A focused review on recent advances in diagnosis and management of fibrolamellar hepatocellular carcinoma
Mahmoud Aryan, Nicholas Forrister, Nishah Panchani, Bijal Vashi, Zahara Chowdhury, Haider A.Mejbel, Mohamed Shoreibah
1Tinsley Harrison Internal Medicine Residency, Department of Medicine, University of Alabama at Birmingham, 1720 2nd Avenue South, BDB 327, Birmingham, AL 35294, USA.
2University of Alabama at Birmingham School of Medicine, Department of Medicine, 1720 2nd Ave S., Birmingham, AL 35294,USA.
3University of Mississippi Medical Center School of Medicine, Department of Medicine, 2500 N State St., Jackson, MS 39213,USA.
4Division of Surgical and Molecular Genetics Pathology, Department of Pathology, University of Alabama at Birmingham, 619 19th St S, Birmingham, AL 35233, USA.
5Division of Gastroenterology and Hepatology, Department of Medicine, University of Alabama at Birmingham, 1808 7th Avenue South, BDB 391, Birmingham, AL 35294, USA.
Abstract Fibrolamellar hepatocellular carcinoma (FHCC) is a rare primary malignancy of the liver for which data remain limited. This tumor is more often diagnosed in younger patient populations in the absence of underlying cirrhosis and hepatitis. These lesions can be diagnosed on computed tomography scan or magnetic resonance imaging with common findings including central calcifications, a central stellate scar, and radiating fibrotic bands. Laboratory markers have not proved useful for diagnosis; however, pathologic analysis can be implemented to aid in diagnosis with findings including ample granular eosinophilic cytoplasm, nuclei with open chromatin and prominent macronuclei, hyaline and pale bodies, and dense lamellar fibrosis that divides the cells into cords or trabeculae.FHCC demonstrates aggressive malignant potential with nodal spread. Treatment patterns have remained mainly surgical; however, systemic therapies have been implemented and are under further investigation with clinical trials. Locoregional therapies and radiation therapies have been trialed sparingly. In this focused review, we discuss the most up-to-date perspective on epidemiology, clinical presentation, diagnostic approach, differential diagnosis,treatment regimens, prognosis, and future directions of FHCC.
Keywords: Fibrolamellar hepatocellular carcinoma, diagnosis, treatment, surgery, systemic, histology, review
INTRODUCTION
Fibrolamellar hepatocellular carcinoma (FHCC) is a rare primary malignancy of the liver that was first identified by Dr. Hugh Edmondson in 1956[1]. This malignancy was initially discovered primarily in the pediatric and younger adult population[2]. The lesion itself was not termed FHCC until the 1980s when Craiget al.performed a case series on 23 patients who, at that time, were thought to have a unique variant of hepatocellular carcinoma (HCC)[3]. Further research since that time has now categorized FHCC as a separate primary malignancy rather than a specific variant of HCC. In 2010, FHCC was identified as a standalone clinical entity by the World Health Organization (WHO)[4].
Despite the increased focus on FHCC over the past decade, much remains unknown about this tumor.Often presenting in those without cirrhosis or evidence of hepatitis, the underlying origin of FHCC is still under question[5]. This tumor also has unique histologic and radiographic findings that distinguish it from HCC. Diagnostic approaches can vary based on clinical presentation, with management also impinging upon the overall tumor burden. This focused review of literature provides an up-to-date assessment of the epidemiology, clinical presentation, diagnosis, management, and future direction of FHCC.
Epidemiology
In studies looking at data from the Surveillance, Epidemiology, and End Results (SEER) registry, FHCC contributed to about 0.4%-5.0% of all liver cancers of primary origin[6-8]. The age-adjusted incidence rate has been described to be 0.02 per 100,000 between the years 2000 and 2016 for patients with FHCC. Primarily a disease of the young, most cases of FHCC occurs in individuals between the age of 15-40 years and a smaller peak around 70 years of age[9]. Males (0.03 per 100,000) are more likely to have FHCC than females (0.02 per 100,000). However, Caucasians have a higher frequency of reported cases when compared to other races(78%)[9]. Interestingly, despite HCC where high rates are seen in those of Asian descent, the prevalence of FHCC has been reportedly higher in Europe and North America than in Asian countries[10,11]. Cases have been investigated in Japan, China, and Korea[12-14].
Other studies have performed the analysis of patients with FHCC using the National Cancer Database.Findings are similar to studies that utilized SEER, with most patients diagnosed under the age of 40 (60.7%),and males being the predominant gender to be affected (56%) compared to females. Using the Charlson-Deyo Comorbidity Score, 77.2% of patients in the study had a score of 0, indicating that these patients were predominantly healthy with non-cirrhotic livers[15]. Smaller-scale studies have shown no predilection for sex[16,17].
