The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis
Mohamad Khalil, Maria Calasso, Leonilde Bonfrate, Agostino Di Ciaula, Maria De Angelis, David Q. H.Wang, Piero Portincasa
1Clinica Medica “Augusto Murri”, Department of Biomedical Sciences and Human Oncology, University of Bari Medical School,Bari 70124, Italy.
2Department of Soil, Plant and Food Sciences, University of Bari Aldo Moro, Bari 70126, Italy.
3Department of Medicine and Genetics, Division of Gastroenterology and Liver Diseases, Marion Bessin Liver Research Center,Einstein-Mount Sinai Diabetes Research Center, Albert Einstein College of Medicine, New York, NY 10461, USA.
Abstract Consumption of natural products such as herbs, spices, plant-derived compounds, and foods is on the rise globally.The use of these substances is widely recognized as an integral part of culture and tradition, with the philosophy being “no benefit is no harm”. The utility of medicinal plants and extracts is under scrutiny, and the scientific community needs to clarify many conceptual gaps. Medicinal plants are rich in bioactive phytochemicals that produce chemopreventive effects at different levels, including cellular, animal, and clinical. The ultimate translational value is often missing, and some studies suggest that botanicals may contain toxic compounds that cause acute or chronic toxicity. In this regard, the liver is the center, and herbal products can show protective effects or induce hepatotoxicity, thereby promoting liver cancer. In this review article, we examine a range of herbal products implicated in hepatocarcinogenesis and extend the discussion to herbal products that may be potentially involved in the prevention and treatment of liver carcinoma.
Keywords: Herbs, hepatocellular carcinoma, liver cancer, natural compounds
INTRODUCTION
The liver is the largest internal organ and gland in the human body (approximately 2.5% of an adult’s body weight) and is involved in the regulation of physiological metabolism of carbohydrates (glucose, glycogen,and fructose), lipids (bile acids, cholesterol, fatty acids, triglycerides, and phospholipids), proteins, and vitamins[1]. The liver is also essential for regulating hormonal function and bile secretion, as well as metabolism of xenobiotic substances and drugs, with the capacity of detoxifying endogenous (waste metabolites) and exogenous substances (toxic compounds).
Individuals may use herbal products and dietary supplements that are believed to have health benefits[2].Although regulations exist regarding the use, indications, safety, and efficacy of specific herbal preparations,compounds also have potential hazards due to several factors such as inaccurate labeling, poor quality,pollution, unclear efficacy and safety, side effects, and interactions with other drugs[3]. The liver becomes the center of the detoxification function of herbal products and metabolites, but it can also become the target of damage from drugs, herbs, and supplements, leading to acute or chronic hepatotoxicity[4]. The effects of herbal products can also interact with mechanisms associated with hepatocellular carcinoma (HCC), the most common type of primary liver cancer[5].
HCC is one of the most prevalent life-threatening solid tumors, with more than one million new cases diagnosed worldwide each year, and it remains the third leading cause of cancer-related death[6].
The causes of liver cancer include viral hepatitis (HBV or HBC) infection and toxin exposure such as alcohol and aflatoxin. In addition, fatty liver diseases and related metabolic disorders such as obesity,diabetes, and chronic liver inflammation are potential risk factors for liver cancer[7,8].
Eating herbs that contain toxic compounds or mutagenic and carcinogenic substances such as aristolochic acid is associated with a higher HCC risk. Compounds from other plant sources may be toxic to the liver,and, in the presence of other risk factors, they may promote the development of HCC.
In contrast, many natural compounds derived from plants are used as anticancer agents and are currently undergoing medical development[9]. Many plants and plant-derived compounds can have beneficial effects against tumors with low toxicity to normal tissues. Some of these compounds are used as anticancer agents against HCC[10]. Generally, the most common phytochemicals in cancer treatments include alkaloids such as vinblastine and vincristine fromCatharan roseusfor the treatment of lymphomas, leukemias, and solid tumors; terpenes such as taxol fromTaxus brevifoliafor the treatment of lung, breast, and ovarian cancers;and quinolones such as camptothecin fromCamptotheca acuminatefor the treatment of leukemia[11]. Recent evidence suggests that plants, such asSilybum marianum,Solanum nigrum,Claviceps purpurea, Nigella sativa,andThymbra spicata,and isolated natural compounds, such as curcumin, resveratrol, carvacrol, and thymoquinone, alone or in combination with anticancer drugs exert potential antitumor and chemoprotective effectsin vitroandin vivo[11-14].
In this review article, we present evidence-based research on herbal and natural products that may cause cancer and damage to the liver, as well as plants with antitumor effects against HCC.
LIVER TUMORIGENESIS: PLANTS AND PLANT-DERIVED COMPOUNDS MAY INDUCE LIVER CANCER
In traditional medicine, herbal preparations are widely used to prevent or treat many diseases and as food supplements. The World Health Organization estimated that approximately 80% of the population relies on complementary and traditional medicines, including herbal medicine, for their primary healthcare[15]. The idea of using herbs and herbal products is appealing, and people prefer using medicinal plants rather than chemical drugs because herbs are considered safe and efficient. However, herb-induced cardiotoxicity,hepatotoxicity, and nephrotoxicity due to long-term use are also observed, which indicates that some herbal formulations are not completely safe. Other plant-derived constituents show genotoxic and/or carcinogenic effects and may raise safety concerns. In this regard, many hepatocarcinogenic compounds have been reported, including aristolochic acid, alkenylbenzenes, and pyrrolizidine alkaloids.
In this section, we report a panel of herbs and herb-derived compounds that have been reported to induce liver cancer. Table 1 presents studies addressing the possible promoting effects of herbs and their compounds on liver tumorigenesis.
