloys as a novel bioabsorbable biomaterials for orthopedic applications
Fi Xing ,Shng Li ,Dongi Yin ,Jihng Xi ,Pol Mri Rommns ,Zhou Xing ,Ming Liu,∗,Ulrik Ritz,∗
a Orthopedic Research Institute,Department of Orthopedics,West China Hospital,Sichuan University,No.37 Guoxue Lane,Chengdu,Sichuan 610041,China
b Department of Plastic and Burn Surgery,West China Hospital,Sichuan University,No.37 Guoxue Lane,Chengdu,Sichuan 610041,China
c Key Laboratory of Advanced Technologies of Materials,Ministry of Education,School of Materials Science and Engineering,Southwest Jiaotong University,Chengdu,Sichuan 610031,China
d Laboratoire Roberval,FRE UTC-CNRS 2012,Sorbonne universités,Université de Technologie de Compiègne,Centre de recherche Royallieu,CS60319,Compiègne CEDEX 60203,France
eDepartment of Orthopaedics and Traumatology,Biomatics Group,University Medical Center of the Johannes Gutenberg University,Langenbeckstr.1,Mainz 55131,Germany
Abstract Traditional orthopedic metal implants,such as titanium (Ti),Ti alloys,and cobalt-chromium (Co-Cr) alloys,cannot be degraded in vivo.Fracture patients is must always suffer a second operation to remove the implants.Moreover,stress shielding,or stress protection occurs when traditional orthopedic metal implants are applied in fractures surgery.The mechanical shunt produced by traditional orthopedic metal implants can cause bone loss over time,resulting in decreased bone strength and delayed fracture healing.Biodegradable metals that ‘biocorrode’ are currently attracting significan interest in the orthopedics fiel due to their suitability as temporary implants.As one of the biodegradable metals,magnesium (Mg) and Mg alloys have gained interest in the fiel of medicine due to their low density,excellent biocompatibility,high bioresorbability,and proper mechanical properties.Additionally,Mg ions released from the metal implants can promote osteogenesis and angiogenesis during the degradation process in vivo,which is substantially better for orthopedic fixatio than other bioinert metal materials.Therefore,this review focuses on the properties,fabrication,biological functions,and surface modificatio of Mg-based alloys as novel bioabsorbable biomaterials for orthopedic applications.
Keywords: Magnesium;Mg alloys;Biomedical implants;Bone regeneration;Surface modification
1.Introduction
Due to the increased incidence of sports-,trauma-,inflammatory and age-related musculoskeletal injuries and defects,the demand for orthopedic implants has greatly increased [1].The traditional metal implants are usually made of stainless steel,titanium (Ti),Ti alloys,and cobaltchromium(Co-Cr)alloys,which have been widely used as orthopedic implants in clinical treatment due to their biocompatibility,low immunogenicity,strong corrosion resistance and excellent mechanical durability [2].However,traditional orthopedic metal implants cannot be degraded in vivo,and fracture patients need to undergo a second operation to remove them [3].Moreover,stress shielding or stress protection occurs when traditional metal implants,such as bone plates and screws,are used to repair fractures surgery [4].The mechanical shunt produced by traditional metal implants can cause bone loss over time,resulting in decreasing bone strength and delayed fracture healing.Additionally,the traditional orthopedic metal implants with the bioinert surface are ineffective for bone formation and osseointegration [5].Moreover,the orthopedic metal implants that remain in vivo for years will increase the risk of implant-related infection,which is commonly occult and will lead to poor prognosis [6].All these poor prognoses bring considerable physical pain and financia burden for fracture patients [7].
As an important alternative material of traditional orthopedic metal implants,synthetic polymers,such as ultrahigh molecular weight polyethylene,polyetheretherketone,polyurethanes,poly(methylmethacrylate),poly(glycolic acid),and polycaprolactones (PCL),have been gradually being used in orthopedic-related operations [8].Compared with traditional metal implants,synthetic polymer materials also exhibit good biocompatibility.However,synthetic polymers have disadvantages regarding the toxic residual monomers,wear debris,and accumulation of the acidic inter-mediate degradation products,resulting in pathological bone resorption [9].Furthermore,poor mechanical properties of synthetic polymer materials limit their further clinical application.In addition,traditional orthopedic metal implants and synthetic polymers implants also show significan limitations when dealing with osteoporotic fractures,femoral head necrosis,and large bone defects [10].Thus,biodegradable metals that ‘biocorrode’ are currently attracting significan interest in the orthopedic fiel due to their suitability for being used as temporary implants,as they are capable of dissolving after having achieved their required function [11].Compared with traditional metal implants,biodegradable metals release metal ions in the process of metal degradation to promote the repair of surrounding tissues [12].Compared with synthetic polymers or bioceramics,the biodegradable metal has better mechanical properties,including Young’s modulus of elasticity,tensile yield strength,ultimate tensile strength,and toughness [13,14].
Among these biodegradable metals,Magnesium (Mg) and Mg alloys have been focused on and preferred in the fiel of orthopedic metal implants [15,16].Mg has an excellent combination of properties,including high thermal conductivity,low thermal expansion,high damping capacity,excellent electromagnetic shielding,and machinability [17,18].Additionally,Mg and Mg alloys have bone-like elastic modulus close to that of natural cortical bone,effectively decreasing stress-shielding effects [19].Mg-based alloys corrode in tissue and body fluid and release Mg ions,OH ions,and alloying elements,which are nontoxic and can be eliminated from the body [20].Mg ions rereleased from Mg-based alloys directly enhance the differentiation of stem cells into osteoblasts and the expression of osteoblast-related gene proteins by osteoblasts.Moreover,Mg ions enhance vascularization in new bone tissues,which are crucial for exchanging nutrients and waste in bone tissues.Interestingly,Mg ions can even inhibit bone tissue destruction by regulating the osteoclasts.The addition of alloying elements greatly improves the physical and chemical properties of pure magnesium,and the successfully constructed magnesium alloy has a wider range of applications in medical implants.Various functional coating technologies have made Mg-based alloy implants a big step forward in clinical application.Mg-based alloy is a revolutionary medical implant,but to achieve early clinical large-scale applications,researchers still need to do a lot of work to solve the current deficiencie of Mg and Mg alloys.Therefore,this review focuses on the properties,fabrication,biological functions,and surface modificatio of Mg-based alloys as novel bioabsorbable biomaterials for orthopedic applications.Furthermore,the challenges and prospects of biodegradable Mgbased alloys as orthopedic implants are also discussed.
2.Alloying elements selection in Mg-based alloys for orthopedic implants
Pure Mg is rarely used in biomedical applications.Mg exhibits significan property improvement via alloying.The alloying elements impart to strengthen the properties by grain-refinement precipitation hardening,and solid solution strengthening.The commercial cast Mg alloys are mainly classifie into Mg-Al-,Mg-Zn-,Mg-Ca-,Mg-Sr-,and Mg-Li-based alloys [21].However,the selection of alloying elements in Mg alloys for orthopedic implants should take the mechanical properties,degradation,biocompatibility,and osteoinductive bioactivity of alloying elements into account.The most common nutritional alloying elements utilized in biodegradable Mg alloys for orthopedic implants include calcium(Ca),zinc(Zn),manganese(Mn),strontium(Sr),tin(Sn),and silver(Ag) [22].
As one of the main elements in the human body,Ca plays an important role in numerous essential functions.Additionally,Ca is mainly stored in bones and teeth and can promote bone healing.Alloying Mg and Ca can be a positive effect on bone healing [23].Ca refine the microstructure and improve Mg’s strength and creep properties via the formation of stable intermetallic phases.Additionally,acid picking is an effective surface treatment method to tailor the biomineralization and degradation behaviors of the Mg-Ca alloy in the physiological environment [24].The degradation mechanism of Mg-Ca alloy treated or untreated with acid was shown in Fig.1A.Notedly,Nidadavolu et al.[25] have successfully fabricated Mg-0.6Ca specimens with different porosities through powder metallurgy(PM)technique.The results found that a single large pore occupies a huge part of the investigated pore volume in Mg-0.6Ca specimens with 21% porosities,indicating high pore interconnectivity of porous Mg alloys (Fig.1B).
As an essential trace element,Zn is involved in many biological processes,such as immune reaction,carbohydrate catabolism,wound healing,bone development,and growth[26].Infants and adults need around 2 mg/day and 6.5-15 mg/day of Zn [27].Additionally,more than 85% of Zn is stored in the muscles and bones.As another biodegradable metal,the degradation rate of Zn is slower than Mg and faster than Fe [28].Zn can improve the mechanical strength of Mg alloys implants depending on Zn concentration [29].The microstructure of Mg-Zn alloys is associated with corrosion resistance and degradable properties of Mg alloys.Taking Mg-5 wt.%Zn as an example,extruded Mg-5 wt.%Zn alloy aged for 96 h showed high corrosion resistance,good biocompatibility for L929 cells and excellent ability to maintain sample integrity during the immersion of Ringer’s solution[30].