Clinical presentation
Patients with FHCC present with signs and symptoms that can be vague and nonspecific, but mainly include abdominal distension, nonspecific pain, nausea, weight loss, and fatigue. These symptoms are likely attributed to the growth of the hepatic mass[18]. Physical exam can be significant for hepatomegaly[19]. A few case studies have reported presentations of hyperammonemia encephalopathy and gynecomastia as well[19,20]. Tumor sizes are typically larger than HCC, with reported tumor mass sizes in the 5cm-10cm range[8,21,22]. In addition, multiple studies have reported a significantly higher frequency of metastases and stage IV disease (primarily to lymph nodes) with FHCC compared to HCC[15,16,23]. Patients usually do not have a history of liver dysfunction or inflammation. There is also no documented association with alcohol use[18]. Although isolated reports have described hepatitis B viral proteins or DNA within FHCC, no significant association has yet to be identified[24-26].
INVESTIGATION
Different tests and modalities can be implemented to diagnose FHCC. A variety of laboratory tests, imaging studies, histopathologic analysis, and molecular data have been used to aid in diagnosis.
Laboratory studies
Generally, patients with FHCC lack derangements in aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) levels, but these values can seldom be elevated[2,27-29].ALP elevation usually indicates the extension of the tumor into the biliary tree[29]. Unlike HCC, alphafetoprotein (AFP) levels are rarely elevated. In a review of 41 patients by Stipaet al., only 7% of patients with FHCC had levels of AFP > 200 ng/mL[30]. Several case reports have been cited suggesting other potential biomarkers of FHCC, such as transcobalamin I[2,27,31]. These markers’ diagnostic accuracy and clinical utility have yet to be elucidated from large studies[27].
Imaging
Ultrasound (US) can identify well-defined hepatic masses with differing echogenicity[28]. Typically, crosssectional imaging is required for more specific characterization. Recent studies have shown that contrastenhanced US may have improved the ability to identify FHCC and differentiate it from other liver masses[32,33].
Computed Tomography (CT) scans using intravenous (IV) contrast to conduct multiphase imaging of the liver is of great utility in the investigation of possible FHCC. Typically, the FHCC lesions are large. One case series noted an average of 13 cm in diameter[34]. The tumor is well-defined, heterogenous, and lobulated[27,28,35]. Central calcifications are frequently reported between 44%-68% of fibrolamellar carcinomas[Figure 1]. Central stellate scar is common as well and a feature of 65%-70% of FHCCs[28,35]. This scar with radiating fibrotic bands, in addition to other central calcifications, can be useful distinguishers of FHCC[28]from other hepatic masses. Across several series of different cases, there is a general trend amongst contrast enhancement patterns. Pre-contrast the majority are hypoattenuating. During the arterial phase, most lesions are hyperattenuating. The portal phase is variable, with the majority being isoattenuating (50% and 48% by Ganeshanet al.[28]and Ichikawaet al.[34]respectively); however, some remained hyperattenuating,while other lesions become hypoattenuating. Different reports have shown all 3 possibilities in the delayed phase. The central scar was noted often to be hypoattenuating during the pre-contrast, arterial, and portal phases but seldom showed any enhancement during the delayed phase (12% of cases per Ganeshanet al.[35]).
On magnetic resonance imaging (MRI), FHCC presents as hypointense lesions in T1 and hyperintense lesions in T2[2,28]. The central scar provides differentiation from focal nodular hyperplasia (FNH), as it is hypointense in both T1 and T2 with FHCC, whereas in FNH, it is hyperintense in T2. FHCC follows a similar contrast uptake pattern in MRI as it does in CT. Figure 2 depicts an FHCC lesion on MRI.
Nuclear medicine tests are not as well studied. One review based on several patient cases found that fluorodeoxyglucose (FDG)-positron emission tomography (PET) could potentially be used as FHCC demonstrates uptake of FDG in a large percentage of patients[2]. Another review postulates that FDG PET can be used in staging at various times in the disease process[2,28].

Figure 1. Central calcifications seen on CT with contrast.

Figure 2. FHCC measuring up to 7.3 x 6.2 cm on MRI abdomen.
