The duration of the conventional chemoembolization for hepatocellular carcinoma: factors affecting the procedural time
Matteo Renzulli, Mattia Gentilini, Giovanni Marasco, Nicolò Brandi, Alessandro Granito, Silvia Lo Monaco, Anna Maria Ierardi, Antonio De Cinque, Francesco Tovoli, Laura Bartalena, Daniele Spinelli,Fabio Piscaglia, Rita Golfieri
1Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy.
2Internal Medicine and Digestive Physiopathology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138,Italy.
3Division of Internal Medicine, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna 40138, Italy.
4Diagnostic and Interventional Radiology Department, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Università degli Studi di Milano Milan, Milan 20122, Italy.
Abstract
Keywords: Chemoembolization, hepatocellular carcinoma, procedural time, angiographic room, management
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) has been constantly increasing over the last decade: in fact, HCC currently represents the fifth most common cancer worldwide[1], with a very low survival rate(2.5%-19.6% survival rate in 5 years)[2-3], ranking as the most common cause of death in cirrhotic patients[4-5]. The treatments associated with a higher 5-year survival rate, such as liver transplantation,ablation, and surgical resection, can only be applied in the very early and early stages of the disease[6],accounting for a mere 20% of cases at presentation[7]. Therefore, the vast majority of subjects are still diagnosed at intermediate and/or advanced tumoral stages, resulting in unsuitable for these treatments. For patients in intermediate to advanced stages, the only therapeutic options available are transarterial chemoembolization (TACE), radioembolization, or systemic therapies[8-12]. Among these, TACE, up to date,remains the most common treatment modality in HCC patients; in fact, about 46.4% of TACE was performed for initial tumors[13].
TACE is the elective therapeutic choice for HCC patients in every stage of the subclassification of the Barcelona Clinic Liver Cancer (BCLC) Intermediate Stage (B), but it can also be applied to unresectable early stage (BCLC A) and, in some cases, advanced-stage patients, often associated with systemic therapy(BCLC C)[6,14-17]. Moreover, the diagnosis of HCC is currently carried out by imaging[18], and, apart from the histopathological and immunohistochemical features, these lesions are directly referred to treatments, such as TACE[6,19]. Despite the guidelines referring to the TACE procedure in generic terms, various chemoembolization regimens currently exist, which differ in many aspects[20]. The two principal TACE techniques are conventional TACE (cTACE) and drug-eluting bead TACE (DEB-TACE)[21]. In cTACE, a drug carried by lipiodol (a poppyseed oil-based solution) is administered as an arterial injection followed by arterial embolization[22], while DEB-TACE uses drug-eluting beads (DEBs) that were developed to slowly release chemotherapeutic agents and increase intensity and duration of the ischemia[14]. However, the superiority of DEB-TACE to cTACE has not been proven, and the two regimens seem to have the same outcomes in terms of patient response, survival, and safety[14]. Furthermore, cTACE has proven itself to be slightly less expensive overall[23-24]. Moreover, although DEB-TACE was developed later as an attempt to drastically improve cTACE, the vast majority of hospitals still perform cTACE[25].
The recent outbreak of the novel coronavirus (SARS-CoV-19) pandemic has had a strong impact on health systems worldwide, as it has not been seen since the last world war. In this scenario, the radiology practice model has also been heavily impacted. In March 2020 alone, most practices in the United States saw an unprecedented drop in their volume of greater than 50%[26], with similar trends recorded all over the world[27]. Even in countries with a relatively low hospitalization rate due to the novel coronavirus, major modifications had to occur in the management of the patients to reduce the risk of spreading the disease,especially among those patients who were the most fragile, such as oncological patients[28]. Due to the SARSCoV-19 pandemic, the timing aspect has now become more crucial than ever, because it has shown us the importance of time planning and scheduling to keep performing procedures and treatments at the best efficiency rate possible while still maintaining the patient’s safety with the proper distancing, as imposed by the pandemic[28].
The lack of such planning during the pandemic has brought catastrophic scenarios, such as the ones recently reported in the United Kingdom, where in certain cases, patients who had to undergo “priority 2”surgery (such as cancers and urgent cardiac surgery, which are deemed medically necessary to be done within 28 days) have had their surgery canceled[29].
A better planning schedule based on a more accurate estimate of the time duration of any medical act could have significant benefits for the workflow managers (improving productivity and reducing downtime), for the hospital directors, and subsequently for healthcare workers and patients.
Unfortunately, despite the well-established use of cTACE, the mean time of a standard cTACE and which parameters affect the duration of this procedure are still unknown.
The aim of the present study was to evaluate the time duration of cTACE in a large series of patients who underwent this procedure, analyzing possible factors affecting the procedural time.