Fig.1.(A) Schematic representation of the degradation mechanism of Mg-Ca alloy [24].Bare Mg-Ca alloy,BM.Nitric acid treated Mg-Ca alloy,NA.Phosphoric acid treated Mg-Ca alloy,PA;(B) Rendered 3D images of segmented pore features from similar μCT volumes and largest pore volumes in Mg-0.6Ca specimens with different porosity values [25].
Mn is an essential trace mineral in the human body and takes part in metabolic activity in the human body.Mn can also play a vital role in bone metabolism and producing bone formation-related enzymes.Mn can improve the corrosion resistance of Mg alloys[31].Sr has similar metallurgical,chemical,and biological functions as Mg [32].Almost all of the Sr in the human body is stored in the bone tissue.Sr can improve the mechanical properties and corrosion resistance of the Mg alloys by improving its surface properties when the Sr content in the alloys is less than twowt.%[33].Additionally,Sr could promote osteoblast viability and proliferation [34].Sn plays a vital role in synthesizing proteins and nucleic acids,which are crucial for body growth.Additionally,Sn can improve the compressive strength and corrosion resistance of Mg alloys [35].Additionally,the addition of Sn improves both the mechanical and corrosion properties of Mg/HA composites[36].Tin has not been found to have any specifi biological functions in the human body.The biological role of tin is still unknown and undefined The use of alloying elements to improve the mechanical properties and corrosion resistance of Mg alloys is currently one of the main concerns.
Lithium (Li) has been widely used as a mood stabilizer in treating of depression and bipolar disorder for the past fift years [37].Li+could promote the proliferation and osteogenesis of osteogenic cells in vitro,simultaneously stimulating bone formation in vivo [38].Additionally,the Li element is also found to have a significan inhibitory effect on osteosarcoma [39].Therefore,Mg-Li-based alloys could be a potential implant therapy after osteosarcoma surgery due to their properties of reliable mechanical support,promoting bone formation,and inhibiting tumor growth [40].The increasing Li content could reduce the dynamic recrystallization of (hexagonal close-packed) HCP Mg-Li alloys during hot extrusion[41].Simultaneously,the groove-like corrosion of HCP Mg-Li alloys is weakened with increasing Li content [41].The orientation maps and pole figure of Mg alloys with different Li content are shown in Fig.2.
To fully improve the physical and chemical properties of Mg alloys,more and more researchers not only use a single metal element and Mg to build Mg alloys but also use a variety of different metals elements and Mg to build Mg alloys[42,43].Mg-Zn-Sn alloy has advantages of pH responsiveness and ion-associated antibacterial properties,which can be applied in antibacterial implants for bone-implant-associated infection therapy [44].Mg-Zn-Gd alloy can be possessed into a biodegradable membrane with guided bone regeneration for rabbit calvarial defect repair [45].The addition of Gd in Mg-Zn alloy could improve the yield strength and ultimate tensile strength of Mg alloys [46].The previous study found that adding Mn and Ca increased the yield strength and ultimate tensile strength of Mg-4Zn alloys via strengthening from grain refinement dislocation strengthening of unDRXed region,and nano-scale secondary phase particles [47].In another study,four different Mg-Zn-Ca-Mn alloys were produced using the gravity die casting method.Mn content was kept constant in all alloys,while Zn and Ca were added in two different ratios[45].The researchers found that grain size decreased signifi cantly with increasing Zn content,Zn promoted Ca2Mg6Zn3phase formation,corrosion resistance increased with increasing Zn/Ca atomic ratio,hot rolling resulted in much higher tensile strength but much lower ductility and toughness [45].

Fig.2.(A) Orientation maps of as-extruded Mg-1Li,Mg-3Li and,Mg-5Li alloys analyzed by electron back-scattering diffraction (EBSD) [41];(B) Pole figure of as-extruded Mg-1Li,Mg-3Li and,Mg-5Li alloys analyzed by electron back-scattering diffraction (EBSD) [41].
Currently,various Mg alloys,such as AZ31,AM50,AZ80,and AZ91,are used as orthopedic medical implants.Unfortunately,these Mg alloys contain aluminum(Al),which is identifie to be neurotoxic and causes Alzheimer’s disease [48].Therefore,eliminating the adverse side of Al in the Mg alloying process still needs further studies.Additionally,during the process of Mg alloy synthesis,Fe,Ni,Co,and Cu,which are considered impurities,should be avoided to be added to the Mg alloys [49].The impurities in these alloys will be released during the degradation process,causing toxic side effects to the human body [50].
Careful selection of alloying elements is the firs step in designing Mg alloys for orthopedic implants [51].To ensure that Mg alloys for orthopedic implants can continue to exert their biological functions after being implanted into the body,the alloying elements selection of Mg alloys should prioritize the elements already existing in the human body,especially the elements necessary for the body’s metabolism.Additionally,various alloying elements endow Mg alloys with different corrosion properties.Combined with the various biological properties of the alloying elements,Mg alloys with different corrosion properties can be implanted in different parts,including cancellous bone,cortical bone,load-bearing bone,and non-load-bearing bone.Rare-earth (RE) elements as alloying elements can effectively improve the mechanical,anti-corrosion,and biological properties of Mg alloys.A low concentration of RE ions has been proved to promote bone formation in vitro.Considering that RE elements are not essential trace elements for the human body and the cytotoxicity caused by excessive amounts,when designing Mg-RE alloys for orthopedic implants,Mg alloys with low content of RE elements are recommended.Furthermore,the addition of other non-metallic elements,such as phosphorus(P),sulfur(S),flu orine(F),also significantl improve the biocompatibility and mechanical properties of Mg alloys for orthopedic implants.
3.Physical and chemical properties of Mg and Mg alloys for orthopedic implants
Mg is considered to be the best green material in the 21st century [52].Mg is the fourth most abundant cation element found within the human body and takes part in more than 300 known cell enzymatic reactions,including mitochondrial activity,protein,and nucleic synthesis [53].Mg is also one of the seven essential macro minerals required for a healthy body.Additionally,more than 50%of the body’s Mg is stored in bone tissue [54].As the lightest metallic material with a density of 1.738 g/cm3,Mg is approximately 60% of that of aluminum (Al,2.7 g/cm3) and 20% of that of iron (Fe,7.9 g/cm3) [17,55].This part introduces the physical and chemical properties of Mg and Mg alloys.
3.1.Mechanical properties
To withstand various biomechanical forces,ideal orthopedic implant materials should have good mechanical properties,including yield strength,elastic modulus,and ultimate tensile strength.Traditional orthopedic metal implants,such as stainless steel,Co-Cr alloys,and Ti alloys,can provide strong mechanical support for bone tissue,which may increase the occurrence of stress shielding.Pure Mg is the simplest form of bioresorbable metal materials [56].Mg has poor strength in cast conditions [57],but proper alloying and processing conditions can strengthen the mechanical properties of Mg [58].Additionally,the strength of Mg can be improved by the grain refinemen and solid solution strengthening mechanism of alloying elements [59].Compared with traditional orthopedic metal materials,such as stainless steels (189~205 GPa) and Ti materials(110~117 GPa),the elastic modulus of Mg and Mg alloys (41~45 GPa) is much closer to that of human bone(3~20 GPa),which indicated that Mg and Mg alloys reduced the stress shielding of implants [2,60] (Fig.3A).The results of a finit element analysis model consisting of the femur and inserted Ti or Mg-based screws found that compared with Mg-based screws,higher stress distribution in the Ti screws and a higher level of bone tissue around the Ti screws were observed,which indicates that Mg-based screws can effectively reduce the stress shielding at the implant-bone interface [2] (Fig.3B).Compared with other biodegradable materials,such as ceramics,polymers,and bioactive glasses,Mg and Mg alloys have higher Young’s modulus and tensile strength,so that Mg and Mg alloys can provide sufficien mechanical support at the early stage of bone reconstruction [22,61].Even in the weight-bearing sites,large bearing stress is required at the fracture site,and the Mg-based alloys screws had sufficien mechanical strength to bear the stress[62].
Although pure Mg medical implants can effectively avoid the occurrence of stress shielding,their mechanical properties still cannot meet the mechanical requirements of lower extremity load-bearing bones.Currently,many methods,including the intentional addition of alloying elements,heat treatments,and plastic deformation,have been utilized to the mechanical properties of Mg alloys [63].Alloying is the most used method to strengthen the mechanical properties of Mg alloys [64].The mechanical properties of Mg alloys for orthopedic implants are shown in Table 1.Heat treatment is also one of the ways to effectively strengthen the mechanical properties of Mg alloys [65].Heat treatment modulates the mechanical properties of Mg alloys by changing the microstructure of the alloys [66].The temperature of the alloy transformation point determines the heat treatment temperature.In addition,avoiding the degradation of mechanical properties caused by grain growth during heat treatment still needs further research.Plastic deformation,including extrusion,forging,and rolling,improves the plasticity of Mg alloys by activating more dominant slip systems,thereby improving the mechanical properties of Mg alloys.

Table 1 The mechanical propertied of Mg alloys for orthopedics implants.
3.2.Properties of corrosion resistance
Compared with other metal materials,pure Mg has a fast corrosion rate.Mg would degrade in the body flui environment,and the resulting products are magnesium hydroxide and hydrogen gas [19].The corrosion reaction is given below:

The hydroxide layer formed on the surface of implants acts as a protective coating to prevent further corrosion under standard environmental conditions.Chloride is an inorganic anionic halogen distributed exclusively within the extracellular flui compartment,which comprises the blood/plasma(or serum) compartment and the interstitial flui compartment [76].Magnesium hydroxide reacts with chloride ions to produce soluble magnesium chloride in the physiological environment with high chloride concentration [60].The highly soluble magnesium chloride accelerates the dissolution of Mg implants,promoting degradation of Mg implants [77].The degradation products of Mg implants formed under physiological conditions are depicted in Fig.4 Eqs.(1)-(3).
Under the condition of corroded by body fluid many degraded products,including Mg(OH)2,Mg3(PO4)2,MgCO3,CaCO3,and Ca3(PO4)2,aggregate at the surface of Mg-based alloys [77].Macrophages can phagocytose part of degradations,and the other degradations can be temporarily stored in the bone matrix [78].The Mg2+stored in the bone matrix binds with hydroxyapatite crystals,and the combination of Mg and hydroxyapatite increases the solubility of hydroxyapatite crystals and improve the bone strength [79].Besides,following the slow degradation of Mg alloy implants,the temporarily aggregated Mg2+can be released through either local bone regeneration or circulation system,without fluctuatin blood serum level of Mg2+[80].
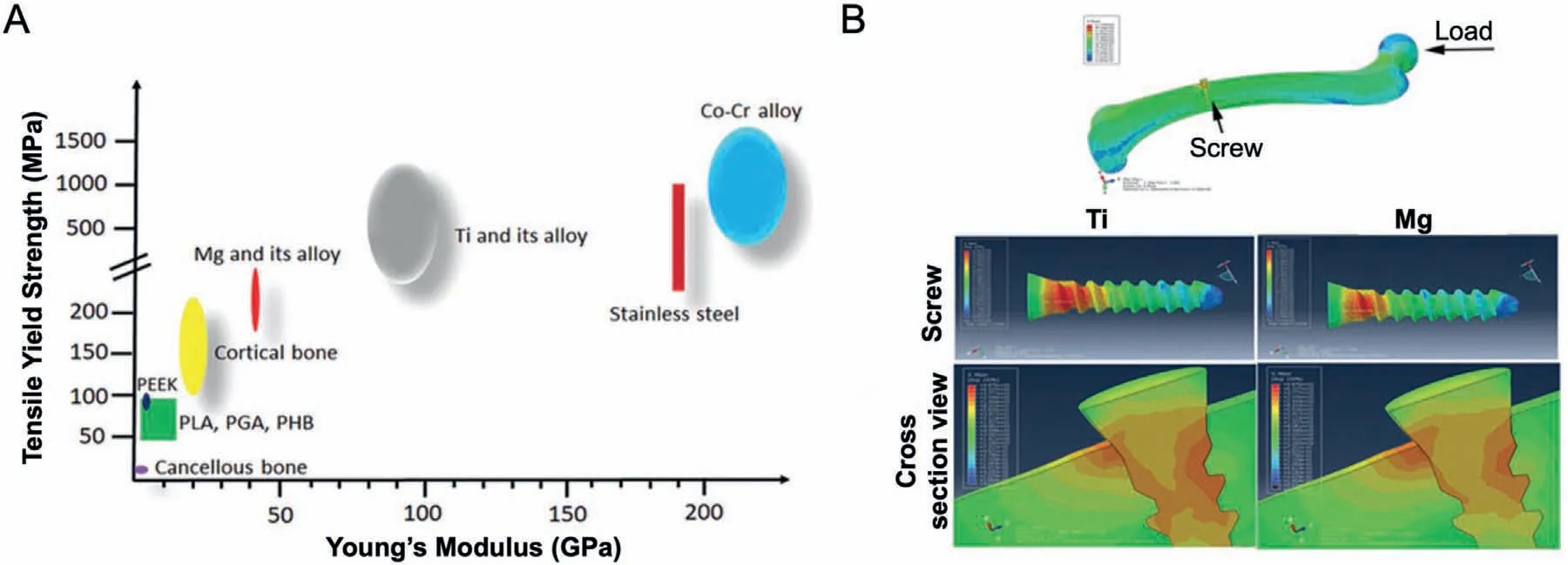
Fig.3.(A) The distribution range of tensile yield strength and Young’s Modulus of natural bone,commercially available orthopedic implants made of polymers and inert metals,and biodegradable Mg and Mg alloys [2];(B) The peak stress distribution in a finit element analysis model composed of a femur and an inserted Ti or Mg screw [2].
Although the degradation product of pure Mg in the body is nontoxic,and the degradation product Mg2+can also promote bone regeneration,too fast degradation of Mg implants in the body can easily lead to failure of fracture treatment[81].Therefore,improving the corrosion resistance of Mg implants is crucial for prolonging the fixatio time of Mg implants in vivo.Adding other elements to Mg can change the microstructure of Mg alloys,such as grain size,type,and structure of compounds,thereby improving the corrosion resistance of Mg alloys.Table 2 summarizes the corrosion rates of Mg alloys with other metal elements.Additionally,various processing conditions,coating,and heat treatment also have been used to improve the corrosion resistance of Mg alloys [49].The in vitro corrosion resistance of Mg alloys has been confirme by many studies [82,83].However,the in vivo anti-corrosion performance of Mg alloy for orthopedic implants still needs further research.In addition,precisely controlling the corrosion rate of Mg alloys for orthopedic implants to match the process of bone regeneration is still a challenge for researchers.
4.Mg in bone regeneration
Mg and Mg alloys became good candidate materials for bone defect repair,not only owing to their superior biodegradation,biocompatibility,and adequate elastic modulus,but also due to the osteoinductive activity of their degradation products [89,90].Additionally,unlike the traditional metal orthopedics implants,such as Ti,Ti alloys,Co-Cr alloys,Mg is an essential element in the construction of soft tissue and bone,involved in numerous biochemical reactions [91].A healthy adult is recommended to take 310-420 mg Mg ions daily to maintain normal life activities and store 24~30 g Mg ions to maintain basic metabolism [92].Most of the Mg ions in the human body are stored in bone tissue [93].If the Mg ion content in the bone tissue decreases,the mechanical properties of the bone tissue will be weakened,the trabecular bone will be broken,and the fragility of the bone tissue and risk of fracture would increase [94].Even though excessive Mg ion is taken in the body,it can be promptly excreted by the circulation and urinary systems without causing any damage [95].
4.1.Mg promotes osteogenic differentiation of osteogenic cells
Bone reconstruction is a complicated process,which demands the coordination of multiple stem cells and a suitable microenvironment.Many researchers have investigated the effects of Mg and Mg alloys on various kinds of osteogenic cells.Díaz-Tocados et al.[96] demonstrated that increasing Mg ion concentrations (0.8,1.2,and 1.8 mM) enhanced the osteogenesis of mesenchymal stem cells (MSCs)in vitro (Fig.5A).Additionally,Mg ion also enhance the expression of osteogenic related mRNA,such as alkaline phosphatase (ALP),RUNX Family Transcription Factor-2 (Runx-2),osteocalcin,via activation of the Notch signaling pathway.In another study,Hung et al.[97] found that the cell culture medium with 10 mM Mg ion effectively promoted osteogenic differentiation of human mesenchymal stem cells derived from bone marrow (BMSCs) osteogenesis and increase mineralization and expression of ALP in the extracellular matrix by activating Wnt signaling pathway.Wang et al.[98] also demonstrated that Mg ions promoted the expression of osteogenic differentiation markers in BMSCs,such as bone morphogenetic Protein-2(BMP-2),collagen-1(Col-l),Runx-2,via MARK signaling pathway.Liu et al.[99] found that Mg promoted mouse embryo osteoblast precursor cells (MC3T3-E1) expression and secretion of platelet-derived growth factor(PDGF),which promotes osteoblast development and bone formation.In another study,Gao et al.[100]revealed that Mgcoated Ti6Al4V co-culture with MC3T3-E1 cells can improve cell proliferation,adhesion,extracellular matrix (ECM) mineralization,and ALP activity compared with bare Ti6Al4V cocultivation (Fig.5B,C).Furthermore,Mg also promoted the osteopontin gene expression of adipose-derived stromal cells (ADSCs),an essential gene for the early biomaterialcell osteogenic interaction [101].Mg can promote the osteogenic differentiation of various osteogenic cells,which is the fundamental theoretical basis for applying Mg in the fabrication of orthopedic metal implants.The bidirectional balance between osteoclastogenesis and osteogenesis plays a central role in bone regeneration [102].In addition to regulating osteoblasts to promote bone regeneration,Mg can also inhibit the action of osteoclasts and bone resorption.Zhai et al.[103] found that Mg leach liquor suppressed osteoclasts’ action and prevent osteolysis in vivo via inhibiting activation of nuclear factor-kB (NF-κB) and activated T-cells cytoplasmic1 (NFATc1) signaling pathway (Fig.5D).In another study,Wu et al.[104] demonstrated that the cell culture medium with 2 × dilution of Mg extract promoted osteogenic differentiation of osteoclasts and inhibit differentiation of osteoclasts.
Furthermore,Mg can also promote the process of bone regeneration in vivo.Hamushan et al.[105] demonstrated that a high-purity Mg pin promoted distraction osteogenesis and shorten the consolidation in rat femur (Fig.6A).Diffusion of implant-derived Mg2+enhances bone consolidation in distraction osteogenesis via regulating Ptch protein activating Hedgehog-alternative Wnt signaling (Fig.6B).Gao et al.[106] constructed a composite chitosan-magnesium (CS-Mg)membrane by dip-coating Mg alloys into chitosan solution for guided bone regeneration.The in vivo results showed that the CS-Mg membrane shows great potential for application as a biodegradable metallic GBRM with excellent osteogenic activity when dealing with rabbit calvarial defects [106].Mg has also been utilized to improve tendon-bone healing.Wang et al.[107] demonstrated that compared with stainless steelpretreated periosteum,Mg-pretreated periosteum significantl enhanced the osteointegration of the tendon graft into the bone tunnels in rabbits.In another study,Cheng et al.[108] compared the differences of Ti screw and high-purity Mg screw to fi the semitendinosus autograft to the femoral tunnel in a rabbit model of ACL reconstruction.The in vivo results showed that the high-purity Mg group had significantl higher expression of BMP-2 and VEGF than the Ti group in the early phase of tendon-bone healing,followed by enhanced expression of fibrocartilag markers and GAG production.
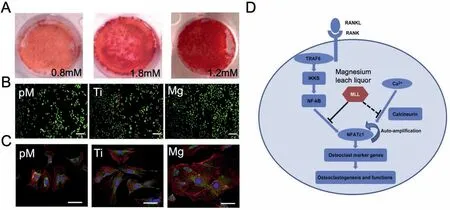
Fig 5.(A) The Alizarin Red S staining of matrix mineralization at different Mg ion levels [96];(B) Calcein-AM/PI staining of MC3T3-E1 cells with different mediums after one week cultivation [100];(C) F-actine/Vinculin/DAPI staining of MC3T3-E1 cells with different extraction after incubation for 48h Pure complete medium (pM);Ti6Al4V sample extraction (Ti);Mg-coated Ti6Al4V sample extraction (Mg) [100];(D) Schematic diagram of the mechanism by which Mg leach liquor inhibits osteoclast differentiation and function [103].
4.2.Mg promotes angiogenesis during bone regeneration
Being a dynamic tissue,bone is not only a complex heterogeneous tissue with intricate hierarchical architecture but also a highly ordered and vascularized tissue with vascular networks,which is connected to the blood system by transverse channels [109].It is essential to construct functional vascular networks,which supply the exchange of oxygen,nutrition,and waste products between new bone tissue and the host[110].Vasculature networks play a critical role in the formation and regulation of bone tissue.The process of bone formation involves the connection between bone cells and blood vessels [111-113] (Fig.7).Bone vasculature networks take part in two general ways of bone regeneration,intramembranous and endochondral ossificatio [114].During the process of intramembranous ossification the vasculature network extends into the mesenchymal region of the periosteum,leading to the differentiation of osteoprogenitors into osteoblasts[115].During the process of endochondral ossification the non-proliferative chondrocytes in the cartilage template secrete pro-angiogenic factors to stimulate blood vessel growth.As the vasculature expands,cartilage tissue is gradually replaced by bone tissue,leading to the formation of new bone[116].Therefore,how to promote vascularization in vivo is very important for bone regeneration.
Mg deficien y would impair angiogenesis in vivo.Mg can regulate the biological functions of various endothelial cells.The previous study demonstrated that human umbilical vein endothelial cells (HUVECs) exhibit a high proliferation rate,cell morphology,and viability in a certain amount of Mg ion [117].Additionally,a certain concentration of Mg ion can also increase the expression levels of angiogenic biomarkers [117].In another study,Pan et al.[118] found that MgCl2enhanced the migration and invasion capabilities of HUVECs and promoted epithelialmesenchymal transition through the Wnt/β-catenin pathway.Additionally,the results demonstrated that MgCl2treatment promoted cytoskeletal reorganization,with an increased long axis/short axis dimension ratio compared with the control(Fig.8A).In another study,Bernardini et al.[119] found that high Mg stimulates proliferation and migration and sensitizes microvascular cells.Li et al.[120] evaluated the effects of extracts of Mg-Zn-Mn alloy on the angiogenesis of human umbilical vein endothelial cells (HUVECs).The in vitro results demonstrated that 6.25% Mg-Zn-Mn alloy extract induces the angiogenesis of HUVECs via the FGF signaling pathway.Yu et al.[121] fabricated a potential dental implant material-dual Zn/Mg ion co-implanted titanium to improve implants’ osseointegration and long-term survival.The in vitro results showed that Mg ions from Zn/Mg-PIII increased Mg2+influ by upregulating the expression of MagT1 transporter in HUVECs,and then stimulated the transcription of VEGF and KDR via activation of hypoxia inducing factor(HIF)−1α,thus inducing angiogenesis [121].The previous study demonstrated that Mg coating on the surface of the Ti6Al4V scaffolds significantl improved the ability of scaffolds to form blood vessels in vivo [100] (Fig.8B).Zheng et al.[89] demonstrated that oral supplements daily of Mg significantl increased the blood perfusion in the proximal tibia and decrease the leakage particles in the distal tibia in the steroid-associated osteonecrosis rat model (Fig.8C).Additionally,Co-IF-staining of ZO-1 and NF-κB p65 showed the coincidental effects of Mg on protecting endothelial cell monolayer integrity and reducing the inflammatio induced by lipopolysaccharide (Fig.8D).Lai et al.[90] formulated a porous poly (lactide-co-glycolide) (PLGA)/β-tricalcium phosphate (β-TCP)/Mg (PTM) scaffolds with Mg powder,PLGA,β-TCP using low-temperature rapid prototyping (LTRP) technology.The results demonstrated that Mg increased the in vivo bone formation ability of PLGA/β-TCP scaffolds when dealing with a segmental ulnar bone defect in the rabbit model.Simultaneously,the results of micro-CT-based angiography showed that more newly formed vessels in the defects implanted with PTM scaffolds than PT scaffold after surgery in the steroid-associated osteonecrosis rat model (Fig.8E).