Histopathology
Upon light microscopic examination, the neoplastic cells are relatively monomorphic yet quite large. Cells of FHCC appear 3x larger than normal hepatocytes and are even 1.6x larger than HCC cells[2,35,36]. The neoplastic cells have ample granular eosinophilic cytoplasm, with an accumulation of Mallory bodies, and show nuclei with open chromatin and prominent macronuclei [Figure 3]. Hyaline and pale bodies are also commonly present in FHCC[2,37]. Dense lamellar fibrosis that divides the neoplastic cells into cords or trabeculae is a characteristic histologic feature of FHCC [Figure 4][2]. This often results in a scar-like appearance on gross examination [Figure 5]. As with clinical presentation, cirrhotic morphology of the tissue is absent. Rarely FHCC can demonstrate a pseudoglandular pattern bearing similarities to some cholangiocarcinomas[29].
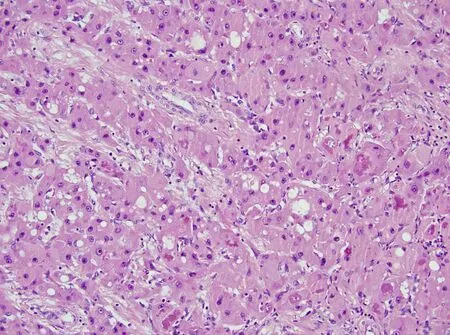
Figure 3. Neoplastic cells with granular eosinophilic cytoplasm and accumulation of Mallory bodies that show nuclei with open chromatin and prominent macronuclei (hematoxylin and eosin, original magnification 40x).

Figure 4. Bands of Lamellar fibrosis divide the neoplastic cells into trabeculae. The tumor cells exhibit patchy macrovesicular fat(hematoxylin and eosin, original magnification 20x).
Immunohistochemistry
FHCC has several immunohistochemical patterns which have been described. Hep Par1 is a marker of hepatic neoplasia and is positive in FHCC and HCC[29]. Other cell differentiation markers are often positive such as CD68, which has a known sensitivity of 96%, and CK7 which is 100% sensitive for diagnosing FHCC; however, they can also be positive in HCC and intrahepatic cholangiocarcinoma[2,27,29]. Combined negative cytokeratin 7 and CD68 helps nearly invalidate the diagnosis of FHCC[38]. Similar to serologic studies, almost all FHCC lesions are negative with AFP stain[2,38].
Molecular diagnostics
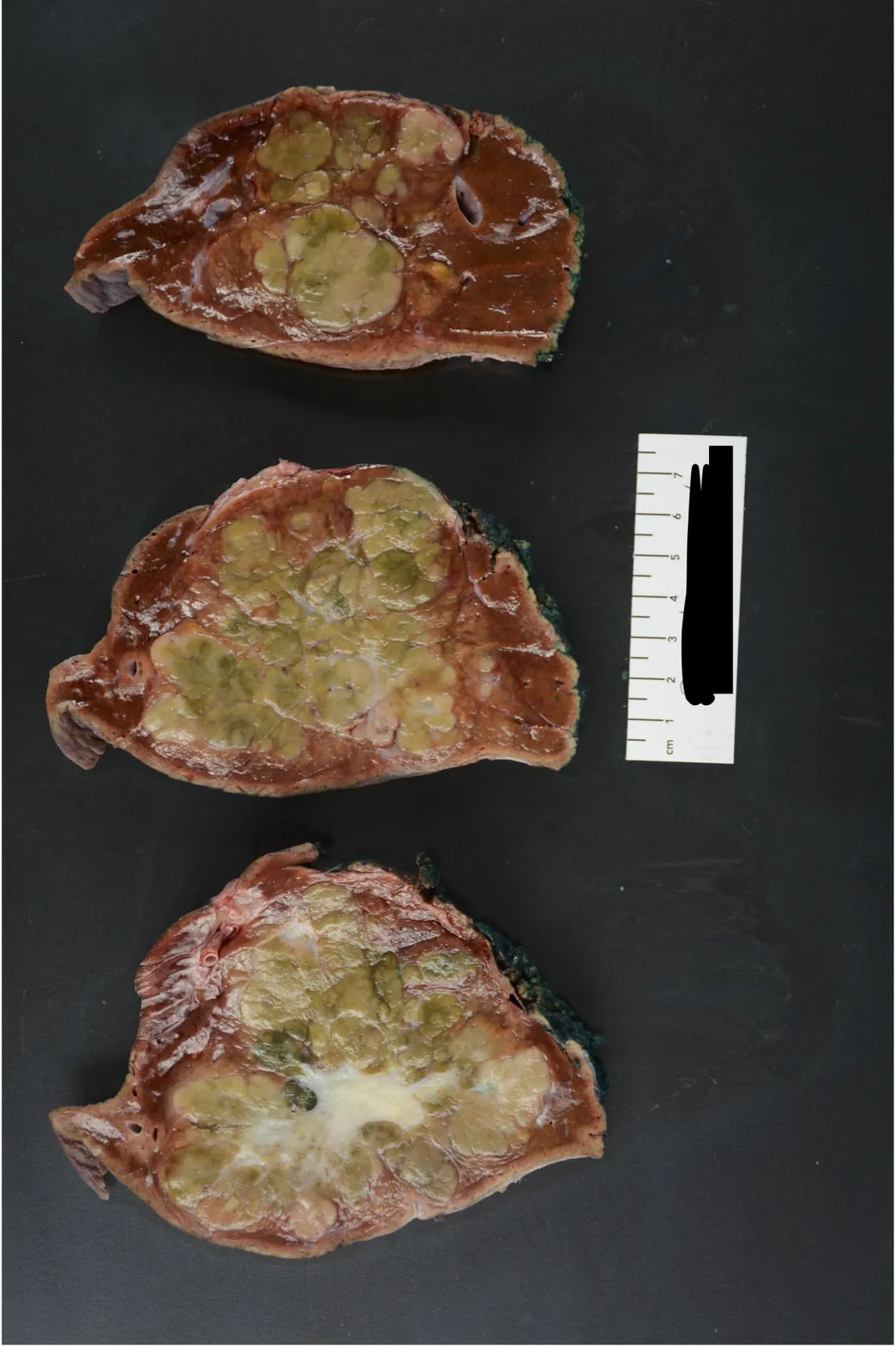
Figure 5. Gross pathology images of FHCC.