METHODS
Patients and cTACE procedure
Patients consecutively referred to our radiology unit from 1 January to 31 December 2019 to undergo cTACE for HCC were prospectively enrolled. cTACE was performed according to the EASL-EORTC guidelines[6]. Cirrhosis is defined as the histological development of regenerative nodules surrounded by fibrotic tissue and is considered as the final evolution stage of any progressive liver disease[30-31]. All cases that did not fit the EASL-EORTC guidelines were discussed by the dedicated multidisciplinary tumor board.All angiographic procedures were performed by a team of four interventional radiologists with more than fifteen years of experience in cTACE. The cTACE procedures were performed as described in detail in previous papers and used Farmorubicin (Pfizer, Latina, Italy) as a chemotherapeutic agent[20,32]. In particular,a selective cTACE procedure was the preferred modality whenever it was technically feasible, placing a highly flexible coaxial microcatheter (2.7-2.8 Fr Terumo Progreat microcatheter or a Boston Scientific Renegade HI-FLO microcatheter) into the hepatic arterial branch afferent to the segment, in which the tumor was located and passed through a 4 Fr catheter previously placed approximately in the hepatic artery itself.
The local institutional review board approved this prospective study (protocol code 098/2014/U/Oss), and written informed consent was obtained from all the patients. This study was conducted according to the Declaration of Helsinki for clinical studies. Diagnostic tests and therapeutic procedures were priced according to the Italian National Health Service (INHS).
Time and other Parameters of cTACE procedure
The time per patient spent in the angiographic room for performing cTACE can be divided into three time intervals: the first (pre-procedural) and the last (post-procedural) ones usually last a fixed (standard)amount of time. In fact, pre-procedural time consists of the time when the patient is carried into the angiographic room, positioned onto the bed, and peripheral venous access is positioned and connected to all the devices, which will allow the operators to control the patient’s vital parameters. Subsequently, the sterile field is prepared, as well as all the devices needed for the procedure. This time, always the same for all cTACE procedures, was estimated as a mean of 15 min. Post-procedural time consists of the closure of the arterial access until the patient is carried outside, with subsequent disinfection of the angiographic room.This time, again, the same for all cTACE procedures, was estimated as a mean of 30 min. On the contrary,the central period of the occupation time of the angiographic room by cTACE is determined only by the duration of the chemoembolization itself. The overall procedural time of cTACE was the time between the insertion and the removal of the angiographic sheath[33].
The following features related to the tumor burden and angiographic procedures, which could be related to the procedural time, were recorded on a dedicated database: initial time (insertion of the angiographic sheath) and ending time (removal of the angiographic sheath), after which the procedural time was automatically calculated subtracting initial from ending time; the number of nodules treated per patient; the number of nodules per segment; the number of nodules in the same segment; lobe affected by HCC (right,left, or right plus left); nodule size (< 3 cm, 3 cm ≤ nodule < 5 cm, or ≥ 5 cm); Milan Criteria (within or out of); and the number of segments treated.
Statistical analysis
All recorded data were reported in an electronic database. For the main purpose of the study, all demographics, HCC-related features, and cTACE parameters were reported with descriptive statistics.Continuous variables were reported as medians with interquartile range (IQR) or mean and standard deviation (SD), while categorical variables as numbers and percentages. Since the mean cTACE duration was 58.1 min, we a priori decided to dichotomize our data rounding up a cTACE duration of 60 min in order to obtain a definite procedural time useful for clinical and managerial purposes. Student's t-test,Mann-Whitney, Fischer test, and Chi-square were used for comparisons between groups when appropriate.Subsequently, factors associated with cTACE duration ≤ 60 or > 60 min were explored using univariate analysis. The estimated odds ratios (OR) with their 95% confidence intervals (CI) were calculated. If more than one variable resulted associated with procedural cTACE duration at univariate analysis, to confirm the independent predictive value of these variables, those withP< 0.1 were inserted in a multivariate logistic model. Associations with aP-value < 0.05 were considered statistically significant. The statistical analysis was performed using STATA13 (Stata Corp., College Station, TX, USA).
RESULTS
The final study population was composed of 175 patients who underwent cTACE during the study period.Their demographic characteristics are detailed in Table 1. The median age of the enrolled subjects was 68 years (IQR 60.2-77.4 years), 120 patients (68.5%) were males, and 146 (83.4%) were in Child-Pugh class A.All included patients with alcohol abuse (11%) were also affected by chronic hepatitis C virus (HCV)infection; 33 patients (18.9%) were regularly drinking alcoholic beverages but did not reach the criteria for alcoholism.
The procedural time required to perform cTACE resulted in a mean of 58.1 min (SD 21.6 min; range 21-150 min). The procedural data and the tumor features of the study population are detailed in Table 2. cTACE time according to the tumor and procedural characteristics is reported in Table 3.
No difference in procedural time was reported among operators (P= 0.921); in particular, the mean procedural time for Operator 1 was 63.1 min (SD 23.4), 57.8 min (SD 18.8) for Operator 2, 56.8 (SD 23.5)for Operator 3, and 58.9 (SD 24.9) for Operator 4 [Figure 1].