Fig.6.(A) 3D micro-CT images of the distraction zone after surgery 3,6 and 9 weeks [105].Stainless steel (SS);High-purity magnesium (HP Mg);(B)Schematic diagram showing diffusion of implant-derived Mg ion enhances bone consolidation in distraction osteogenesis via regulating Ptch protein activating Hedgehog-alternative Wnt signaling [105].

Fig.7.The schematic illustration of complex bone tissue with vascular structure [112].
4.3.Osteoimmunomodulatory properties of Mg

Fig.8.(A) The F-actin/DAPI staining of HUVECs [118];(B) Microangiography analysis of newly formed blood vessel around the scaffolds [100];(C) 3D images of micro-CT based vessel architecture and CD31 IHC staining images in proximal tibia and distal tibia 2 weeks after steroid-associated osteonecrosis(SAON) induction in each group [89];(D) Co-IF-staining of ZO-1 and NF-κB p65 [89].Lipopolysaccharide (LPS);(E) 3D reconstructed images of micro-CT-based angiography in this distal femur of the SAON rabbits at 4 and 8 weeks after scaffold implantation [90].

Fig.9.Schematic illustration of effects of Mg2+ on promoting bone regeneration.
Immune responses are vital for bone regeneration and play an essential role in the fate of biomaterials after implantation.Recently,in the process of bone tissue engineering,the osteoimmunomodulatory property of biomaterials is particularly emphasized for osteoclasts and osteoblasts of defect regions,which guides the outcome of bone regeneration [122,123].There is a complex relationship between the immune and skeletal systems.Additionally,the immune and skeletal systems share various cytokines,receptors,and transcription factors that regulate signal transduction pathways involved in osteoclastogenesis and immune system activation,including the receptor activator of nuclear factor-κB ligand/receptor activator of nuclear factor-κB/osteoprotegerin (RANKL-RANKOPG) pathway [124].As a vital role of immune system,macrophages include pro-inflammator M1 type and antiinflammator M2 type.M1 cells take part in acute inflamma tion and,thereby,are more closely associated with the early stage of tissue repair.On the contrary,M2 plays a role in the later stages of bone healing,leading to active repair or the production of fibrou tissue [125].Additionally,M1 type and M2 type can switch to each other in response to various kinds of biomaterials.While,during the process of macrophage subtype transition,various osteogenic related molecules can be released to take part in the new bone formation.Mg and Mg alloys as orthopedic metal implants have a great influenc on osteogenesis and osteoclasts,forming the basis for the study of osteoimmunology.When Mg and Mg alloys are placed into a host,an immune response around the metal implants is triggered.The Mg2+,derived from implants,regulates osteoimmune microenvironment by enhancing macrophage polarization to the M2 phase.The M2-type macrophages secrete IL-10 to initiate the process of bone repair.Furthermore,Mg2+suppressed M1 macrophages with a consequent decrease in proinflammator factors(TNF-α,IL-6),thus acting as an antiinflammator agent.Fig.9 summarizes the effects of Mg2+on promoting bone regeneration by regulating the osteoimmune microenvironment.
The osteoimmunomodulatory properties of Mg and Mg alloys depended on Mg ion concentration in the surrounding microenvironments.Zhang et al.[126] found that microscale Mg ions (100 mg/L) possess osteoimmunomodulatory properties that favor bone formation.Additionally,microscale Mg ions induced M2 phenotype changes of macrophages and the release of anti-inflammator cytokines by inhibiting the TLR-NF-κB signaling pathway.Wang et al.[127] fabricated magnesium calcium phosphate cement (MCPC) for bone regeneration by combining it with CPC powder,magnesium phosphate cement (MPC) powder,and liquid phase (deionized water).The in vitro results showed that compared with the group of CPC extracts,the proinflammator cytokines,including TNF-αand IL-6,were significantl less expressed,and macrophages significantl up-regulated the bone repair-related cytokine of TGF-β1 in the group of MCPC extract.Wang et al.[128] constructed a two-way communication,interactive,transwell system to observe the influence of degradable Mg on paracrine signaling between immune cells and MSCs.The results demonstrated that Mg collaborated with human umbilical cord perivascular cells (HUCPV) to attenuate inflammatio via a moderate cytokine release,accompanied by the pro-healing M2 macrophage phenotype.In turn,Mg in synergy with immune cells could stimulate HUCPV migration,while Mg could influenc the proosteogenic potential of HUCPV but not immune cells [128].CD68 and CD80 subsets were biological markers of type 1 macrophages,while CD68 and CD206 subsets for type 2 macrophages.The previous study demonstrated that Mg alloys significantl stimulated macrophage polarization at the implant-bone interface 5 and 10 days after surgery [129](Fig.10).