A major study in the genetics of FHCC was conducted by Honeymanet al., which found a 400 kb deletion of chromosome 19 leading to the DNAJB1-PRKACA gene fusion[39]. This was found in 100% of the 15 patients examined by the study. Further research has investigated the specific function of these genes.DNAJB1 produces Hsp40 protein, and PRKACA produces a protein kinase A (PKA) subunit[40]. PKA is associated with immune regulation and many other cellular processes[41]. This chimeric protein appears to drive the oncogenesis of FHCC[37,40]. FHCC has been shown to share some similar chromosomal abnormalities as HCC, including chromosomes 7, 8, and 18, but the frequency of these chromosomal abnormalities is more frequent in HCC[42].
Interestingly minimal disruptions to the genome overall aside from this deletion have been discovered[40].Newer studies examining tumorigenesis seek to elucidate more of the pathology this fusion product creates.A model conducted with zebrafish showed worsened inflammation and hepatomegaly with the introduction of the DNAJB1-PRKACA gene into healthy hepatocytes[41]. This gene combination has been isolated in other pancreatobiliary tumors but not traditional HCC[43].
Fluorescent in situ hybridization (FISH) has been implemented for the DNAJB1-PKACA gene fusion for the diagnosis of FHCC. A retrospective analysis by Grahamet al.of 123 patients across 3 continents was performed on tissue previously under histologic investigation for FHCC[44]. A FISH probe demonstrated a 99% sensitivity for FHCC. In the same study, 88 tumors histologically consistent with HCC were all negative under the FISH probe. They postulate that a combination of histology, immunohistochemistry, and molecular assays should be used to definitively diagnose FHCC[44].
DIFFERENTIAL DIAGNOSIS
FHCC is a rare and often underrecognized diagnosis for hepatic lesions[45]. Understanding the differences between FHCC and other hepatic lesions can aid in the accurate diagnosis of FHCC.
Heptocellular carcinoma
FHCC was initially classified as a subtype of HCC but has now been recognized as a distinct entity[36]. HCC commonly occurs in the setting of cirrhosis, hepatitis, or other liver diseases, whereas only 3% of cases of FHCC have underlying cirrhosis[45,46]. HCC is increasingly prevalent within Asian countries representing up to 76% of worldwide cases, given the high hepatitis B and C rates[47-51].The current serum diagnostic marker for HCC is an AFP above 400ng/dl; however, these levels tend to only occur in patients with cirrhosis and large HCC lesions. AFP has a sensitivity of 41%-65% and a specificity of 80%-94%, and recent studies show promise of various microRNAs, such as miR-122, miR-192, miR-21,miR-223, miR-26a, and miR-801, being used as markers with sensitivities and specificities as high as 82%and 84% respectively. This is in contrast with FHCC, which tends to have a normal AFP in most cases[52].
Currently used diagnostic radiologic tests are US, multiphase CT, and MRI[53]. The preferred imaging modality of HCC is multiphase perfusion CT or MRI[52,54,55]. HCC on CT appears hypervascular throughout the hepatic arterial phase and hypodense through the delayed phase. The hypervascular nature of HCC allows for angiography to be used in alongside CT or MRI for confirmation. Percutaneous liver biopsy is only used when imaging results are inconclusive[52,56]. Histology of HCC can vary, but classic features include neovascularization, wide trabeculae, distinct acinar pattern, loss of reticulin network, and Kupffer cells[46].