Table 1. Demographical characteristics and tumor burden of the study population at baseline
Short and long cTACE duration
As mentioned above, the study population was divided into two groups {Group 1, procedural time ≤ 60 min[Figure 2]; Group 2, procedural time > 60 min [Figure 3]}. The tumor features and procedural data of these two groups are reported in Table 4. Significative differences were found between the two groups; in particular, longer procedural time was associated with a number of nodules treated per patient ≥ 2(P< 0.001), a progressive increment as the number of nodules increases, the number of segments with nodules ≥ 2 (P< 0.001), a progressive increment as the number of segment involved increases, the presence of more than 1 nodule in the same segment (P< 0.001), the location of the tumor in the left lobe (P= 0.001),the exclusion from the Milan criteria (P< 0.001), the number of segments treated ≥ 2 (P< 0.001), and a progressive increment as the number of segments increases.
Factors associated with longer cTACE duration
The influence that every single tumor and procedural parameter has had on the cTACE procedure’s duration according to the univariate and multivariate logistic regression analysis is shown in Table 5. In particular, the increment of cTACE’s time was significantly correlated to the progressive increase of the number of nodules treated per patient (OR 2.927, 95%CI: 2.015-4.251,P< 0.001), the progressive increase of the number of segments with nodules (OR 2.388, 95%CI: 1.651-3.452,P< 0.001), and the progressive increase of the number