Fig.10.Immunohistochemical analysis of macrophage polarization over time [129].Significan differences were presented as ∗p<0.05.
5.Fabrication of Mg-based alloys for orthopedic implants
Various material processing technologies determine the mechanical properties and corrosion resistance of Mg alloys.The processing sequences of Mg and Mg alloys from raw materials to the fina medical implants are extremely important.The primary processing of Mg alloys for orthopedic implants includes solid and liquid state technologies.Powder metallurgy is the primary solid-state synthesis technique used to fabricate both particle and whisker-reinforced metal matrix composites [130].The powder metallurgy process includes mixing and blending of Mg powder and other metal powders,followed by the compaction of the mixed powder and in the last sintering of the compact under controlled conditions.Power metallurgy has advantages of high production rate,controllable composition,and close dimensional tolerance.Jiang et al.[131] successfully fabricated Mg-Ti composites with bicontinuous by powder metallurgy and ultrasonic infiltratio for biomaterial potential.In another study,Balog et al.[132] also fabricated a Mg-Ti bioactive composite,which decreased the occurrence of stress-shielding and has better biocompatibility than that of the traditional Ti and Ti alloys used for dental implants.Zheng et al.[133] successfully fabricated Mg/Ca composites by powder metallurgy for medical applications.However,power metallurgy has disadvantages of high production cost,poor plastic properties,and poor ductility.As one of the liquid state technologies,casting is a manufacturing process in which a liquid material is usually poured into a mold containing a cavity of the desired shape and then allowed to solidify.During the process of casting,molten liquefie metal is poured into the cavity and then ejected from the mold to get the desired form,such as rods,wires,tubes,and plates.The casting process can be divided into sand casting,continual casting,squeezing die casting,lost foam casting,low-pressure casting,gravity die casting,and investment casting.The casting process has advantages of low production cost and shape customization.Hong et al.[134] fabricated Mg-Al alloy scaffolds for biomedical application via a camphene-based freeze-casting process with precisely controlled heat treatment.However,the implants manufactured by the casting process have a rough surface.Additionally,it is not possible to achieve close dimensional accuracy via casting.Hence further machining is required.The secondary processing of Mg alloys,including machining,joining,sterilization,and packaging,is utilized to fabricate Mg and Mg alloy medical implants with specifi biological functions.
6.Application of Mg-based alloys in vivo
6.1.Pre-clinical applications of biodegradable Mg-based alloys for bone regeneration
Medical metal implants,including plates,screws,and intramedullary hook pins,are made of various metal materials that have been utilized to facilitate bone healing in orthopedic surgery,such as fracture,osteotomy,bone transport.Medical metal implants made of Ti alloys are most widely used due to their excellent mechanical strength.However,due to their non-degradability,Ti alloy implants often require removal,which entails additional surgery,resulting in repeated surgical trauma and financia burden to the patient [135].As a nontoxic,biodegradable material,Mg has gradually been used as a raw material in constructing orthopedic biodegradable implants.
High-purity Mg has been fabricated into biodegradable metal implants with various shapes.Chaya et al.[136] successfully constructed fixatio plates and screws with 99.9%pure Mg and compared them with titanium devices in a rabbit ulnar fracture model (Fig.11A).The histomorphology results after four weeks in vivo showed no difference in bonebridging fractures fi ed with Mg and Ti devices [136].Additionally,a large amount of new bone tissue formed around Mg plates and screws.In another study,Han et al.[58] also found good osseointegration surrounding high-purity Mg screws,and increased bone volume and bone mineral density were detected at the fracture gap of the femoral intercondylar fractured rabbit model (Fig.11B).Cheng et al.[137] applied biodegradable high-purity Mg screws in the rabbits model of anterior cruciate ligament (ACL) reconstruction.The in vivo results demonstrated that high-purity Mg screw effectively inhibited graft degradation and improved biomechanical properties of tendon graft during the early phase of graft healing(Fig.11C).Yu et al.[138]implanted as-rolled high-purity Mg screws in rabbit tibiae for up to 52 weeks to evaluate their long-term in vivo degradation and the local and systemic effects of their degradation products.They found that highpurity Mg screws had a slow degradation rate and a uniform degradation morphology in rabbit tibiae during 52 weeks(Fig.11D).New bone formation at bone-implant contact was observed at 26 weeks and 52 weeks post-implantation.In addition,during the 26-week monitoring period,no abnormal increase in serum Mg and urinary Mg levels was detected,and no liver or kidney dysfunction was detected.
Many studies have proven the promotion of the new bone formation of high-purity Mg.However,the poor mechanical strength of Mg is still one of the major challenges for its clinical application.Therefore,many researchers use the strategies of adding alloying elements in Mg to increase their mechanical properties.Table 3 summarize the in vivo studies of Mg-based alloys for orthopedic implants.Levorova et al.[139] demonstrated that bone healing around the WE43 Mg alloy screws was of similar quality as around titanium screws after implantation of rabbit tibia.Cho et al.[140] found that Mg-Ca-Zn alloy bone screws showed a faster degradation rate and better histopathological response than self-reinforced poly L-lactide (SR-PLLA) (Fig.12A).Waizy et al.[141] investigated the biocompatibility and degradation rate of extruded Zn-Mg-Sr alloy screws by implanting them into one of the tibiae of rabbits.The in vivo results showed that more than 50% of screws were covered by fibrou tissue,and osteolysis of the surrounding bone was not observed.In another study,Wang et al.[142] prepared Mg-Zn-Sr alloys and tested their potential for attenuating peri-tunnel bone loss in ACL reconstruction.The in vivo results showed that compared with the poly-lactide (PLA) screw group,peri-tunnel bone loss was significantl lower in the Mg-Zn-Sr group (Fig.12B).Interestingly,the bony ingrowth rapidly fille the cavity left by the complete degradation of Mg-Zn-Sr screws at 16 weeks.Angrisani et al.[143] analyzed the degradation progress from nine months to 3.5 years after implantation of cylindrical LAE442-based Mg alloy pins into the medullary cavity of New Zealand White rabbits.The in vivo results showed a very slow degradation rate of up to 72 weeks.Three-point-bending results revealed a percentage loss of F(max) of 88.47% for LAE442-based Mg alloy pins after 3.5 years implantation.Although the total amount of alloying elements detected in the rabbit organs is very low,compared with rabbits without any implanted Mg alloy,rabbit organs with LAE442-based Mg alloy pins show an increase in the concentration of the alloying elements lanthanum,cerium,neodymium,and praseodymium by 10-20 times [143] (Fig.13).

Table 3 The in vivo studies of Mg-based alloys for orthopedic implants.
6.2.Clinical applications of biodegradable Mg-based alloys in orthopedics

Fig.11.(A) Magnesium and titanium plates and screws [136];(B) Micro-CT scanning of rabbit femur with Mg or PLLA screws at 4,8,16 and 24 weeks post-operation [58];(C) Micro-CT view of the Ti or Mg screws in f xation of tendon graft in femoral intracondyle [137];(D) In vivo 3D morphology of the HP Mg screws implanted in rabbit tibiae at different time points from micro-CT [138].DL,deposition layer.

Fig.12.(A) Micro-CT of Mg or PLLA screws [140];(B) Representative histology of the peri-tunnel tissue in the PLA and Mg-6Zn-0.5Sr groups at 6 and 16 weeks after surgery [142].F:fibrou tissue;T:tendon graft.
Fracture is one of the most common diseases in orthopedics.Traditional stainless steel or Ti alloy implants currently used in orthopedics have the disadvantage of excessively high elastic modulus,biological inertness,stress shielding,and metal artifacts.Additionally,after implantation,traditional metal implants need to be removed by a second operation.Mg regained more attention for the treatment of orthopedic implant application.Unlike permanent implants,Mg implants corrode inside the animal or human body with simultaneous replacement of native bone [152].Mg and Mg alloys can completely change the design and function of existing medical metal implants and bring new medical effects[155,156].At present,some orthopedic metal implants made of Mg alloys have been used to treat clinical orthopedic diseases.Regarding functional and radiological outcomes,bioabsorbable Mg and Ti screws had similar therapeutic effica y in medial malleolar fracture fixatio [157].However,the rate of implant removal was higher with Ti screws [157].The preoperative ankle radiograph of Mg screws implanted into medial malleolus fracture was shown in Fig.14.At the 1.5-month follow-up,gas shadows were observed around screws(white arrows).The fracture was united at the 7th-month visit,and a significan decrease in gas shadows was seen.At the 20th month follow-up,almost no gas shadows were visible.At the 39th follow-up,a faded silhouette of screws was hardly noticeable.Zhao et al.[158] applied biodegradable and osteogenic pure Mg screws for fixin vascularized bone flap during osteonecrosis of the femoral head surgery via a prospective and randomized pilot clinical trial.The results showed that Harris hip score was significantl improved in the Mg screw group compared to the control group.Additionally,the postoperative serum levels of Ca,Mg,and P,relevant for liver and kidney function,were within the normal physiological range in all patients of both groups[158].Plaass et al.[159] applied Mg-based MgYREZr screws to treat for fixatio of modifie Chevron osteotomies in hallux valgus surgery.The results showed that Mg-based alloy implants degrade without an implant-directed inflammatio reaction and possess higher strengths than degradable polymer implants.In another study,Windhagen et al.[160] demonstrated that Mgbased MgYREZr screws are equivalent to standard titanium screws for fixatio during chevron osteotomy in patients with a mild hallux valgus via a prospective,randomized,clinical pilot trial.Additionally,no foreign body reactions,osteolysis,or systemic inflammator reactions were detected in the group of Mg-based MgYREZr screws.Lee et al.[161] applied the Mg-5wt.%Ca-1wt.%Zn screw in the treatment of the fracture.The results showed that all patients involved in the clinical study showed a normal healing rate without any sign of pain,which demonstrated the endless possibility of biodegradable implant devices.In another study,the MgYREZr alloy screw was used in eleven patients with a medial malleolar fracture[155].All the fracture patients achieved solid radiographic union without disfunction and pain.Mg and Mg alloy orthopedic metal implants currently used in clinical work are mostly screws.Additionally,most Mg and Mg alloy screws are used for non-weight-bearing bone fractures.Up to now,no clinical research on Mg and Mg alloy plates has been reported.

Fig.13.Qualitative element distributions of magnesium,aluminum,calcium,phosphorous,lanthanum and cerium after implantation duration [143].