Hepatic hemangiomas
Hepatic hemangiomas (HH) are the most common benign tumors of the liver[57]. Typical hemangiomas are small, ranging from a few millimeters to 3 cm, and do not tend to increase in size which can aid in the diagnosis. Giant liver hemangiomas range from 10cm and beyond and often are symptomatic[58,59]. HH commonly occurs in the right liver lobe, and tumor markers such as AFP, carbohydrate antigen (CA) 19-9,and carcinoembryonic antigen (CEA), are normal[57].
US is the first imaging modality often used with a specificity of 80% and a sensitivity of 94% for small nodules[60]. On US, typical HH appears as a well-defined hyperechoic, homogenous nodule along with posterior acoustic enhancement[58]. Atypical, larger HH may appear inhomogeneous, with mixed echogenicity[60,61]. Furthermore, CT imaging can identify HH lesions larger than 5cm with the appearance of well-defined hyperdense lesions. On MRI, both HH and HCC appear hyperintense on T2 weighted images.Elevation of echo time can help distinguish the two, with HH increasing and HCC decreasing[62]. Given the hypervascular nature of HH, biopsy is seldom used[63]. Histology shows dilated vascular channels lined by a single endothelial layer[57].
Focal nodular hyperplasia
FNH is the second most common benign tumor of the liver[64,65]. Approximately 80% of these lesions occur in women, commonly in their reproductive years[66]. Laboratory markers are normal; however, mRNA angiopoietin genes ANGPT1 and ANGPT2 can be altered. The ratio of ANGPT1 to ANGPT2 in FNH is increased when compared to normal hepatic architecture, cirrhosis, and other hepatic lesions[66,67]. Dioguardi Burgioet al.highlighted a diagnostic strategy for FNH with 5 key assessments through CT and MRI with a specificity of 98% and sensitivity of 70%[68]. These 5 components are similar signal intensity, homogeneity,enhanced arterial phase before washout, central scarring, and the absence of a capsule. On CT and MRI,FNH appears as isointense on T1 and T2 weighted images and homogenous on both pre and postcontrast images[68]. Furthermore, CT and MRI imaging will often show enhancement during the arterial phase, with isodensity or slight hyperdensity in the portal venous and delayed phases[69]. The central scar will appear hypointense on pre-contrast T1 weighted imaging and hyperintense on T2[70]. This stellate central scar is seen in both FNH and FHCC[45]. The presence of many, defined arterial vessels radiating from the center to the periphery is known as the “spoke-wheel” and is associated with FNH in about 40% of cases[68]. Like HH and HCC, biopsy is reserved for atypical cases of FNH. Histology shows bile duct hyperplasia, hepatocyte rearrangement, and prominent Kupffer cells[64,66]. The absence of calcification in FNH can help distinguish FNH from FHCC and other metastatic lesions[68].
Hepatocellular adenoma
Hepatocellular adenoma (HCA) is a rare, benign, epithelial tumor that has been commonly linked to estrogen[45,71]. HCA is most common in young women during their reproductive years with a history of estrogen-based, oral contraceptives, and steroid use[71,72]. Laboratory markers are normal, but genetic abnormalities have been associated with the development of HCA, including hepatocyte nuclear factor 1A(HNF1A) inactivation and beta-catenin-activated adenomas[66]. These two genetic mutations form 2 of the 4 classifications for HCA, with inflammatory HCA and unclassified HCA being the other 2 subtypes.Excisional biopsy is the gold standard for diagnosis but is reserved for cases with inconclusive imaging[73].CT shows heterogeneity through early phase peripheral contrast enhancement and centripetal contrast enhancement in the portal venous phases[72,74]. Gadoxetic-enhanced MRI shows HCA hypointense during the hepatobiliary phase, distinguishing it from FNH[75].
HNF1A inactivated HCA tends to have downregulation of liver fatty acid-binding protein (LFABP), which serves as a marker in immunohistochemistry of the lesion[66]. C-reactive protein (CRP) and serum amyloid alpha (SAA) are markers to identify inflammatory HCA[76]. Beta-catenin mutated HCA is diagnosed through beta-catenin and glutamine synthetase (GS) staining, having an absolute specificity and a sensitivity of 75%-85%. Unclassified HCA is diagnosed based on the exclusion of key findings of the other 3 types of HCA[66].