of segments treated (OR 2.365, 95%CI: 1.704-3.283,P< 0.001). Moreover, the presence of multiple nodules in the same segment (OR 5.737, 95%CI: 2.567-12.823,P< 0.001), the exclusion from the Milan criteria (OR 6.406, 95%CI: 3.822-18.101,P< 0.001), and the number of segments treated(OR 1.860, 95%CI: 1.416-2.445,P< 0.001) were also significantly correlated with longer procedural time.Furthermore, both the nodule size and its location in the left lobe did not significantly influence the procedure’s duration. The number of nodules treated per patient was also significantly associated with a longer procedural time in multivariate analysis (OR 2.927, 95%CI: 2.015-4.251,P< 0.001).
DISCUSSION
cTACE, up to date, remains the most common modality used in the treatment of HCC for initial tumors[13,34], despite many novelties in all types of treatments for this cancer, such as surgical, locoregional,systemic, or combined ones[35]. However, the duration time of the TACE procedure is not known, which is important for correct planning of the workflow of an angiographic room, especially during the pandemicera, to ensure patient safety. In the literature, cTACE’s duration has only been reported once as a secondary finding in one non-targeted study[31]. Furthermore, in the absence of dedicated studies, the factors affecting the procedural time of cTACE procedures remain unknown. The main aim of the present study was to determine the mean time of cTACE in a large series of patients, highlighting the possible factors affecting the procedural time.
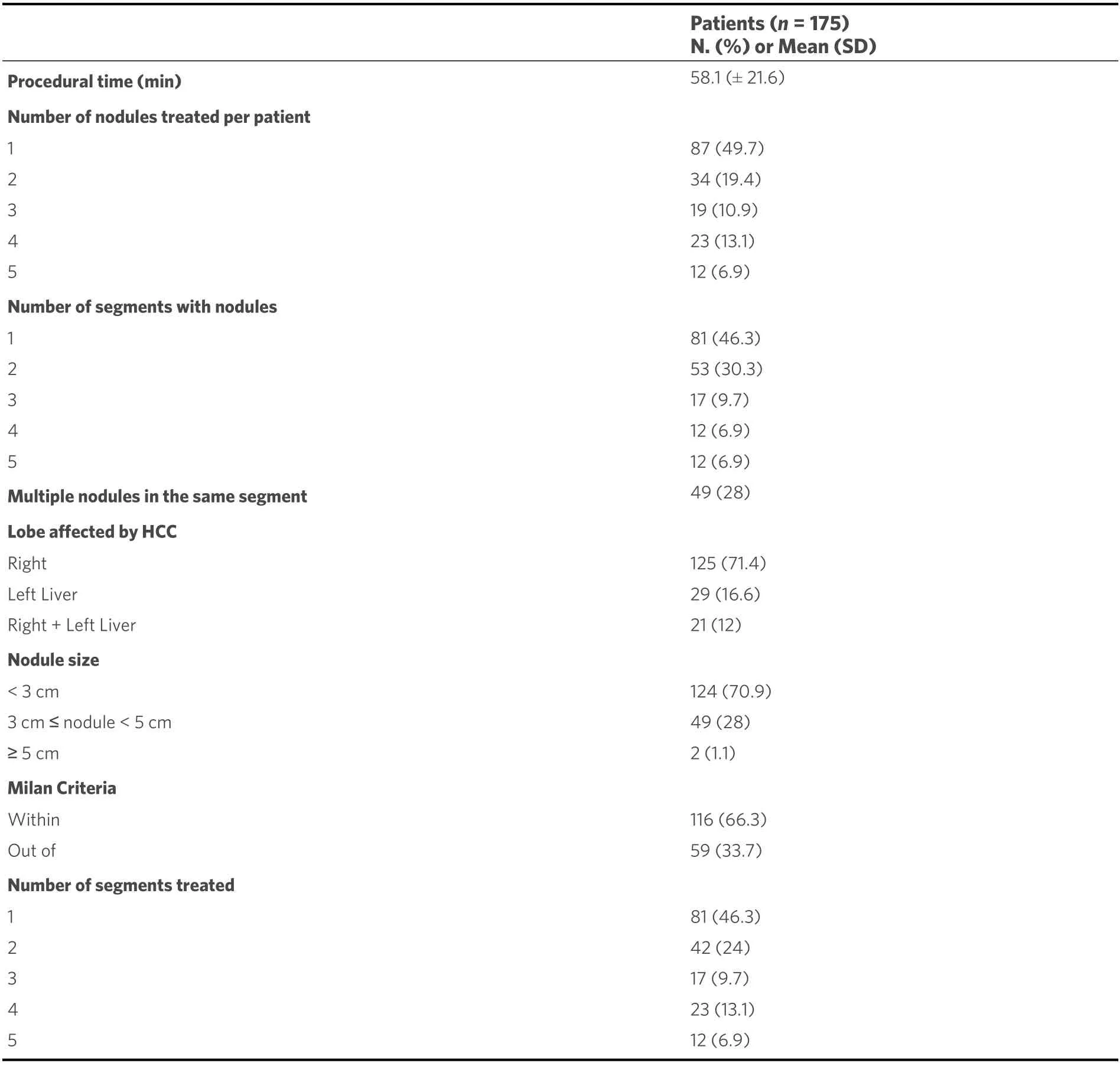