Aristolochic acid
Aristolochic acid (AA) is found in different plant families such asAsarum, Bragantia,andAristolochia.These plants are traditionally used in Chinese herbal medicine. Based on IARC monographs on the identification of carcinogenic hazards to humans, AA and AA-containing plants are classified as Carcinogenic Group 1. The genotoxic effect of AA has been demonstrated in different studies. In a cellular model with the human hepatoma HepG2 cells, AA caused a significant increase of DNA migration and micronuclei formation. The AA-induced DNA and chromosome damage was associated with an increase in nitric oxide (NO) level and the formation of 8-hydroxydeoxyguanosine. This observation confirms that AA could exert a genotoxicity effect, likely through increasing NO and its derivatives[16]. In addition, in Hepa1-6 cells, AA promoted hepatocellular carcinoma invasion and migration and enhanced epithelial-mesenchymal transitionviaSnail, N-cadherin, and vimentin upregulation and E-cadherin downregulation; those effects were mediated by the C3a/C3AR activation pathway[17]. Furthermore, the liver tumor-promoting effect of AA was studied using animal models. Mice exposed to AA alone or combined with CCl4 (a well-known hepatotoxic agent) developed HCC or combined HCC and intrahepatic cholangiocarcinoma. The study showed that AA caused DNA damage and AA-DNA adduct formation, leading to the initiation of different liver tumors through adenine-to-thymine transversions[18]. In dogs, 10-day oral administration of AA caused hepatic premalignant alterations and tumor progenitor-like cell formation by overexpression of c-Myc oncoprotein and oncofetal RNA-binding protein Lin28B. The activation by phosphorylation of forkhead box O1 also increased upon AA exposure. Furthermore, the c-Myc signaling pathway seems related to microRNAs in the liver, where let-7 miRNAs and miR-23a are significantly dysregulated in animals treated with AA[19].
A human cohort study of HCC Chinese patients has found an AA-associated mutational signature in 25.8%of livers[18]. Another cohort study investigated the association between the use of AA containing Chinese herbs and HCC risk among HBV-infected patients. A significant dose-dependent relationship was observed between herbs containing AA consumption and HCC in patients with HBV, suggesting that aristolochic acid in HBV patients is crucial for HCC pathogenesis[20]. Another HCC cohort study showed that the AAassociated mutational signature is correlated with different tumor-immune markers such as high PD-L1 expression and CD3+ T-cell infiltration[21].

Table 1. Studies of the possible promoting effects of herbs and their compounds on liver tumorigenesis
Ginkgo biloba
Since prehistoric times, theGinkgo bilobatree has been used in Chinese folk medicine for treating several illnesses[22,23]. Moreover, extracts fromG. bilobaare diffused as natural supplements[24]. Accumulative studies over the last decades explored the possible bioactivity ofG. biloba,which includes neuroprotective and cardioprotective effects and anticancer proprieties[25]. Despite this, some components of ginkgo are suspected of possessing mutagenic properties[26]. Indeed, the IARC monographs on the identification of carcinogenic hazards to humans classifyGinkgo bilobaextract as Carcinogenic Group 2B. Due to its longterm and high-dose consumption,G. bilobaextract (GBE) was studied by the National Cancer Institute for carcinogenic tests in rodents. B6C3F1/N mice and F344/N rats received GBE by gavage for two years. The study found an increased incidence of hepatocellular adenoma in male F344/N rats. Moreover, the study indicated that administration of GBE also caused dose-dependent carcinogenic activity in livers of mice with high incidences of HCC, hepatoblastoma, and hepatocellular adenoma[27].
To better address the mechanism of GBE-induced hepatocarcinoma, Hoenerhoff and colleagues[28]showed that oral GBE exposure to B6C3F1 mice resulted in a dose-dependent increase of centrilobular hypertrophy risk after 90 days and hepatocellular necrosis and hepatocellular tumors by two years. The study showed that, despite the similarity between GBE-exposed and spontaneous HCC at the morphologic level, the hepatocarcinogenesis alterations were different; in particular, high-dose GBE exposure is associated with Ctnnb1 mutation. In addition, GBE exposure is also associated with dysregulation of the WNT pathway.This finding provides a molecular and genetic profile behind GBE-induced hepatocarcinogenesis in mice[28].
The mode of action for GBE hepatocarcinogenesis was further clarified by investigating the involvement of the constitutive androstane receptor (CAR) using CAR-knockout (CARKO) and wild-type mice[29]. The results show that HCC DNA replication increased only in wild-type mice after one-week exposure to GBE;in contrast, no significant increase in HCC DNA replication in CARKO mice was observed. Additionally,long-term GBE exposure (4 or 13 weeks) led to a higher increase in hepatic Cyp2b10 expression and hepatocellular hypertrophy in wild-type mice than in CARKO mice. In addition, using a well-established hepatocarcinogenesis model by diethylnitrosamine treatment, GBE exposure promoted more eosinophilic foci and hepatocellular adenomas in wild-type mice than in CARKO mice. These findings indicate that the constitutive androstane receptor is essential for GBE-mediated hepatocarcinogenesis.
Phenylpropanoid (Alkenylbenzenes) compounds
The IARC monographs on the identification of carcinogenic hazards to humans classify some phenylpropanoid compounds such as methyleugenol and safrole as Carcinogenic Group 2B. Eugenol,isoeugenol, and methyleugenol are produced by different plants such as calamus, savory, basil, and clove.They are used in foods and beverages as flavoring compounds, as well as fragrance compounds in numerous hygiene products, perfumes, creams, and detergents. Due to the large human exposure and their similar chemical structure to safrole, which is known to be a mutagenic compound, methyleugenol and isoeugenol were studied by the National Toxicology Program for a carcinogenic test. In 1999, it was announced by the Committee of Experts on Flavoring Substances of the Council of Europe that methyleugenol exhibits DNAbinding propriety, and it was identified as genotoxic carcinogenic natural. For that purpose, male and female F344/N rats and B6C3F1 mice received pure (≥ 99%) isoeugenol[30]or methyleugenol[31]for two years.The studies reported that isoeugenol exerted a carcinogenic effect on B6C3F1 male mice by increasing HCC and/or hepatocellular adenoma[30]. In addition, the methyleugenol displayed an evident carcinogenic effect in mice by increasing liver neoplasms incidence[31]. Methyleugenol has a mutagenic effect on the β-catenin gene. In detail, Devereuxet al.[32]showed that, in B6C3F1 mice, 20 of 29 methyleugenol-induced hepatocellular neoplasms had β-catenin gene point mutations.