Metastatic lesions
Metastatic lesions remain more common than primary tumors of the liver. Colorectal and lung cancers are the more common sources of metastatic lesions to the liver, but pancreatic, breast, and prostate cancers are also common sources[77]. Liver enzymes may be elevated due to hepatocyte injury. AFP level can be elevated for certain metastatic origins, including gastric cancer and germ cell tumor[78-80]. Vascular properties of the metastatic lesions depend on the origin of the tumor, which will impact the enhancement of imaging. MRI is often used in the diagnosis of hepatic metastases through Gadoxetate disodium-enhanced MRI[81,82].Lesions of gastric, colon, lung, or breast origin are typically hypovascular, translating to perilesional enhancement. Renal cell carcinoma, neuroendocrine tumors, thyroid tumors, melanoma, and a few breast tumors usually lead to hypervascular lesions that show the greatest enhancement in the arterial phase. These lesions can be harder to differentiate from HCC as they may light up in a similar pattern[80,83]. Although variable patterns may arise, the findings of peripheral ring enhancement with diffusion restriction and hypointensity in hepatobiliary phase represent the most consistent features of liver metastases[80]. Histology of metastases varies based on the primary tumor origin. In cases where imaging cannot distinguish metastases from other primary hepatic lesions, immunohistochemical staining is required[77]. A summary of the differential diagnostic patterns amongst all these lesions is represented in Table 1.
MANAGEMENT
Treatment of FHCC is contingent upon specific clinical factors in addition to the underlying tumor burden.Surgical approaches in the form of liver transplant (LT) or tumor resection have been implemented.Systemic therapies including chemotherapy agents and locoregional therapies consisting of transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) have been applied to patients with FHCC. Given the rarity of such malignancy, data remain limited on the management approaches, and like HCC treatment, often requires a multidisciplinary approach[84].
Surgical resection
Given the younger age of presentation, in addition to the often absence of underlying cirrhosis upon diagnosis, surgical resection has served as the mainstay of therapy for FHCC. Optimal surgical approach is aimed at achieving negative surgical margins with concurrent lymph node dissection in situations ofregional spread[2]. Surgeries include localized resection in addition to partial hepatectomy through left hepatectomy (resection of segments II-IV) or hemi-hepatectomy (resection of segments V-VIII). Extended hepatectomy (resection of ≥ 5 liver sections) through right versus left lobectomies has also been implemented in scenarios[2]. National trends from the United States (US) indicate that nearly 37% of FHCCs undergo a hemi-hepatectomy, and around 19% undergo a partial hepatectomy. Extended resections are less often implemented (~17% of patients)[85].
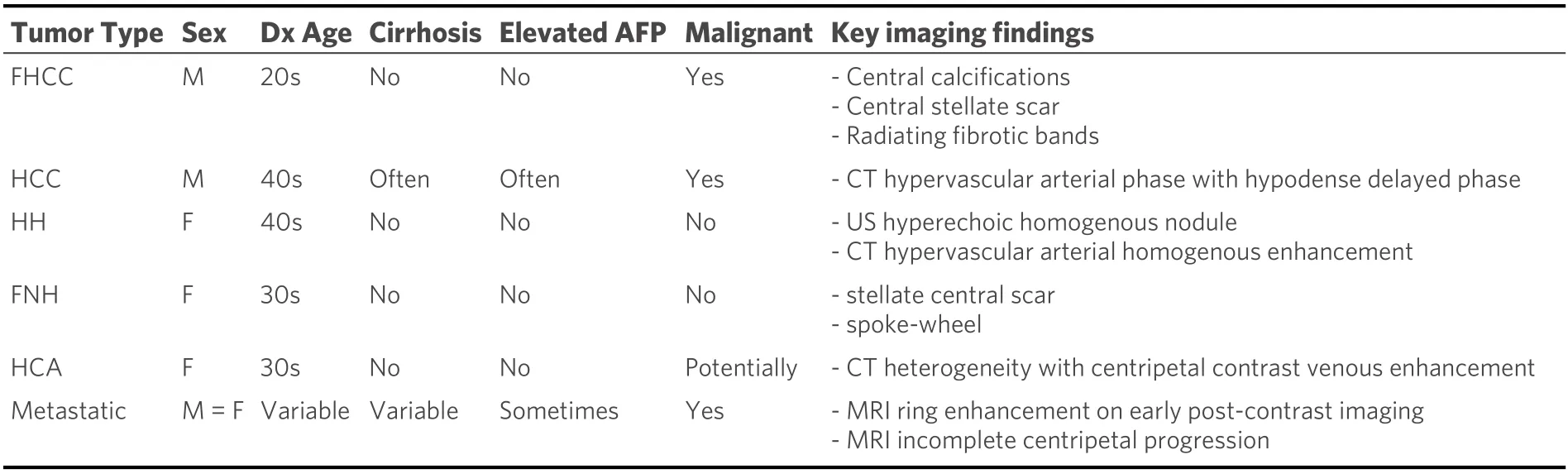
Table 1. Differential diagnosis table of key demographic and diagnostic patterns for hepatic lesions[9,51,63,65,72]
Literature has indicated that surgical resection provides mortality benefits when compared to other treatments. Systematic review by Mavroset al.depicts a 5-year overall survival (OS) rate of 70% in FHCC patients who underwent partial hepatectomy compared to 5-year survival rates of 34% in LT patients and 0% in those managed without surgery[86]. Furthermore, more recent retrospective data from Chakrabartiet al.demonstrate that surgical resection was associated with prolonged OS[16]. Given the rarity of FHCC, data remain conflicting regarding whether aggressive surgical resection should be applied in advanced disease. A study performed by Pinnaet al.with nearly 90% of patients receiving surgical resection having stage IV-A or IV-B disease demonstrated a survival rate as high as 66.2% at 5 years and 47.4% at 10 years[87]. However,in a cohort of patients with advanced disease, Hermanet al.report 5-year OS rate of only 28% following surgical resection[88].