Table 2. Procedural data and tumor features of the study population
This study reported the procedural time of 175 cTACE, which is approximately the yearly number of consecutive cTACE performed in the interventional radiology unit of our tertiary center for liver diseases during 2019. We analyzed consecutive patients to overcome any bias in the evaluation of procedural time of cTACE. Furthermore, fortunately, the year evaluated (2019) was pre-pandemic, and therefore it was possible to perform the analysis on a larger number of patients as compared to the (pandemic) year 2020.
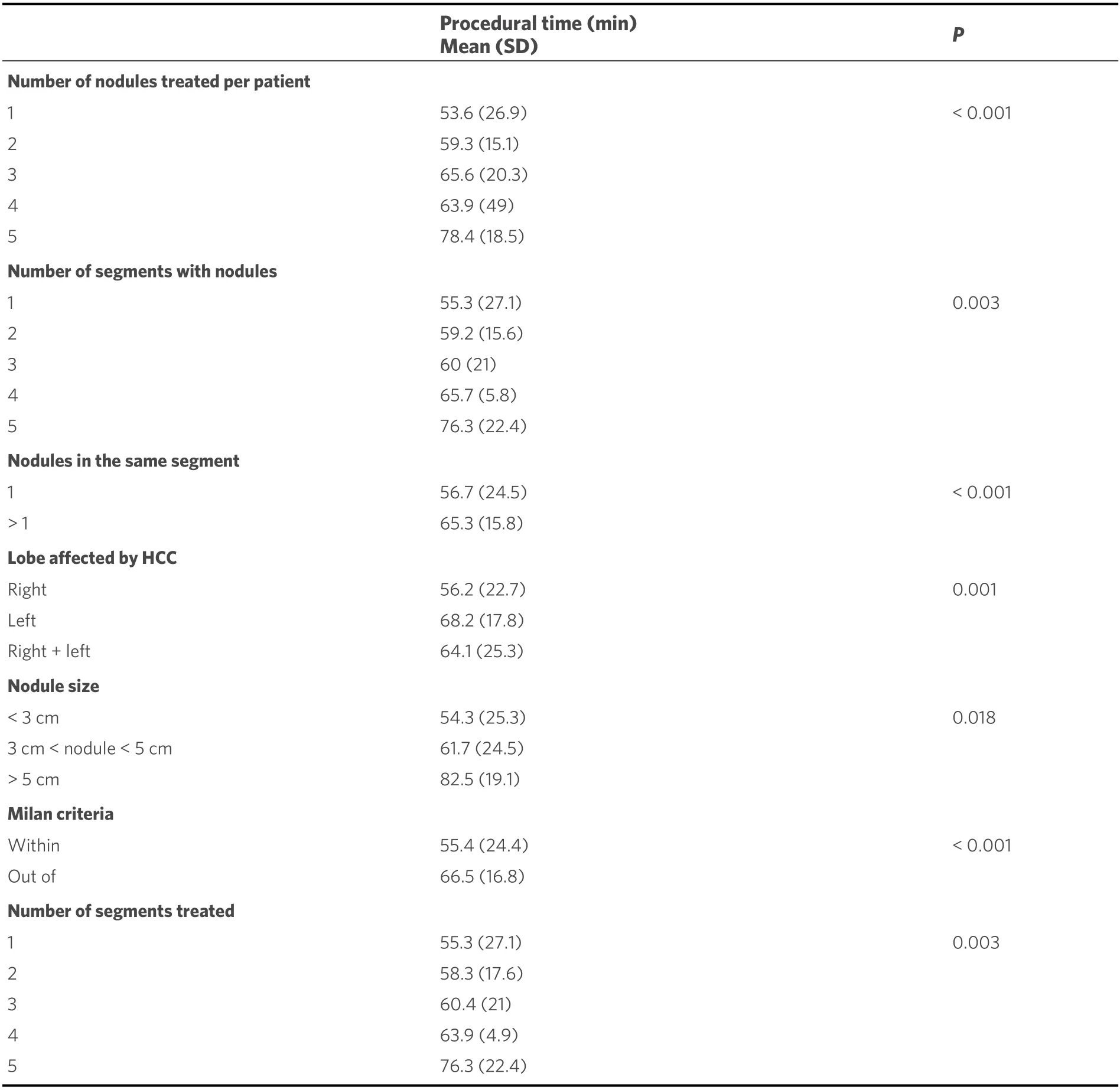
Table 3. Chemoembolization time according to tumors and procedural characteristics
The patients in the present series who underwent cTACE had a median age of 68 years and about 84% of cases were Child-Pugh A. These patient characteristics are in line with other published series[32,36]. Other studies, such as that of Chenet al.[37], reported a lower mean age (58 years) in patients all in Child-Pugh A:in our opinion, these data, analyzed in terms of the procedural time, probably do not imply an increase of the duration time of TACE due to the overall clinical conditions, which was better in the series of Chenet al.[37]as compared with our one.
The procedural time required to perform cTACE resulted in a mean of 58.1 min, according to our results. It is difficult to discuss these data because there are no studies focused on this topic. To the best of our knowledge, the unique paper reporting the procedural time of cTACE is that of Iwazawaet al.