Methyleugenol and other alkenylbenzenes represent the main component of the aromatic composition of basil[33]and are also present in basil-containing sauce (e.g., Italian pesto). The daily exposure to alkenylbenzenes by regular pesto consumption may have a carcinogenic effect. The assessment of pesto sauce risk was performed based on the detected level of methyleugenol in different pesto sauces[34]. The study concluded that pesto sauce consumption does not represent a cancerogenic risk; however, a safety concern might be considered upon long-term daily consumption of pesto sauce.
Safrole, another member of the phenylpropanoid family of natural products, is found in sassafras and other plants; it is also found in many Chinese herbal drugs derived fromAsarumspecies. It is considered a “weak”hepatocarcinogen, and its effect is related to stable safrole-DNA adduct formation. Safrole also caused oxidative damage, chromosome aberrations, and sister chromatid exchanges in rat liver, which is linked to hepatic injury and hepatocarcinoma[32,35]. In male BALB/c mice, 16 weeks of exposure to safrole resulted in histopathologic alteration including centrilobular hepatocytes hypertrophy. In contrast, long-term exposure to safrole induced hepatocellular adenomas in 7 of 10 mice[36]. In addition, 24 weeks of safrole exposure was able to induce the formation of hepatocyte basophilic and acidophilic foci, and 36 weeks of exposure led to the formation of neoplastic nodules[37].
Turkey egg genotoxicity assay showed that high doses of alkenylbenzenes caused DNA adduct formation[38,39]. The DNA binding ability of these compounds was also investigated in CD-1 mice[40],showing that methyleugenol, estragole, and safrole had a strong binding ability to liver DNA. Interestingly,the evidence suggests that the metabolic activation of alkenylbenzene compounds is crucial for their genotoxic and carcinogenic properties. In detail, sulfotransferases (enzyme for 1'-hydroxylation of allylbenzenes) inhibition resulted in the binding inhibition of safrole to liver DNA[41].
Areca catechu
Areca catechu(betel palm) is originally native to South and South-East Asia. Betel nuts (A. catechuseeds)have been widely used in herbal medicine practices for their antiparasitic properties. The nuts are also used for the treatment of diarrhea, throat inflammations, and urinary disorders. However, long-term betel nuts consumption is linked to the development of several diseases including cancer and metabolic dysfunction[42,43]. In this regard, in a cohort study, Chouet al.[44]found a positive correlation between nut chewing and liver disease (fibrosis) in persons with nonalcoholic fatty liver disease.
The widespread betel nut chewing habit may represent a risk factor for the development of liver diseases such as cirrhosis and HCC. A population-based study in Taiwan with a high prevalence of hepatitis B/C infection conducted by Wuet al.[45], showed increased risks of cirrhosis and HCC in betel chewers independently from hepatitis B/C infection. Subjects with hepatitis B/C infection had a higher HCC risk[45],and similar results were observed in a community-based cohort study[46]and in case-control studies[47-49].The pathogenesis of betel-quid-induced liver cirrhosis and HCC is still not clear. Recently, the betel nut chewing cancerogenic effect appears to be mediated by arecoline, a nicotinic acid-based mild parasympathomimetic stimulant. Arecoline, the major alkaloid of betel quid, has been reported as one of the abundant constituents of areca nut, and it can act as an atherogenic[50], hepatotoxic[51], and cancerogenic agent[52]. A study using normal rat hepatocytes showed that arecoline had genotoxic properties and increased the expression of TGF-β[53]. Similarly, Wanget al.[54]showed that arecoline and its metabolite arecoline N-oxide induced DNA damage.
PROTECTION ON LIVER TUMORIGENESIS: PLANTS AND PLANT-DERIVED COMPOUNDS MAY PREVENT/ALLEVIATE LIVER CANCER
Besides their safety profile, some herbs and plant-derived compounds possess multitarget effects, making them optimum preventive and/or therapeutic/adjuvants agents against HCC proliferation, progression, and metastasis. Table 2 depicts the studies on the possible preventive effects of herbs and their compounds on liver tumorigenesis.
Silybum marianum L.
Silybum marianum, a flowering plant from the Mediterranean basin, is commonly known as milk thistle (or blessed milk thistle) and is used in folk herbal medicine. Silymarin, the common name for the mixture extract ofS. marianum,has been found to exert marked antioxidant, anti-inflammatory, and hepatoprotective activities; it is mainly consumed by patients with chronic liver disorders such as fatty liver,cirrhosis, and HCC.
The potential chemopreventive activities of silymarin against N-nitrosodiethylamine-induced HCC in rats were evaluated[55]. In this study, silymarin pre- and post-treatment showed a significant attenuation of oxidative stress by decreasing the MDA level and increasing GSH and liver antioxidant enzymes SOD, GPx,and GR. Interestingly, silymarin was also able to reduce the increased levels of GGT, AST, ALT, and VEGF.
Silibinin, a major bioactive flavanone in silymarin, is one of the most known hepatoprotective agents; its protective effect is found in many liver injuries such as fatty liver, viral hepatitis, bile duct inflammation,and cirrhosis. Silibinin showed a repressive effect on HepG2 and Hep3B cell proliferation, which was associated with apoptosis induction and cell cycle arrest by decreasing cyclin (D1, D3, and E) as well as cyclin-dependent kinase (CDK-2/4) levels[56]. In addition, silibinin inhibits cytochrome p4502E1 induction and ROS generation in ethanol-insulted HCC cellsin vitro, which suggests that silibinin is beneficially effective against ethanol-dependent increases in HCC cell proliferation in culture[57].In vivo,silibinin showed a weak antitumor activity in an HCC cell transplanted animal model. Therefore, the anti-HCC activity of co-treatment with silibinin and sorafenib (standard HCC chemo-drug) has been investigated as a potential therapeutic strategy for HCC[58]. In detail, the combination of silibinin and sorafenib markedly inhibited the cell proliferation of different HCC cells and exhibited significant proapoptotic activity. In addition, in an animal model of HCC subcutaneous transplantation, the co-treatment suppressed thein vivotumor growth. These effects were accompanied by AKT and STAT3 phosphorylation inhibition, as well as downregulation of antiapoptotic protein expressions such as Mcl-1 and Bcl-2. Using HCC stem cells,silibinin and sorafenib co-treatment reduced the expression of stemness-related proteins, resulting in the reduction of the self-renewal of HCC stem cells[58]. Silymarin, in combination with doxorubicin, also markedly reduced the telomerase activity of HepG2 cells[59].