Fig.14.The preoperative ankle radiograph of Mg screws implanted into medial malleolus fracture [157].
7.Functional surface modificatio of biodegradable Mg-based alloys for orthopedic implants
The surface properties of biodegradable medical implants play an essential role in the interaction between the implant and surrounding tissue because the surfaces contact the host environment directly and regulate the biological behaviors of cells on the surface of implants.The novel Mg-based alloys acting as orthopedic implants have drawn much attention due to their spontaneous degradability,favorable mechanical properties,and excellent biocompatibilities.In addition,in vivo degradation products of Mg-based alloys can effectively promote bone formation.However,the rapid degradation rate of pure Mg and Mg alloys in vivo will cause a strong local inflammator response that can inhibit the integration of implant-to-tissue,and result in the failure of internal fixa tion [162].Therefore,how to control the degradation rate of Mg-based alloys and achieve slow and controllable longterm degradation of implants is still a challenge.Currently,many methods,including heat-treatment,alloying,high-purity,plastic-deformation,amorphous,and surface modificatio[163,164].Except for the surface modification other methods regarding improving corrosion resistance were at the expense of mechanical property [165].Besides,the corrosion contact between theα-Mg matrix and the electrolyte solution cannot be completely prevented via microstructure adjustment [166].As a typical method to improve the corrosion resistance,surface modificatio can effectively isolate the Mg-matrix from the electrolyte by establishing the corrosion barrier without changing the microstructure characteristics [167].In addition,the biological behaviors of Mg-based alloys for orthopedic implants can also be enhanced by surface modificatio[168].
7.1.Various surface modificatio methods of Mg-based alloys for orthopedic implants
Constructing anti-corrosion coatings on the surface of Mgbased alloy implants using surface modificatio technology is beneficia for maintaining implant structure stability and performance in the early stage of implantation [168-170].Recently,many studies have reported various surface modificatio methods for constructing coating on the surface of Mg-based alloys.Generally,according to process methods,the surface modificatio of Mg-based alloys can be divided into four types:mechanical,physics,chemical,and biological coating.The scheme and details of coatings are shown in Fig.15.The surface modificatio can be classifie into conversion coatings and deposition coatings in actual operations.As a common surface modificatio method,conversion coating is an in-situ coating formed by a chemical reaction between an Mg substrate and a coating solution.Conversion coatings are created in a complex interaction of metal dissolution and precipitation [171].The conversion coatings include chemical conversion coating,ionic liquid conversion coating,alkali heat treatment (AHT) conversion coatings,plasma electrolytic oxidation coating (PEO),and hydrothermal coating.Among these methods,chemical conversion coating is the most commonly used method due to its simple operation and inexpensive cost [172].Before conversion coatings,Mg-based alloys surfaces need to undergo a series of surface activation treatments.In addition,the morphology and properties of the conversion coatings are related to the substrate alloy.Deposition coatings refer to ex-situ coatings in which the substrate does not participate in the formation of the coating[173].The deposition coatings include physical vapor deposition (PVD)coating,Atomic layer deposition (ALD) coating,electrolytic deposition layer,dipping or socking coating [174].The bonding force between the coating and the substrate prepared by the deposition coatings is derived from intermolecular and mechanical forces,resulting in feeblish adhesion of the coating on the substrate.In contrast,the adhesion between the substrate and the coatings constructed by conversion coatings is pretty good,which is the primary distinction between conversion coating and deposited coating.Therefore,how to solve the problem of peeling off under stress (hydrogen bubbling) still needs further studies.
7.2.Specifi functionalized coatings for orthopedic applications
With the acceleration of global population aging and the rising of traffi accidents,the number of trauma patients,especially geriatric fracture patients and open fracture patients with vascular and nerve injury,increases each year [175].Elderly fractures and open fractures are two common diseases that trauma orthopedics need to face.Elderly fracture patients often suffer from postoperative nonunion or delayed union due to aging and comorbidities.Improving the osteogenic activity of orthopedic implants can effectively reduce the occurrence of nonunion in elderly patients.In contrast,patients with severe open fractures often suffer from postoperative bone infection or osteomyelitis.Enhancing the anti-infective properties of orthopedic implants can effectively reduce the occurrence of bone infections.Currently,Mg and Mg alloys are receiving immense attention as promising orthopedic implants due to their degradability,high bioactivity,excellent biocompatibility,and comparable mechanical properties to bone.Simultaneously,various coating methods have been applied to the surface modificatio of Mg and Mg alloys.All these coatings can not only improve the corrosion resistance of Mg-based alloys but also endow magnesium alloys with specifi functionalized coatings for orthopedic applications,including promoting osteoblast differentiation and anti-infective properties.
7.2.1.Functionalized coatings for enhancing osteogenic activities
Calcium phosphate (CaP) coating is currently the most commonly used functional coating to enhance osteogenic activities of Mg-based alloys.As a component of natural bone tissue,CaP processes osteoinductive and osteoconductive capabilities that are beneficia for bone regeneration [176].As the major inorganic component of bone tissue,hydroxyapatite (HA) is the main phase of CaP and takes part in the metabolic processes of bone tissue [177,178].In addition,the synthetic HA could strengthen the apatite group of periimplant bone tissue [179].Therefore,the CaP-coating is expected to become multiple-functions film outside Mg alloys,such as better bioactivity and osteoconductivity as faster integration with host bone tissue,protecting implants from body fluid [176,180].
So far,various surface modificatio methods have been used to construct CaP coatings for Mg and Mg alloys.Hahn et al.[181] produced an HA-based coating on the Mg alloys to enhance the biocompatibility and corrosion resistance by spray techniques.Abbas et al.[182] reported a study on the Mg alloys with laser coating,in which the corrosion resistance of Mg alloys was improved,and weight was reduced.The laser coating is beneficia to the transfer of stoichiometry between Mg alloys and the film while the splash effects along with the technique lead to poor fil quality [183].The electrochemical technique is known for simplicity and costeffectiveness.Similarly,the HA coating prepared by the electrochemical method formed HA crystal on Mg and Mg alloys as a dense covering,which effectively suppressed the discharge of Mg ion [184] (Fig.16A).The schematic illustration of the formation and growth mechanism of the HAp coating on the surface of Mg is shown in Fig.16B.However,the coating deposited by the electrochemical method was found to be more porous and less adherent,which decreased the coating quality [176].Sol-gel coating technology is mainly studied to be a suitable technique for clinical applications.Rojaee et al.[185] synthesized the HA coating produced by the sol-gel method,which increased the corrosion resistance of Mg alloys and promoted cell growth.The behavior of in vitro biomineralization of n-HAp coated specimens following immersion in SBF solution is shown in Fig.16C.The main disadvantage of this process is the quick evaporation of the residual water and solvent due to the porous and cracking coating [176].Moreover,it is difficul to maintain the thickness of fil because of the specimen holder’s uneven dipping and withdrawing speed in the sol-gel solution[183].The multistep coating is an effective way to improve the corrosion resistance of Mg alloys.Ten et al.[186] revealed that multiple coating greatly improved the anticorrosion properties of Mg alloys and compromised the porosity level.However,the different coating compositions of different coating layers bring challenges to achieving optimum bonding strength between coating layers and substrate [176].Although there are always some arguments about choosing among these methods,a feature must be possessed in all the methods is the uniform fil thickness [187].The dense and uniform coating can prevent the body flui from penetrating the Mg and Mg alloys.Furthermore,plasma electrolytic oxidation (PEO),also known as micro-arc oxidation (MAO),is a high voltage plasma-assisted anodizing process used to modify the surface of valve metals like Mg and Mg alloys [188,189].PEO can provide porous and biocompatible coatings for implants among various techniques [190,191].Azarian et al.[192] have successfully fabricated a bioceramic PEO layer containing Si,P,Ca,Na,and F on AZ31 Mg alloy.They found that applying a voltage of 350-400 V (50-100 V higher than the breakdown limit)could greatly facilitate the synthesis of a PEO ceramic coating with fewer defects and more uniform morphology.Additionally,the growth mechanism of the PEO coating on AZ31 Mg alloy includes a sequence of steps,as shown schematically in Fig.16D.In another study,Sampatirao et al.[193] fabricated a less porous and corrosion-resistant ceramic composite coating on a ZM21 alloy by incorporating ZrO2nanoparticles into the PEO coating by PEO-EPD process followed by a laser texturing process.The results showed that laser surface textured PEO and PEO-EPD coated samples unveiled enhanced corrosion resistance and cell growth,thereby enabling a suitable prototype for biodegradable implant applications [193].

Fig.16.(A) Surface and cross-sectional images of pure Mg treated for various times [184];(B) Schematic illustration of the formation and growth mechanism of the HAp coating [184];(C) SEM photomicrographs of sol-gel derived n-HAp coating on Mg substrate after soaking in SBF solution at different times[185];(D) Schematic view of the PEO process on AZ31 Mg alloys [192].
Recently,the mechanisms and degradation processes of Mg-based alloys coated with CaP coating in vitro and in vivo have been widely studied.All these results are vital in accumulating the data of Mg-based alloys coated with CaP coating for further clinical applications.Compared with uncoated Mg and Mg alloys,HA coating could effectively slow down the degradation rate of Mg and Mg alloysin vitroandin vivo[194].Compared with uncoated Mg alloys,cell adhesion,cell differentiation,and cell proliferation on Mg and Mg alloys with HA coating were greatly increased [195] (Fig.17A).For example,the viability and proliferation of BMSCs were increased on the HA-Mg scaffold,better cell adhesion and proliferation of osteoblast cells were reported on HA-coated Mg alloys,and MC3T3-E1 cells showed a high growth rate on HA-coated Mg-alloys [196-198].Peng et al.[196] fabricated a bilayer coating (HA/GO#) with hydroxyapatite as the inner layer and graphene oxide as the out layer was prepared on AZ31 alloy (Fig.17B).In addition,MC3T3-E1 on the AZ31 sample showed poor spreading,while on HA and HA/GO coating samples showed obvious filopodi and lamellipodia.Simultaneously,the spread area of MC3T3-E1 on the HA/GO coating sample was significantl larger than that on the HA sample,which indicated that HA/GO bilayer coating can enhance cell spreading (Fig.17C).Besides,through the iconography method,general repair processing,and mineralization can be observed.Wu et al.[199] found that both volumes of new bone around CaP coated Mg-implants and the volume of residual implants in rat calvarial defect models were more in CaP coated group through Micro-CT(Fig.17D).The results in vitro revealed that the CaP coating improved the hydrophilicity,cell affinit,and Ca affinit of the Mg and Mg alloy medical implants and lower degradation,resulting in a higher osteointegration and bone affinit CaP coated Mg and Mg alloy implants[199].In another study,Hiromoto et al.[200]fabricated HA coating layers on the surface of pure Mg,Mg-0.8mass% Ca (MgCa),WE43,Mg-3mass% RE-1mass%Y (EW31) and Mg-4mass% RE (RE4) rods,and compared the differences of uncoated and HAp-coated Mg/Mg alloy rods in rat femurs.The surface and cross-section and overall SEM images of HAp-coated pure Mg and MgCa,WE43,EW31,and RE4 alloy rods were shown in Fig.17E.The in vivo results showed that HAp coating on the surface of Mg and Mg alloy enhanced bone regeneration and suppressed the corrosion initiation and corrosion progress of Mg substrates,which attributed to the fewer gas cavities formed from corrosion at the peri-implant tissue.Singh et al.[201] fabricated a TiO2-HA-PCL hybrid coating on ZM21 alloy using a facile wet chemical method to obtain uniformly distributed interconnected pores in PCL layers (Fig.18A).The results showed that the hybrid TiO2-HA-PCL coating effectively enhanced the corrosion resistance of Mg alloys in SBF.The mechanism of corrosion resistance is shown in Fig.18B.