Despite mortality benefits with surgical resection, recurrence rates are overall high with areas of malignancy recurrence including the liver and lungs as well as lymph node and peritoneal spread[88]. Even in instances of negative resection margins through surgery, recurrence remains increasingly frequent, with rates estimated to be as high as 71%[16,88].
Liver transplant
While surgical excision is the primary modality of treatment for FHCC, LT can be implemented in situations of recurrence following surgical resection or with lesions that are too large for direct excision[2].Data remains limited on the utility of LT in these patients, with most literature consisting of case series or isolated reports; however, some patients have benefitted from LT.
Trends from the US between 2000-2010 showed that those with FHCC below the age of 40 were most likely to receive LT[8]. Systematic review by Atienzaet al.of 63 cases from United Network for Organ Sharing(UNOS) of FHCC undergoing LT showed an OS of 96%, 80%, and 48% at 1, 3, and 5 years, respectively[89].Rate of tumor recurrent was 10%. Interestingly, the 5-year OS following LT was greater in those with HCC than FHCC (68%vs.48%)[89]. In the 35 patients examined by Mavroset al.undergoing LT for FHCC, there was an observed OS rate of 34% at 5 years and a median OS of 32 months[86].
When comparing surgical resection versus LT in this patient population, metanalysis by Njeiet al.indicates that those undergoing LT had worse clinical outcomes with regard to OS[90]. Additionally, there was no significant difference in post-transplant survival between HCC patients and FHCC patients (47.5vs.51.4 months)[90]. Furthermore, the role of nodal spread in conjugation with LT remains under investigation. Case report by Inceet al.in a patient with hilar nodal metastasis who underwent LT for FHCC alongside node dissection demonstrated the patient having 22 months of tumor free survival and 26 months of OS[91]. In a cohort of both FHCC and HCC patients undergoing LT, nodal involvement was associated with worse survival[92]. Amongst the limited published data on this FHCC, a definitive consensus on the mortality benefits associated with LT is lacking. Some patients have shown clinical improvement following LT, and it remains an acceptable method of treatment for FHCC.
Systemic therapy
Given the high incidence of metastasis and recurrence of FHCC, the use of targeted chemotherapy agents has been implemented to hinder overall tumor progression. Unlike formal HCC with extensive data on various systemic regimens, data on FHCC remain extremely scarce. Minimal reports on the use of agents such as cisplatin, oxaliplatin, epirubicin, 5-fluorouracil (5-FU), interferon, doxorubicin, and nivolumab have been published[93-96]; however, no standard systemic therapies have been established for FHCC.Systemic therapy has been applied as a neoadjuvant bridge through tumor downstaging prior to surgery[97],an adjuvant treatment measure, a primary treatment modality, and a palliative regimen, but there remains no defined treatment approach as to how these agents are best applied[86].
Despite limited outcome data, clinical benefits have been seen. A phase II trial assessed the use of 5-FU alongside recombinant interferon alfa-2b (IFN-α-2B) on 9 FHCC patients and showed complete and partial radiological response rates of 12.5% and 50%, respectively, with a median OS of 23.1 months[96]. The reason behind this combination is that studies have shown that IFN-α-2B upregulates the activity of thymidine phosphorylase, an enzyme that is essential for the activation of 5-FU[98]. A single-center experience of 94 FHCC cases by Kasebet al.showed that a multimodal approach to FHCC treatment in the form of systemic therapy, both adjuvant and neoadjuvant in timing, was associated with OS benefits[94]. Of these 94 patients,29% were given the 5-FU + IFN-α-2B combination regimen[94,95]. Most recently, Gottliebet al.have implemented a triple therapy regimen of 5-FU, interferon, and nivolumab within a cohort of patients of which, after a median of 18 cycles of treatment, an objective response (clinical remission + partial response)of 50% and tumor control rate (clinical remission + partial response + stable disease) of 93% were seen[93].