To evaluate the effect of silibinin on obesity-induced liver cancer, HepG2 cells were exposed to sera from normal weight, overweight, and obese males with or without silibinin. The results show that silibinin attenuated obesity-induced cell proliferation and invasion. At molecular levels, silibinin decreased inflammatory cytokines (IL-6/1B) and reduced Erk phosphorylation[60].
Silybin is a bioactive flavonolignan component of silymarin extract with reported hepatoprotective and chemopreventive proprieties. Silybin reduced HCC cell proliferation and migration and induced caspase 3-and ROS-dependent apoptosis. Furthermore, silybin treatment decreased the Notch signaling pathway inHCC[61].

Table 2. Studies of the possible preventive effects of herbs and their compounds on liver tumorigenesis
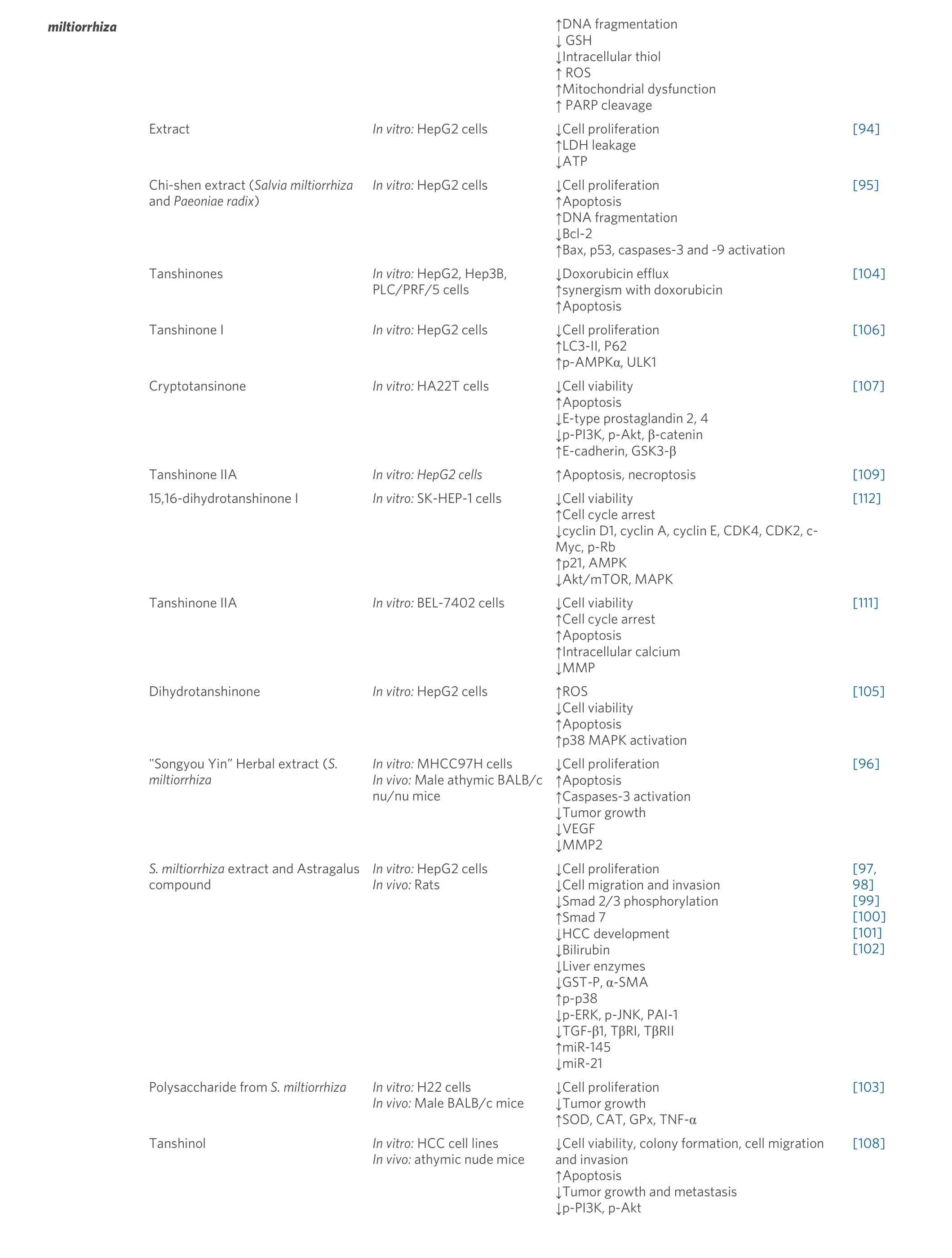
miltiorrhiza ↑DNA fragmentation↓ GSH↓Intracellular thiol↑ ROS↑Mitochondrial dysfunction↑ PARP cleavage Extract In vitro: HepG2 cells ↓Cell proliferation↑LDH leakage↓ATP[94]Chi-shen extract (Salvia miltiorrhiza and Paeoniae radix)In vitro: HepG2 cells ↓Cell proliferation↑Apoptosis↑DNA fragmentation↓Bcl-2↑Bax, p53, caspases-3 and -9 activation[95]Tanshinones In vitro: HepG2, Hep3B,PLC/PRF/5 cells↓Doxorubicin efflux↑synergism with doxorubicin↑Apoptosis[104]Tanshinone I In vitro: HepG2 cells ↓Cell proliferation↑LC3-II, P62↑p-AMPKα, ULK1 Cryptotansinone In vitro: HA22T cells ↓Cell viability↑Apoptosis↓E-type prostaglandin 2, 4↓p-PI3K, p-Akt, β-catenin↑E-cadherin, GSK3-β[106][107]Tanshinone IIA In vitro: HepG2 cells ↑Apoptosis, necroptosis [109]15,16-dihydrotanshinone I In vitro: SK-HEP-1 cells ↓Cell viability↑Cell cycle arrest↓cyclin D1, cyclin A, cyclin E, CDK4, CDK2, c-[112]Myc, p-Rb↑p21, AMPK↓Akt/mTOR, MAPK Tanshinone IIA In vitro: BEL-7402 cells ↓Cell viability↑Cell cycle arrest↑Apoptosis↑Intracellular calcium↓MMP Dihydrotanshinone In vitro: HepG2 cells ↑ROS↓Cell viability↑Apoptosis↑p38 MAPK activation[111][105]"Songyou Yin” Herbal extract (S.miltiorrhiza S. miltiorrhiza extract and Astragalus compound In vitro: MHCC97H cells In vivo: Male athymic BALB/c nu/nu mice In vitro: HepG2 cells In vivo: Rats↓Cell proliferation↑Apoptosis↑Caspases-3 activation↓Tumor growth↓VEGF↓MMP2↓Cell proliferation↓Cell migration and invasion↓Smad 2/3 phosphorylation↑Smad 7↓HCC development↓Bilirubin↓Liver enzymes↓GST-P, α-SMA↑p-p38↓p-ERK, p-JNK, PAI-1↓TGF-β1, TβRI, TβRII↑miR-145↓miR-21[96][97,98][99][100][101][102]Polysaccharide from S. miltiorrhiza In vitro: H22 cells In vivo: Male BALB/c mice Tanshinol In vitro: HCC cell lines In vivo: athymic nude mice↓Cell proliferation↓Tumor growth↑SOD, CAT, GPx, TNF-α↓Cell viability, colony formation, cell migration and invasion↑Apoptosis↓Tumor growth and metastasis↓p-PI3K, p-Akt[103][108]
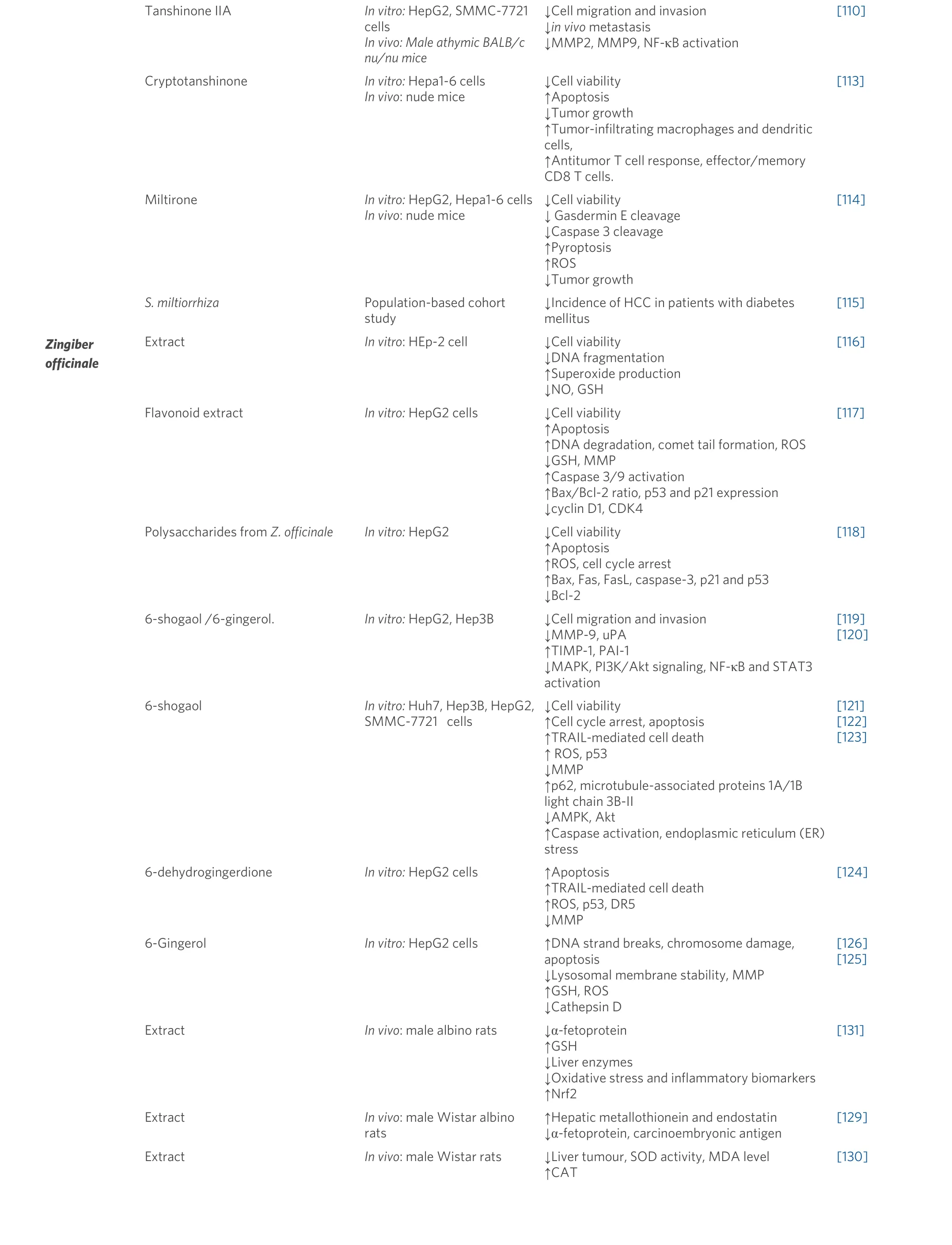