[33], with the limit of the study design not dedicated to the evaluation of the procedural time and the clinical or tumoraldata affecting it. In their study[33], they performed cTACE with and without software assistance: to perform a comparison with our results, it is necessary to evaluate only the group of cTACE carried out without software assistance because cTACE procedured in our series were performed without software assistance.The overall procedural time for single chemoembolization in the group of cTACE without software assistance reported by Iwazawaet al.[33]was a mean of 116 min (standard deviation of 31 min; range 58-228 min). In our opinion, the explanation for this time longer than ours is because in the group of cTACE carried out without software assistance, they[31]performed not only angiograms but also C-arm CT images obtained from the suspected feeder artery to confirm whether the target tumor was located within the treatment area. These C-arm CT images (that we did not achieve) need additional time to be obtained, thus justifying the difference with our series.
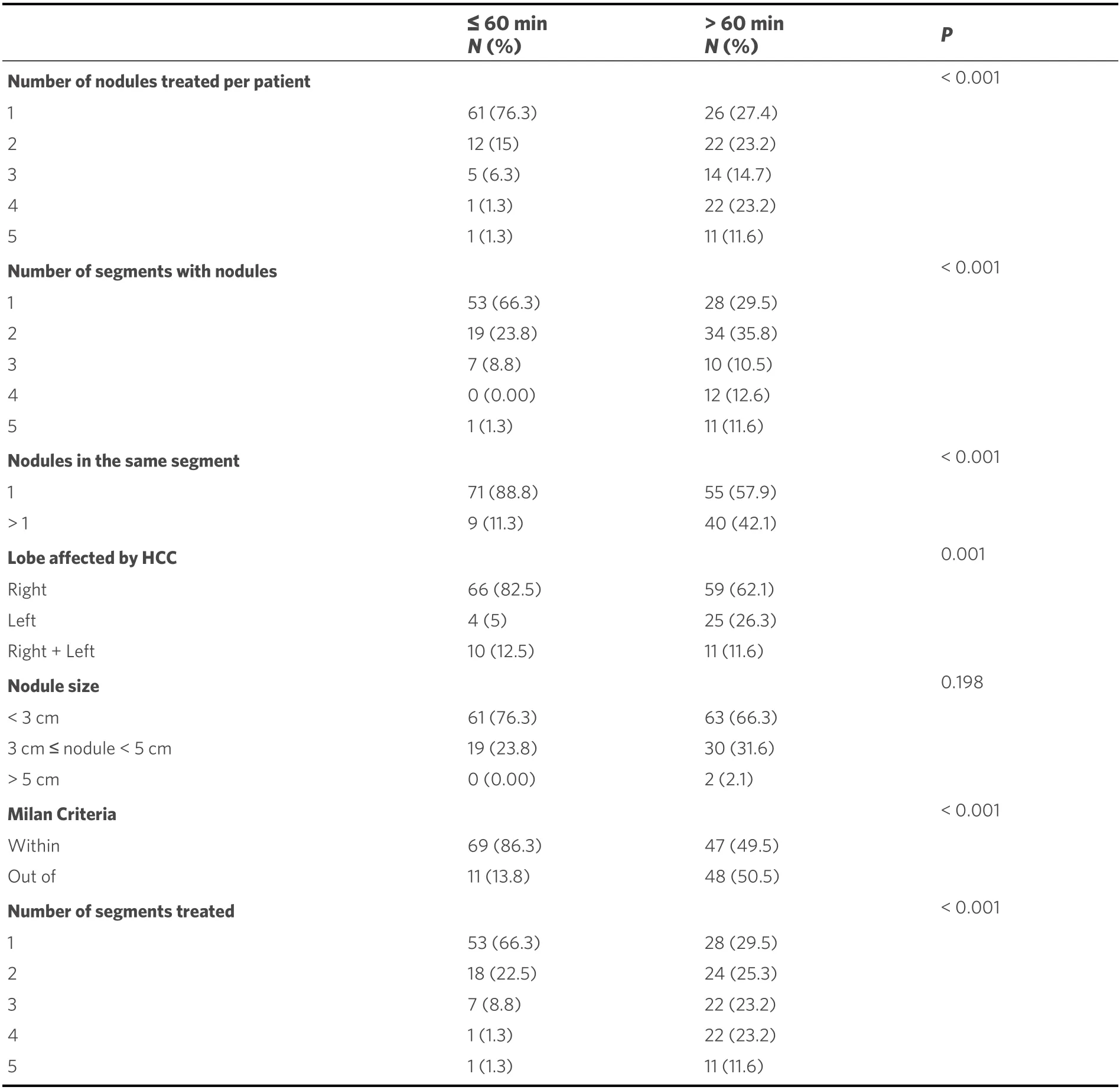
Table 4. Tumor and procedural characteristics of the study population according to the chemoembolization time cutoff
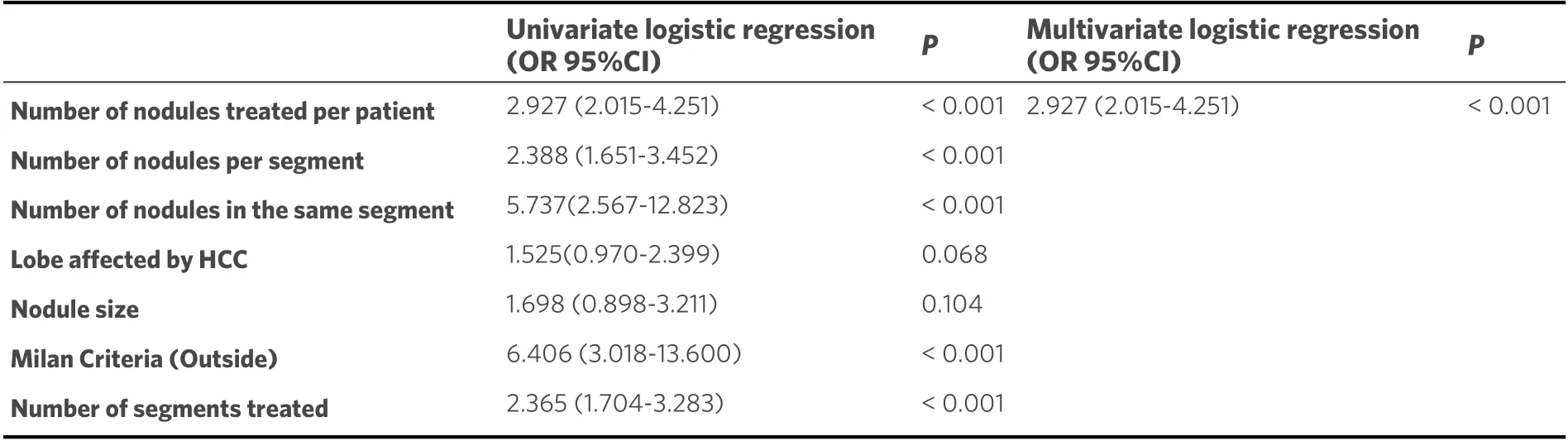
Table 5. Univariate and multivariate logistic regression analyses for the assessment of tumor and procedural factors associated with the chemoembolization time (> 60 min)
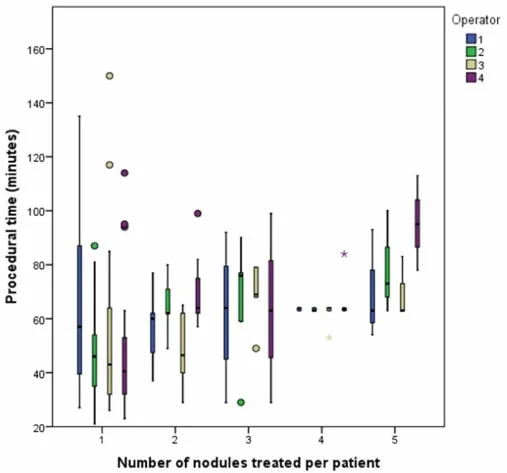
Figure 1. Procedural time according to the number of nodules treated per patient and operator.