With the young patient demographic, FHCC can be diagnosed in pediatric patients; therefore, systemic regimens used for hepatoblastoma have been applied to FHCC. Some of these therapies included combination cisplatin + 5-FU + vincristine, carboplatin + doxorubicin + cisplatin, cisplatin + doxorubicin,and gemcitabine + oxaliplatin[99-101]. Although positive clinical responses can be seen, the rarity of FHCC hinders the establishment of definitive evidence-driven regimens.
Further studies through clinical trials are needed to uncover treatments for these patients. Previous studies focusing on various agents, including ENMD-2076 (an aurora kinase inhibitor)[102], Everolimus (an mTor inhibitor)[103], and Suntinib (a receptor tyrosine kinase inhibitor)[104]have been discontinued after showing no clinical benefits for FHCC. Various clinical trials are currently ongoing, looking at different combinations of regimens which are included in Table 2[105-108].
Radiotherapy
Radiation therapy (RT) has been applied to a myriad of oncologic diseases. Regarding FHCC, RT has been used primarily in instances of metastasis. Peacocket al.describe a case of FHCC metastatic to the lungs that was treated with RT following primary and secondary resections[109]. Over a 6-month period post-RT, an estimated 85% decrease in tumor volume was seen[109]. RT has also been used in multimodality treatment plans either alongside chemotherapy or surgical planning[110]. Overall, the use of RT in FHCC patients remains minimal with the primary treatment approaches being surgical.
Locoregional therapy
Locoregional therapies in the form of TACE and yttrium90 (Y90) TARE have been routinely applied to HCC patients. The TACE process is characterized by a direction injection of chemotherapeutic agents towards a lesion through an arterial vessel followed by ligation of the vessel to contain the agent[111]. TARE is a similar process that instead implements Y90 in small vector beads for delivery to targeted lesions through hepatic vasculature[112,113].
Several case reports have described the use of TARE in both pediatric[114,115]and adult HFCC patients[116]with overall positive results. This modality has been implemented in unresectable FHCC cases both as a primary treatment modality and as a bridge to potential surgery or transplant by downsizing the lesion[117]. Few studies have reported on TACE in FHCC[118], however, this modality has been applied in the perioperative environment to assist with tumor burden either before or after surgery[119]. The majority of literature remains derived from case reports on TACE[120,121].
Prognosis
When compared to HCC, FHCC had once been thought to have an overall better prognosis[6]; however, data remain conflicting, and more recent analysis has shown that the OS rates between these two types of cancer remain similar[99]. From a prognosis standpoint, surgical resection has been associated with prolonged OS. A systematic review from Mavroset al.showed a 5-year OS of 44% in all patients with FHCC; however, 5-year OS rates in those who received surgical excision were as high as 70%[86]. Other positive prognostic indicators have included multimodality treatment, early detection of relapse, resection of recurrent lesions, and metastasectomy[22,94,122]. Negative prognostics indicators have included advanced-stage disease, unresectable disease, lymph node involvement, and macrovascular invasion[22]. Reports remain conflicting regarding a morality difference based on sex[22,94,122].
Conclusions
FHCC remains a clinically significant oncologic disease. Definitive data and treatment guidelines have ultimately been limited by the rarity of such lesion and have required a multidisciplinary approach. While surgical resection remains at the forefront of primary management, active clinical trials investigating systemic therapies, either as primary therapy or multimodal agents, are currently underway. Continued clinical, surgical, and pharmacological studies are needed to better understand this disease and appropriate treatment modalities.

Table 2. Clinical trials of systemic therapy for FHCC
DECLARATIONS
Authors’ contributions
Idea Conception and overall structural design of the manuscript: Aryan M, Forrister N, Panchani N,Shoreibah M
Literature search and manuscript writing: Aryan M, Forrister N, Panchani N, Shoreibah M, Vashi B,Chowdhury Z, Mejbel H
Image processing and table designs: Aryan M, Forrister N, Mejbel H. Vashi B, Chowdhury Z
Critiqued and reviewed the manuscript for submission: Aryan M, Forrister N, Panchani N, Shoreibah M,Vashi B, Chowdhury Z, Mejbel H
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
- Hepatoma Research的其它文章
- The role of minimally invasive surgery in the treatment of HCC
- The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis
- The duration of the conventional chemoembolization for hepatocellular carcinoma: factors affecting the procedural time
- Minimally invasive surgery for HCC
- Liver tumors in childre n