Tanshinone IIA In vitro: HepG2, SMMC-7721 cells In vivo: Male athymic BALB/c nu/nu mice Cryptotanshinone In vitro: Hepa1-6 cells In vivo: nude mice Miltirone In vitro: HepG2, Hepa1-6 cells In vivo: nude mice S. miltiorrhiza Population-based cohort study↓Cell migration and invasion↓in vivo metastasis↓MMP2, MMP9, NF-κB activation↓Cell viability↑Apoptosis↓Tumor growth↑Tumor-infiltrating macrophages and dendritic cells,↑Antitumor T cell response, effector/memory CD8 T cells.↓Cell viability↓ Gasdermin E cleavage↓Caspase 3 cleavage↑Pyroptosis↑ROS↓Tumor growth↓Incidence of HCC in patients with diabetes mellitus[110][113][114][115]Zingiber officinale Extract In vitro: HEp-2 cell ↓Cell viability↓DNA fragmentation↑Superoxide production↓NO, GSH[116]Flavonoid extract In vitro: HepG2 cells ↓Cell viability↑Apoptosis↑DNA degradation, comet tail formation, ROS↓GSH, MMP↑Caspase 3/9 activation↑Bax/Bcl-2 ratio, p53 and p21 expression↓cyclin D1, CDK4 Polysaccharides from Z. officinale In vitro: HepG2 ↓Cell viability↑Apoptosis↑ROS, cell cycle arrest↑Bax, Fas, FasL, caspase-3, p21 and p53↓Bcl-2 6-shogaol /6-gingerol. In vitro: HepG2, Hep3B ↓Cell migration and invasion↓MMP-9, uPA↑TIMP-1, PAI-1↓MAPK, PI3K/Akt signaling, NF-κB and STAT3 activation[117][118][119][120]6-shogaol In vitro: Huh7, Hep3B, HepG2,SMMC-7721 cells↓Cell viability↑Cell cycle arrest, apoptosis↑TRAIL-mediated cell death↑ ROS, p53↓MMP↑p62, microtubule-associated proteins 1A/1B light chain 3B-II↓AMPK, Akt↑Caspase activation, endoplasmic reticulum (ER)stress[121][122][123]6-dehydrogingerdione In vitro: HepG2 cells ↑Apoptosis↑TRAIL-mediated cell death↑ROS, p53, DR5↓MMP 6-Gingerol In vitro: HepG2 cells ↑DNA strand breaks, chromosome damage,apoptosis↓Lysosomal membrane stability, MMP↑GSH, ROS↓Cathepsin D Extract In vivo: male albino rats ↓α-fetoprotein↑GSH↓Liver enzymes↓Oxidative stress and inflammatory biomarkers↑Nrf2[124][126][125][131]Extract In vivo: male Wistar albino rats↑Hepatic metallothionein and endostatin↓α-fetoprotein, carcinoembryonic antigen[129]Extract In vivo: male Wistar rats ↓Liver tumour, SOD activity, MDA level↑CAT[130]
Curcuma longa L.
Curcuma longahas been used for centuries for culinary and medicinal purposes. Several studies investigated the chemopreventive and antitumor effects of turmeric (C. longa L.) and its main polyphenolic compound,curcumin. A panel ofin vitrocellular studies of curcumin alone or in combination with commonly used chemotherapeutic drugs explored its potent anti-hepatocarcinoma effects. These studies showed that curcumin decreased HCC cell proliferation and metastasis and induced apoptotic cell death[62]through different mechanisms including decreasing the hypoxia-induced hypoxia-inducible factor-1α protein level[63]; downregulation of the expression of Snail via suppressing Smad2 pathway and TGF-β1[64]; the mitochondria-dependent pathway related to intracellular accumulation of calcium[65], miR-21-5p, andSOX6[66]; inhibition of the WNT signaling pathway[67-70]; inhibition of metalloproteinases (MMP-2/9);reduced AKT, p38, and STAT3 phosphorylation[71,72]; activation of AMPK signaling pathway[73]; reduction of DJ-1 expression[74]; increasing apoptosis and pyroptosis[75]; inducing endoplasmic reticulum stress and mitochondrial dysfunction[76]; downregulating SREBF1[77]; and induction of FasL-related apoptosis through p38[78]. Moreover, a bioinformatics analysis investigating the crucial genes, transcription factors, and microRNAs associated with the effects of curcumin against HCC showed that curcumin might downregulate LEF1 and CTGF, regulated by miR-19A, and upregulate CDKN1A expression[62].