Fig.17.(A) The SEM and CLSM images of the MC3T3-E1 on the surface of bare Mg and HA-coated Mg [195];(B) Corrosion morphology of AZ31,HA and HA/GO samples after immersed in α-MEM culture medium [196];(C) Skeleton stain of MC3T3-E1 cells after cultured on AZ31,HA and HA/GO samples [196];(D) Cross-sectional elemental analysis of CaP-coated Mg after alkali-hydrothermal treatment for 2 h [199];(E) The surface and cross-section and overall SEM images of HAp-coated pure Mg and MgCa,WE43,EW31 and RE4 alloy rods [200].
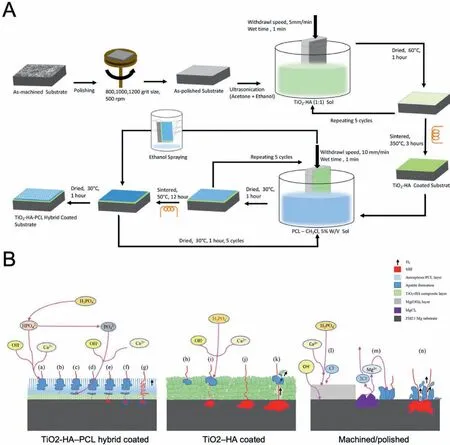
Fig.18.(A) Schematic representation of creating TiO2-HA and TiO2-HA-PCL hybrid coatings on ZM21 Mg substrate [201];(B) Schematic illustration of the Adsorption mechanism of TiO2-HA-PCL hybrid coated,TiO2-HA coated and as machined/polished ZM21 Mg substrate immersed in SBF [201].
Bioactive glasses (BG) coating has gained attention as another osteogenic coating for Mg-based orthopedic implants due to its biocompatibility,excellent osteoinductivity,and good biodegradability [202].The dissolution products of the BG can stimulate the osteoprogenitor cell to promote new bone growth,which contributes to the forming of hydroxycarbonate apatite (HCA) layer,a kind of bone mineral interacting with collagen fibril in host bone[203].The HCA layer is beneficia to proteins absorbing and cell attaching.Furthermore,it promotes the osteogenic differentiation of MSCs and bone matrix produced.Notably,the osteoinductive property of BG is different from the osteoconductive biomaterials.The former stimulates bone growth on the bioactive glass away from the bone-implant interface,while the latter stimulates bone growth along with the bone-implant interface[204].During the regeneration process of bone tissue,blood vessels act as a carrier to transport nutrients.Vascular endothelial growth factor (VEGF) is a vital angiogenic growth factor during tissue regeneration [205].The degradation products of BG also stimulate VEGF secretion of fibroblas and proliferation of endothelial cells [206].
Recently,various coating techniques have been utilized to construct BG coatings for Mg-based alloys [207,208].The sol-gel deposition is a commonly used technique for the BG coating,which has various advantages,including low processing temperature and coating of intricate shapes [209].Zhang et al.[202] have successfully fabricated mesoporous 45S5 bioactive glass-ceramic (45S5 MBGC) coatings on AZ31 Mg alloy substrates through the sol-gel method and followed the dip-coating technique.The results showed that the glassceramic coatings lost the protective effects to the Mg substrate in a short time when the applied compressive stress was greater than 25 MPa,and no crystallized apatite was formed on the surface due to the high Mg2þ releasing and the peeling off of the coatings (Fig.19A).Ye et al.[210] successfully coated BG on Mg alloys with the crack free uniform coating by sol-gel coating methods (Fig.19B).The gradual corrosion in SBF of bioactive 45S5 glass-ceramic coating of Mg alloy is shown in Fig.19C.And the potential corrosion value of coated Mg alloys was increased from −1.60 V to −1.40 V[210].While,there were limitations observed in the sol-gel coating,such as the weak adhesive strength between coating and substrate,the cracks on the surface coating,and a longer processing period [211].Electrophoretic deposition is a lowcost electrochemical process,which can be used on several substrate shapes with a short deposition time [187,212].Rojaee et al.[212] deposited BG coating on Mg alloys by the electrophoretic deposition method at 1000 °C and increased the corrosion potential of coated substrate controlling the release rate of Mg in a safe range of the human body.Additionally,the large surface area of the nanostructured bioactive glass coating would accelerate the deposition of HCAp in the SBF solution (Fig.19D).
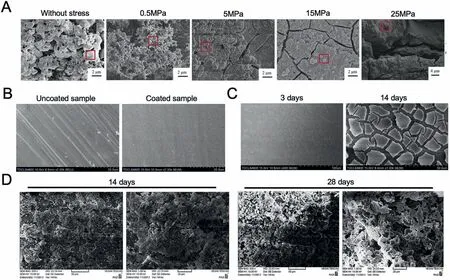
Fig.19.(A) SEM micrographs of 45S5 bioactive glass-ceramic coating immersed in SBF for 8 days without stress and under various compressive stress[202];(B) Surface morphology of uncoated sample and bioactive 45S5 glass-ceramic coated Mg alloy [210];(C) The gradual corrosion in SBF of bioactive 45S5 glass-ceramic coating of Mg alloy [210];(D) The SEM micrographs of the bioactive glass coated on the Mg in SBF [212].
At present,various researchers have devoted themselves to exploring the bioactivities of BG-coated Mg-based alloys.In terms of mechanical properties,BG is hard enough to bone regeneration.Fabris et al.[213] demonstrated that BG-coated implants possessed higher mechanical stimulation for bone maintenance and enhanced corrosion resistance.What’s more,the BG coating can enhance the adhesion strength and cellular performance,including cell proliferation,spreading,and cell viability [214].From the part of bioactivity,the degradation products of BG can promote new bone growth and mineralization,further improving the mechanical properties of fracture sites.Silver et al.[215] evaluated the bioactivity of BG in vitro assessment,findin BG increasing intracellular calcium levels of osteoblasts and up-regulating related genes expression,such as insulin-like growth factor II and alkaline phosphatase.Zhang et al.[216] coated mesoporous BG on metallic implants for bone regeneration in a rabbit model.The micro-CT results showed higher volumes of new bone,more bone matrix apposition,and maturation in the mesoporous BG coated group and neovascularization inside the regenerative tissue.They demonstrated that the release of silicon and calcium from BG coating was the key factor enhancing osteoconduction and vascularization of peri-implant tissue [216].
At present,CaP coatings and BG coatings are still the main coatings used to increase the osteogenic ability of Mgbased orthopedic implants.These two coatings not only increase the mechanical and anti-corrosion properties of Mgbased alloys but also increase the osteogenic and angiogenic abilities of Mg-based orthopedic implants.In addition,degradable polymeric coatings loaded with drug delivery systems have also been recognized as a feasible means to enhance the osteogenic ability of Mg-based orthopedic implants [162].The polymer mainly includes polylactic acid(PLA),poly(lactide-co-glycolic) acid (PLGA),polycaprolactone (PCL),polydopamine (PDA),chitosan (CS),and Collagen (Col) [217].Additionally,degradable polymer coatings cannot directly improve the osteogenic ability of Mg-based orthopedic implants and can only as a carrier of osteogenic drugs to enhance bone regeneration.
7.2.2.Functionalized coatings for enhancing antibacterial properties
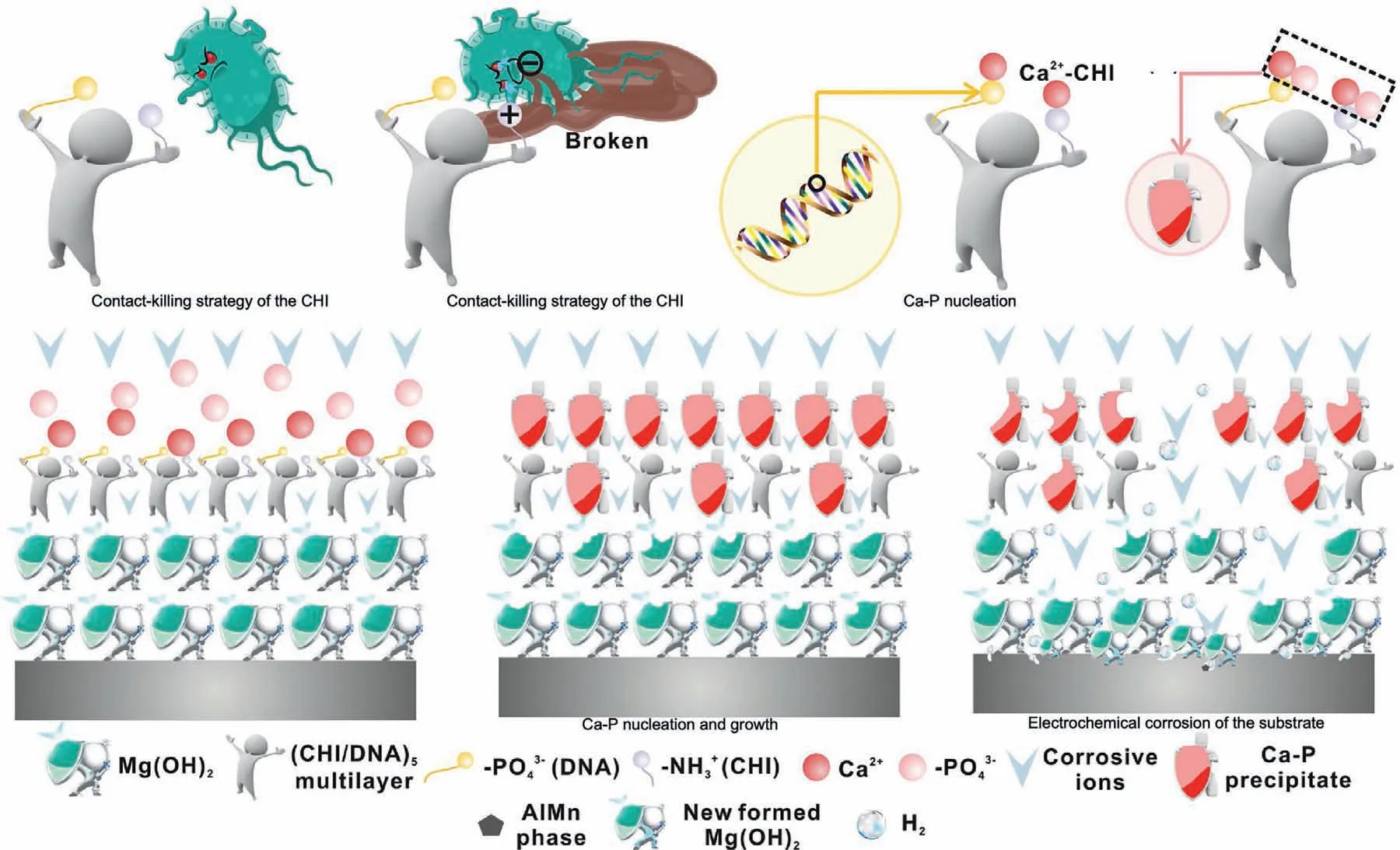
Fig.20.Schematic representation of the antibacterial and degradation mechanism of the (CHI/DNA)5/Mg(OH)2 coating [224].
An orthopedic implant is a kind of widely used medical device that affects the regeneration of surrounding tissues so that there are various requirements for surface properties.Surface modificatio is an available method to modify the biological performance of implant devices.As the main complications after surgery,infection is closely related to the success of surgery,and the therapeutic effect of implants is closely related to its antiinfection properties [218].While from the antibacterial aspect,one of the most effective ways is to load antibiotics on the surface of medical implants [219].Recently,many types of research focused on antibacterial coating constructed by zinc due to its anti-inflammator function[220,221].Chitosan is a semi-natural biopolymer with low toxicity and hydrophilic surface to promote cell adhesion and proliferation,exhibiting antimicrobial activity based on the contact-killing strategy [222].Chitosan coating improves the anti-corrosion and degradation performance of Mg-Ca alloy[223].In another study,a (chitosan/deoxyribonucleic acid)5 coating was constructed by a layer-by-layer (LbL) assembly dip-coating method with Mg(OH)2coating as an inner protective layer on AZ31 alloy,which exhibits good corrosion resistance and antibacterial properties [224].The antibacterial and degradation mechanism of the (chitosan/deoxyribonucleic acid)5/Mg(OH)2coating was shown in Fig.20.Feng et al.[225] fabricated a Zn-contained polydopamine coating on Mg alloys,which possessed excellent resistance againstStaphylococcus aureus,superior anti-inflammator property,and,more important,enhancing the osteogenic differentiation ability of MC3T3-E1.Zhou et al.[221] doped Zn into HA coating on Mg alloys and tested its corrosion resistance and bioactivities.The coated Mg alloys showed better antibacterial properties,higher corrosion resistance and promoted the adhesion and differentiation of BMSCs [221].As well as Zn ions,many metallic ions,such as copper (Cu),silver (Ag),and Mg,have the ability of antibiosis.However,due to a couple of corrosion between two metals,the metallic coating outsides Mg alloys at risk of accelerating corrosion.Furthermore,the releasing rate of metallic coating outsides Mg alloys is out-of-control.Therefore,in many studies,biodegradable polymer and CaP are used to load antibiosis and improve surface properties.Yang et al.[226] incorporated the Cu into a polycaprolactone(PCL)coating system to improve the bioactivity of Mg alloys.Cu ions released slowly from the coating continually inhibit bacterial growth,and the silicon released from coating stimulates osteoblast cells to differentiation and collagen type I synthesis [226].Wang et al.[227] utilized polydopamine and chitosan as adhesive assisting multiple layers of osteoinductive carbonated apatite and antibacterial silver nanoparticles assembling on the surface of Mg alloys.The multilayered coating on the surface of Mg alloys released Ag ions into surrounding tissues,effectively inhibiting the growth ofEscherichia coliandStaphylococcus aureus[227].