Starting with our result of a mean procedural time of 58.1 min, a procedural cut-off time of 60 min was set,identified for its usability from a managerial point of view. In fact, this time can be rounded to 1 h, which represents an easy duration to use as a work planning tool in an angiography room. The parameters that seemed to determine if a cTACE has a duration longer than 60 min are the number of nodules treated ≥ 2,the number of segments with nodules ≥ 2, the involvement of the left lobe of the liver, the tumor burden outside the Milan criteria for liver transplantation, and the number of segments treated ≥ 2. In the absence of studies designed to analyze the features related to the duration of a cTACE, it is difficult to compare the results of the present study with others.
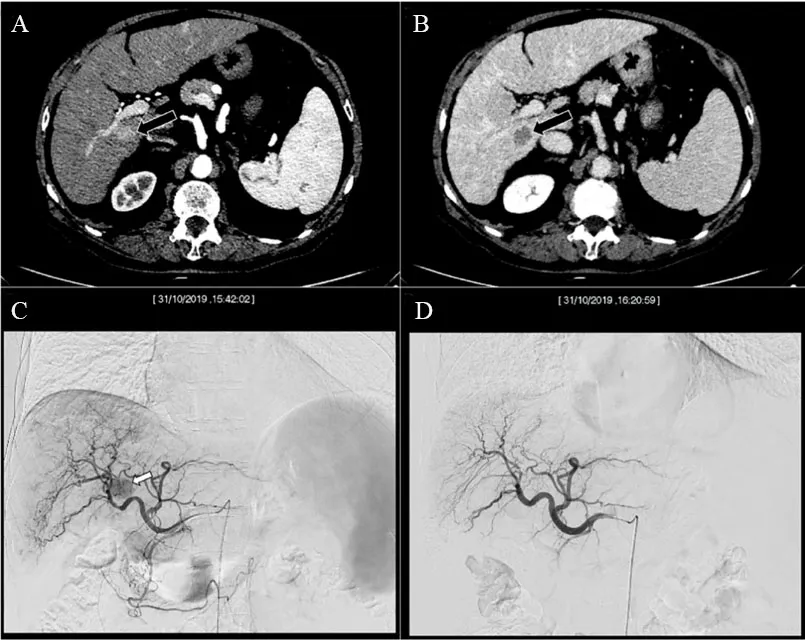
Figure 2. Computed tomography of a cirrhotic patient demonstrated a single nodule of 1.8 cm located in the liver segment VI, with typical features of hepatocellular carcinoma according to the EASL guideline: hyperenhancement in the arterial phase (A) coupled with wash-out of contrast media in the delayed phase (B). The lesion, due to its deep location, in close proximity to inferior vena cava and almost adhering to the right portal branch, was scheduled for cTACE. The angiographic study in the parenchymal phase showed the hypervascular nodule [arrow in (C)]. The image in (D) proved the end of cTACE, with occlusion of the tumor feeding vessels and the patency of the remaining hepatic arteries. The images in (C, D) are Dicom images, reporting in the top portion, the date and time at which those images were taken. It is possible to note that the procedural time, in this case, was 38 min.
It is very interesting how the tumor involvement of the right lobe determined the duration of the cTACE less than 60 min when compared with the involvement of only the left lobe of the liver. It is conceivable that the angulation of the hepatic arterial branches of the right lobe (> 90° with respect to the common hepatic artery) facilitates their catheterization with respect to the left hepatic branches (usually showing an angulation < 90° with respect to the common hepatic artery), thus reducing the overall time of the procedure. Notably, the right location of tumors is the most frequent condition in our series, affecting more than two-thirds of the cases. Obviously, the duration of a cTACE procedure is longer when the disease is bilobar, i.e., HCC located on both the right and left liver lobes, because the tumor burden is greater and, in addition to the catheterization of the right arterial branches, the more “complex” catheterization of the left arterial branches is also needed.
Other parameters determining the duration of cTACE less than 1 h are the number of segments treated ≤ 1,the number of nodules treated ≤ 1, and, especially, the number of segments with nodules ≤ 1, which was significantly correlated with longer cTACE time even in multivariate analysis. It is reasonable to assume that the more lesions there are and the more these are spread to different liver segments, the more the number of arteries that need to be catheterized to treat tumors, leading to an increase in the procedural time. In support of this theory, the inclusion into the Milan criteria for liver transplantation has also been associated with a shorter procedural time. It is very important that all these tumor parameters (number of nodules,location, number of segments involved and treated, and tumor within the Milan criteria) are known before the cTACE takes place as well as at the time the cTACE procedure is requested. Therefore, knowing these data, the workflow of the angiographic room can be scheduled with much better accuracy, enhancing patient safety and improving the managerial organization of the angiographic room (reducing downtime).Moreover, another interesting secondary goal of our results is to improve the early diagnosis of HCC with different strategies[38-40]; in the future, progressively less extended lesions will be treated, and therefore the duration of treatments such as cTACE would be further reduced, decreasing the risks of radiation exposure to which both patients and interventional radiologists are exposed[41].