The chemopreventive effect ofC. longawas investigated in anin vivoanimal study. Administration ofC.longaextract to HBV mice for three months reduced visceral fat and resulted in a smaller and delayed liver disease progression. The study indicated thatC. longacould have beneficial effects on preventing and/or delaying liver carcinogenesis[79]. The preventive effect ofC. longawas also shown in DENA-induced liver carcinogenesis, where dietary supplementation ofC. longadecreased the elevated serum level of liver enzymes and improved hepatic damage[80].
The effects ofC. longaconstituents and phytochemicals on liver cancer have also been investigated. For example, the aromatic turmerone, a volatile turmeric oil isolated fromC. longa,induced ROS-triggered intrinsic and extrinsic apoptosis pathways in HepG2 cells[81].
Curcumin, one of the most abundant polyphenols inC. longa,with multiple pharmacological activities including antitumor effects, showed a potent anti-HCC propertyin vitroandin vivo. The antiproliferative and proapoptotic activities of curcumin as a single agent in HepG2 cells were more evident than those ofC.longamethanolic extract[82]. In addition, curcumin-mediated cell death was observed to be related to the inhibition of NF-κB activation pathway. Furthermore, curcumin inhibited liver cancer stem cells and suppressed tumorigenicity[83]. In HepG2 cells, treatment with curcumin reduced the tumor neocapillary density[84]and decreased COX-2 and serum VEGF[85]. A reduction of VEGF expression and PI3K/AKT signaling was also observed upon curcumin treatmentin vitroandin vivo[86].
In anin vivoanimal model, nanoparticulate curcumin oral supplementation decreased the elevated liver enzymes, α-fetoprotein, and VEGF and reduced inflammatory markers such as TNF-α, MDA, and NFκB[87]. Similarly, curcumin decreased α-fetoprotein and AST concentration and increased serum albumin concentration in rats[88].
At the molecular level, curcumin treatment downregulated miR-21 expression, upregulated tissue inhibitor metalloproteinase protein 3 (TIMP) expression, and inhibited the TGF-β1/smad3 signaling pathway in HepG2 and HCCLM3 cells[89].In vivo, using DENA and CCl4-induced HCC in adult female albino rats,curcumin reduced α-fetoprotein and proinflammatory cytokines and decreased the elevated liver enzymes.In addition, curcumin treatment led to the upregulation of the expressions of Cx43, UCP-3, LC3, and Mito.Q10[90].
The possible effect of curcumin co-treatment with cisplatin on liver epithelial tumor cells was also investigated. Curcumin led to NF-κβ and β-catenin inhibition and decreased cyclin D expression. In animal studies, using a mouse xenograft model, curcumin and cisplatin significantly decreased α-fetoprotein[91].