At present,the improvement of the antibacterial properties of Mg-based orthopedic implants is mainly achieved by loading antibacterial agents into surface coatings.Antibacterial agents in the surface coating of Mg-based alloys can effectively kill and inhibit bacteria,thereby reducing the risk of implant infection.The method of surface coating loaded with antibacterial agents has been proved to be effective by many studies.However,whether the delivery of topical antibacterial agents will adversely affect the cells in the bone tissue still needs further research to investigate.In addition,the biomimetic superhydrophobic or super-slippery surface constructed on the surface of Mg-based orthopedic implants can inhibit the initial adhesion of bacteria,thereby preventing the formation of bacterial membranes.However,this kind of physical antibacterial research is still less,and its natural antibacterial effect in vitro and in vivo still needs further research.
8.Challenges and perspectives
Mg has mechanical properties close to bone tissue to reduce stress shielding and has good biocompatibility and biodegradability.It has gradually become the most concerning new metal material in the fiel of orthopedic biomedical materials.Although the biodegradable Mg and Mg alloy have been confirme as promising alternative materials for orthopedic metal implants,there are still some problems to be solved in the process of commercialization.
Rapid and uncontrollable corrosion rates remain the major problem of Mg used as orthopedic metal implants.Control and regulating Mg’s corrosion resistance is the key step in whether Mg metal implants can enter clinical applications.Additionally,abrupt deterioration in mechanical strength of Mg implants is experienced due to their inhomogeneous corrosion.Many strategies,such as alloying elements,different manufacturing processes,Mg purity improvement,and surface modificatio and coating,have been applied to regulate the corrosion Mg rate in vivo.The selection of alloying elements in Mg alloys for orthopedic implants should also take the degradation,biocompatibility,and osteoinductive bioactivity of alloying elements into account.Decreased grain size has improved the corrosion resistance and mechanical properties of Mg and Mg alloys implants.Various manufacturing processes,including high-pressure torsion,rolling,milling,and equal channel angular extrusion,have been utilized to decrease the grain size of Mg and Mg alloys.Additionally,multimodal grain distribution has been reported to increase Mg and Mg alloy implants [228].Surface modification of Mg and Mg alloy implants,such as hydroxyapatite coating,polymeric coating,and other alloy coatings,have also been treated as an effective technique to improve their corrosion resistance [229].Corrosion resistance improvement of Mg and Mg alloy implants reduced accumulation of detached particles and gas,the degradation products of Mg,which resulted in cavity formation at peri-implant space [229,230].
Long-term follow-up studies focusing on Mg and Mg alloy orthopedic implants in vivo are still very limited.Most animal experiments conducted one-year follow-up observation after Mg and Mg alloy implantation.Mg and Mg alloy implants are designed to be biodegradable and do not require secondary removal operations.It is necessary to conduct the long-term safety assessment of Mg and Mg alloy orthopedic implants in animal fracture models.In the process of Mg alloy degradation in vivo,besides releasing Mg ions,it also causes local accumulation of alloying elements.These alloying elements would enter the liver,kidneys,and other organ tissues through the blood circulatory system.It is still unclear whether the alloying elements would be harmful to these organs and tissues in this process.Although most studies have confirme that after the animals were implanted with Mg and Mg alloys,no obvious abnormality was observed in the level of metal elements in the blood within a short period.However,it is still unknown whether long-term Mg and Mg alloy implantation will change the level of trace elements in the blood.Fully exploring and observing the in vivo degradation and metabolism process of Mg and Mg alloy implants is necessary for promoting biodegradable Mg and Mg alloy implants to clinical applications.
More innovative structural designs,including macrostructure design and microstructure design,are necessary to develop to meet higher technical requirements in clinical use.At present,Mg and Mg alloys are mainly made into metal screws for clinical fracture treatment.More Mg and Mg alloy implants,such as intramedullary nails and locking compression plates,can also be fabricated to enrich the types and applications of Mg and Mg alloy implants.Orthopedic metal implants with intricate shapes can be designed according to the patient-specifi anatomic data using computer-aided designs.Based on this goal,in recent years,additive manufacturing,solid free-form fabrication,and 3D printing are synonymous,have been applied to the construction of personalized medical implants with complex 3D architectures.Additionally,many researchers have begun to utilize AM technology to fabricate 3D personalized Mg and Mg alloy orthopedic metal implants [28].However,the fabrication of 3D printed Mgbased metal implant needs to face two major challenges:high chemical reactivity and vigorous evaporation.Hence,more researchers are needed to modulate Mg-based powder’s physical and chemical properties for additive manufacturing.
9.Conclusion and future perspectives
Mg is a rich and indispensable metal element in the human body,which takes part in the growth and development of multiple systems in the body.Based on this,as raw material,Mg has been used in the construction of orthopedic medical implants in recent years.As potential bioactive medical devices,Mg and Mg alloy implants process superior biocompatibility and suitable Young’s modulus with the natural cortical bone.Enormous studies demonstrated that the local Mg-rich environment formed by the degradation of Mg and Mg alloy metal implants in the body could effectively stimulate bone formation,increase the adhesion rate of osteoblasts,inhibit the activity of osteoclasts and regulate the signal pathway of osteoblast-related cells.Therefore,Mg and Mg alloys have good application prospects in orthopedic implants in the future.Simultaneously,Mg and Mg alloy implants can be an alternative treatment of bone diseases and an innovative treatment of challenging bone disease.Optimized Mg and Mg alloy medical implants would be successfully developed and fabricated for clinical use by further exploring the biological constraints of Mg and Mg alloys.At present,the molecular mechanism of Mg promoting osteogenesis and how to combine it with the clinical application still needs further research.Simultaneously,further control of the degradation rate and local toxicity of Mg and Mg alloys is the key to the clinical application of Mg-based medical implants.At present,researchers can achieve preliminary regulation of the degradation rate of Mg-based metal implants by improving the purity of Mg metal,alloying,and surface modification Additionally,achieving precise regulation of Mg-based metal degradation according to the healing speed of bone tissue still needs further research.At present,a great many in vitro studies and in vivo animal experiments have confirme the osteoinductive properties,biodegradability,and appropriate mechanical properties of Mg and Mg alloy medical implants.However,the number of clinical studies on Mg and Mg alloys is still limited.The long-term biological safety of Mg-based metal implants in vivo still needs further clinical studies to evaluate.In addition,combining different implant processing and manufacturing technologies,such as additive manufacturing,is also one of the research directions for future clinical applications of Mg and Mg alloy medical implants.
Declaration of Competing Interest
There are no conflict to declare.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31870961,81501879),the Sino-German Center for Research Promotion (GZ1219),the International Cooperation Project of the Science and Technology Department of Sichuan Province (Grant No.2015HH0049,No.2017SZ0127,No.2020YFS0140) and the National Clinical Research Center for Geriatrics,West China Hospital,Sichuan University (Z2018A11).
 Journal of Magnesium and Alloys2022年6期
Journal of Magnesium and Alloys2022年6期
- Journal of Magnesium and Alloys的其它文章
- EDITORIAL BOARD
- Aims and Scope
- Surface oxidation study of molten Mg-Al alloys by oxide/metal/oxide sandwich method
- Production and characterisation of new bioresorbable radiopaque Mg-Zn-Y alloy to improve X-ray visibility of polymeric scaffolds
- Quantitative study on the tension-compression yield asymmetry of a Mg-3Al-1Zn alloy with bimodal texture components
- Microstructure analyses and phase-fiel simulation of partially divorced eutectic solidificatio in hypoeutectic Mg-Al Alloys