Figure 3. Computed tomography of a cirrhotic patient demonstrated a single nodule of 5.2 cm located in the liver segments VII-VIII,with typical features of hepatocellular carcinoma according to the EASL guideline: hyperenhancement in the arterial phase (A) coupled with wash-out of contrast media in the delayed phase (B). The patient was scheduled for cTACE according to his clinical (advanced fibrosis stages F3-F4 and previous variceal endoscopic ligation treatment) and tumoral characteristics (location in upper segments).The angiographic study in the parenchymal phase showed the hypervascular nodule (C). The image in (D) proved the end of cTACE,with occlusion of the tumor feeding vessels and the patency of the remaining hepatic arteries. The images in (C, D) are Dicom images,reporting in the top portion, the date and time at which those images were taken. It is possible to note that the procedural time, in this case, was 105 min.
Another interesting result that emerged from our series is that the dimension of nodules did not affect the procedure’s duration. Probably this result can be explained by the fact that the catheterization of the artery that supplies the lesion is what actually slowed down the procedure; in fact, the increase in the size of the nodule does not entail particular changes in the time required for the effective administration of the chemoembolizing agent. However, only two patients had a nodule with a diameter ≥ 5 cm in the present series, and the mean duration of cTACE in these patients was longer (82.5 min) compared with the mean procedural time of all other patients, which was reported below the cut-off value of 60 min (54.3 min for nodules < 3 cm and 61.7 min for nodules with a diameter ≥ 3 but < 5 cm). Therefore, further studies are needed to verify if nodule size is not actually related to a significant difference in cTACE duration, perhaps with a larger number of patients having nodules > 5 cm.
This study suffers from many limitations. First, the study population was characterized by single nodules< 3 cm in more than two-thirds of cases. However, this is a prospective study with the consecutive enrollment of patients who underwent cTACE in a tertiary center for liver tumors according to the EASL guideline[6]. In our center, thanks to the precise adherence to the surveillance program of patients at high risk for the development of HCC, tumors are detected in the very early and early stages, reflecting a good clinical practice as suggested by the dedicated guidelines. Moreover, cTACE was the only feasible treatment for many of the patients included in the present study, since their clinical characteristics contraindicated the use of other therapeutic alternatives. Our center, like many others, recognizes the current limitations of the EASL practice guidelines for the management of HCC; therefore, each nodule that fails to fulfill all the technical indications for ablation (according to the EASL guidelines) was discussed in our multidisciplinary tumor board and, in the absence of surgical indications, directed towards TACE procedure. Another limitation was the use of a technique not supported by the new software assistance. However, up to date,this, in our opinion, is not a limit but a richness because it reflects the standard of care used in the majority of centers dedicated to this topic.
This study demonstrated that cTACE had a procedural time of about 1 h. Considering that the time before artery puncture and the time after removal of the angiographic sheath are standard times, usually the same for each hospital environment, this study provides the mean time of the variable part of a cTACE procedure. Furthermore, our study identified the factors correlated to a longer procedural time, such as the number of nodules treated ≥ 2, the number of segments with nodules ≥ 2, the involvement of the left lobe of the liver, the tumor burden outside the Milan criteria for liver transplantation, and the number of segments treated ≥ 2. All these characteristics, known in the pre-procedural phase when cTACE is requested,represent useful tools for the correct planning of the angiographic room’s workflow during the pandemic era as well as in the future to reduce downtime and increase productivity.
DECLARATIONS
Authors’ contributions
Conception: Renzulli M, Piscaglia F, Golfieri R
Design: Renzulli M, Marasco G, Granito A
Supervision: Renzulli M, Piscaglia F, Golfieri R
Fundings: Renzulli M, Piscaglia F, Golfieri R
Materials: Renzulli M, Gentilini M, Marasco G, Tovoli F, Granito A
Data Collection and/or Processing: Gentilini M, Brandi N, Lo Monaco S, Ierardi AM, De Cinque A,Bartalena L, Spinelli D
Analysis and/or Interpretation: Renzulli M, Marasco G, Granito A
Literature Review: Renzulli M, Brandi N
Writing: Renzulli M, Brandi N
Critical Review: Renzulli M, Piscaglia F, Golfieri R
Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of IRCCS Azienda Ospedaliero-Universitaria di Bologna (protocol code 098/2014/U/Oss). Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
- Hepatoma Research的其它文章
- The role of minimally invasive surgery in the treatment of HCC
- A focused review on recent advances in diagnosis and management of fibrolamellar hepatocellular carcinoma
- The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis
- Minimally invasive surgery for HCC
- Liver tumors in childre n