Salvia miltiorrhiza
Salvia miltiorrhizais widely used in Chinese herbal medicine for liver diseases. Several studies indicate thatS. miltiorrhizaexhibits different biological and pharmaceutical activities including antitumor activity.
In HepG2 cells, Liuet al.[92]showed thatS. miltiorrhizaextract inhibited cell proliferation and induced apoptotic cell death. The proapoptotic effect seems to be mediated by GSH depletion and reduction of mitochondrial membrane potential[92]. In addition, they demonstrated in another study that the proapoptotic effects ofS. miltiorrhizaextract are correlated with a rapid decline of GSH and protein thiol content, mitochondrial dysfunction, and overproduction of ROS in HepG2 cells[93]. Furthermore, HepG2 cells treated withS. miltiorrhizashowed an increase in LDH leakage, decrease in ATP level, and induction of apoptotic-like cell morphology change, which also might be involved in the cytotoxic and apoptotic effects[94].
Accumulating evidence suggests thatS. miltiorrhiza, in combination with other medicinal herbs, displays an appreciated effect against HCC. In this regard, combinations such as “Chi-Shen” extract (S. miltiorrhizaandPaeoniae radix)[95], “Songyou Yin” (containingS. miltiorrhizaand four other herbs)[96], and CASE(compound astragalus andS. miltiorrhizaextract[97-102]) have shownin vitroandin vivoanti-HCC effects mainly by cell proliferation inhibition and apoptosis induction through the modulation of Bcl-2 family and caspase-3 and -9 activation, in addition to inhibition of tumor invasion via downregulation of MMP-2, PAI-1 transcriptional activity, TGF-β/TβR and Imp7/8 protein expression, MAPK, and MAPK-dependent phosphorylation of Smad2/3 and Smad4.
Additionally, fractions or single compounds fromS. miltiorrhizahave also shown potent anti-HCC effects.In a cellular model using H22 cells, the polysaccharide fraction fromS. miltiorrhizaexerted an antiproliferative effect. Moreover, in an animal model, inhibition of tumor growth and TNF-α level was observed in mice treated with the polysaccharide fraction[103]. Tanshinones fromS. miltiorrhiza,such as tanshinone I, tanshinone IIA, cryptotanshinone, and dihydrotanshinone, have been largely studied for their bioactivities including antitumor effect.In vitro, using doxorubicin-resistant human liver cancer cells,cryptotanshinone exerted a repressive effect on doxorubicin efflux, and tanshinone IIA provided a great synergism with doxorubicin[104]. In addition, in HepG2 cells, tanshinones inhibited cell growth and induced caspase-dependent apoptosis accompanied with an increase of ROS[105]. Tanshinone I showed a regulatory effect on autophagic cancer cell death by activation of AMP-activated protein kinase[106]. In HA22T cells,cryptotansinone inhibited cell viability and induced apoptosis. At the molecular level, cryptotansinone inhibited EP-2/4 expression and PI3K and Akt phosphorylation in HA22T[107].
Zhuet al.[108]tested the effect of tanshinol, an aqueous polyphenol isolated fromS. miltiorrhiza, and observed inhibition of HCC cell proliferation, migration, invasion, and colony formation. In addition, when testedin vivo, tanshinol treatment reduced tumor growth and metastasis. Thein vitroandin vivoantitumor effects were associated with the reduction of PI3K and AKT[108]. Tanshinone IIA also caused apoptotic and necroptotic HepG2 cell death through Nec-1 inhibition and FLIPS regulation[109]. In addition, tanshinone IIA showed a repressive effect on HCC invasion and metastasisin vitroandin vivoby inhibiting MMP-2/9 activity[110]. The proapoptotic effect of tanshinone IIA was also related to calcium-dependent apoptosis and MT 1A upregulation[111]. 15,16-dihydrotanshinone I also suppressed SK-HEP-1 proliferation and induced cell cycle arrest at G0/G1 phase and regulated AMPK/Akt/mTOR and MAPK signaling pathways[112].
Cryptotanshinone inhibited the proliferation of Hepa1-6 cells and induced apoptosis. In addition,cryptotanshinone exerted an immunotherapeutic effect, as shown in tumor-bearing mice, where cryptotanshinone promoted the activation of immune cells against tumor tissue[113]. In the same cell line(Hepa1-6) and HepG2, miltirone, a bioactive compound fromS. miltiorrhiza,inhibited cell proliferation and induced gasdermin E and caspase 3 protein cleavage. Miltirone also induced ROS production and decreased MEK and ERK1/2 phosphorylation. Moreover, in a mouse HCC syngeneic model, miltirone exerted a significant tumor growth inhibition[114].
A population-based cohort study showed that the use ofS. miltiorrhizaas traditional Chinese herbal medicine reduces the incidence of HCC in diabetes mellitus patients[115].
Zingiber officinale (Ginger)
Ginger root is widely used as a spice and in folk medicine. The anticancer effect of ginger is documented byin vitrocellular andin vivoanimal studies.
In vitro,using HEp-2 cells, the ginger extract showed a dose-dependent inhibition of cell proliferation and caused marked apoptosis-like morphological and nuclear changes including cell shrinkage and chromosomes condensation. Those effects were accompanied by an increase in ROS production and a decrease in GSH content[116]. Similarly, in HepG2 cells, treatment with ginger extracts decreased cell viability and induced apoptotic cell death mediated by DNA degradation, comet tail formation, ROS production,GSH depletion, mitochondrial membrane potential alteration, caspase 3/9 activation, and increasing of the Bax/Bcl-2 ratio[117]. Cell cycle arrest was also observed in ginger-treated HCC[118].
Ginger extract and bioactive compounds such as 6-gingerol and 6-shogaol exerted an anti-migration abilityin vitroby decreasing MMP-9 activity and increasing TIMP-1 activity[119]. Similarly, 6-gingerol and 6-shogaol strongly reduced HCC invasion and metastasis abilities through MAPK, PI3k/Akt, NF-κB, and STAT3 pathways[120].

Figure 1. (A) Chemical structural of the aristolochic acid and alkenylbenzenes estragole, methyleugenol, and safrole. (B) Mutagenesis mechanism of aristolochic acid by DNA adduct formation. HCC: hepatocellular carcinoma.
6-shogaol also induced TRAIL mediated apoptosis through ROS production, p53 expression, and mitochondrial transmembrane potential alteration; these effects were accompanied by inhibition of autophagy flux by increasing p62 and microtubule-associated proteins 1A/1B light chain 3B-II levels[121].Furthermore, 6-shogaol induced G2/M cell cycle arrest in HCC cells and modulated cyclin expression. In addition, 6-shogaol was able to reduce cancer survival signaling pathways such as MAPK, AMPK, and Akt[122]. The proapoptotic activity of 6-shogaol is also linked to endoplasmic reticulum stress and caspase activationin vitro. In an animal xenograft mice model as well, 6-shogaol reduced invivotumor growth and induced apoptotic death through caspase 3 activation and eIF2α inactivation[123].
Furthermore, 6-dehydrogingerdione, a compound isolated fromZ. officinale,showed a proapoptotic effect on HepG2 through mitochondrial- and Fas receptor-mediated pathways and inhibition of p53 nuclear translocation[124].
In HepG2 cells, 6-gingerol exerted anticancer activity by causing DNA and chromosome damage.Additionally, 6-gingerol altered lysosomal membrane stability and mitochondrial membrane potential[125].Other studies showed that 6-gingerol exerts prooxidant effects by increasing ROS production and inducing apoptosis involving cathepsin D mediator[126].
In vivo,using DEN-induced rat liver cancer, ginger extract displayed chemo-preventative effects by tumor growth inhibition and apoptosis induction[127]. The chemo-preventative effects of ginger could be attributed to its ability to decrease inflammatory mediators such as NF-κB and TNF-α expression in rats’ liver cancer[128]. In Wistar albino rats, ginger administration showed a protective effect against premalignant stages of liver cancer. Moreover, the ginger extract reduced serum α-fetoprotein and carcinoembryonic antigen[129]. In rats with liver cancer induced by a 0.1% ethionine and choline-deficient diet, ginger treatment reduced tumor growth and decreased MDA level[130]. In addition, ginger pre-treatment improved rats’ liver function by restoring GSH levels and decreasing inflammatory and oxidative markers[131].
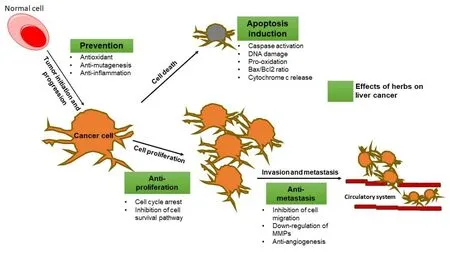
Figure 2. Possible preventive effects and mechanism of herbs and their compounds on liver tumorigenesis. Bax: Proapoptotic protein;Bcl-2: antiapoptotic protein; MMPs: matrix metalloproteinases. Boxes in green represent the herbal effects on liver cancer.
DISCUSSION AND CONCLUSION
Besides the well-established risk factors for liver cancer, hepatotoxic herbs or herbs-derived compounds could represent an additional liver cancer risk factor. For example, AA is an established human carcinogen and is one of the most potent carcinogens. In particular, AA metabolites bind DNA to form adducts resulting in A > T transversion mutations. Although AA is a main risk factor for urinary tract urothelial carcinoma, we report a different science-based link between AA exposure and liver cancer. Results fromin vitroandin vivostudies as well as population-based cohort studies demonstrate the potential hepatocarcinogenic effect of AA. A summary of the properties of AA is presented in Figure 1.
Plants such asGinkgo bilobaand chewing betel-nuts (Areca catechu) as well as plant-derived alkenylbenzene compounds including safrole, methyleugenol, and estragole have been shown to exert hepatocarcinogenic activity in different experimental studies. Here, we summarize several cellular and animal studies concerning their hepatocarcinogenic. In addition, some data from different population-based and casecontrol studies show marked associations between natural products and HCC development.
In contrast, many herbal remedies showed a potent anti-liver cancer activity. In fact, severalin vitrostudies have shown that natural products exhibit strong cytotoxic and proapoptotic activity against HCC, as well as have effects on cell metastasis and invasion [Figure 2].
Hereby, we report evidence from cellular and animal studies that explore the potential anti-liver cancer activity of different herbal products, namelySilybum marianumand its active constituents silymarin,silibinin, and silybin;Curcuma longawith its active compound curcumin;Salvia miltiorrhizaand its principal bioactive compounds tanshinones; andZingiber officinaleand its active polyphenolic fractions, 6-shogaol, and 6-gingerol. In HCC cells, these products inhibited HCC cell proliferation and tumor growth and induced apoptotic cell death. Similarly, in liver cancer animal models, the herbal products reduced tumor growth and metastasis, decreased the elevated level of liver enzymes, and decreased the α-fetoprotein level.In conclusion, herbs and herb-based preparations may provide beneficial outcomes in human health including hepatoprotective effects. However, they can also contain individual ingredients known to be toxic and even genotoxic and carcinogenic to the liver. Despite the available evidence from cellular and animal studies of herbs promoting or preventing liver tumorigenesis, human studies are still missing. In this regard,clinical trials investigating the safety and toxicity of herbs are highly needed. The mechanism of action involved in liver tumorigenicity or hepatoprotection should be clarified by well-establishedin vivoexperimental models.
DECLARATIONS
Authors’ contributions
Conceived the manuscript: Portincasa P
Wrote this manuscript: Khalil M, Portincasa P
Intensive revised the manuscript: Di Ciaula A, Bonfrate L, Calasso M, De Angelis M, Wang DH
Availability of data and materials:
Not applicable.
Financial support and sponsorship
This paper has been partly supported by funding from the European Union’s Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie Grant Agreement No. 722619 (FOIE GRAS) and Grant Agreement No. 734719 (mtFOIE GRAS).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
- Hepatoma Research的其它文章
- The role of minimally invasive surgery in the treatment of HCC
- A focused review on recent advances in diagnosis and management of fibrolamellar hepatocellular carcinoma
- The duration of the conventional chemoembolization for hepatocellular carcinoma: factors affecting the procedural time
- Minimally invasive surgery for HCC
- Liver tumors in childre n

