Effects of Litter Removal and Addition on Soil Nitrogen Mineralization of Eucalyptus Plantation
Wanpeng LU Hongying LIU Chunning LI Bin HE

Abstract[Objectives] This study was conducted to understand the process of soil nitrogen mineralization in Eucalyptus plantations, and to identify the characteristics of soil nitrogen mineralization with different litter inputs. [Methods]With the soil of the Eucalyptus plantation in Fusui County, Guangxi as the research object, the soil nitrogen mineralization of the Eucalyptus plantation under different litter treatments (removing litter, adding litter and retaining litter) was studied by PVC tube closed-top in-situ incubation. [Results] ① After 1 year of litter treatment, the inorganic nitrogen (NH-N + NO-N) in the soil of different treatments ranked as adding litter (20.15 mg/kg) > retaining litter (16.02 mg/kg) > removing litter (11.60 mg) /kg), and the differences reached a significant level (P<0.05). ② After 30 d of in-situ incubation, there were significant differences in soil nitrate nitrogen content before and after incubation in the three treatments (removing litter, adding litter, and retaining litter) before and after incubation (P<0.05), but no significant differences were observed in soil ammonium nitrogen content (P>0.05). Soil nitrate nitrogen contents increased from 1.47, 2.01 and 1.72 mg/kg before incubation to 3.66, 6.73 and 5.02 mg/kg, respectively, and soil ammonium nitrogen content increased from 11.60, 20.15 and 16.02 mg/kg before incubation to 13.65, 21.54 and 17.18 mg/kg, respectively. The net nitrogen mineralization quantities of the three treatments were 4.24, 6.11 and 4.46 mg/kg, respectively, and the net nitrogen mineralization rates from large to small were adding litter[0.180 mg/(kg·d)]>retaining litter[0.141 mg/(kg·d)]>removing litter[0.125 mg/(kg·d)]. Therefore, both removal and addition of litter affected the soil nitrogen input and nitrogen mineralization rate of the Eucalyptus plantation, thereby affecting soil nitrogen availability and the ability of soil to maintain plant-available nitrogen. [Conclusions]This study provides a theoretical basis for nutrient management in Eucalyptus plantations, especially nitrogen nutrient management.
Key wordsEucalyptus plantation; Litter; PVC tube closed-top in-situ incubation; Soil nitrogen mineralization
Nitrogen is one of the essential nutrients for plant growth and development[1], and it is also the most abundant mineral element that plants can absorb from soil. As one of the most important processes in soil nitrogen cycle, nitrogen mineralization regulates the main process of plant utilization of available nitrogen. Organic nitrogen in soil is converted into ammonium nitrogen (NH-N) and nitrate nitrogen ( NO-N) which can be directly absorbed and utilized by plants[1-2]. Therefore, the rate of nitrogen mineralization determines the availability of nitrogen in the soil for plant growth and significantly affects the efficiency of plant nitrogen use in the soil, because nitrogen mineralization is one of the most important processes of nitrogen cycling in forest ecosystems[3-5].
In forest ecosystems, litter input is the most important way of returning nutrients to soil[6], and it is also the main source of soil nitrogen. The total N returned to the soil from litter accounts for about 70%-80% of the N required by plants[6]. Therefore, the input of litter has a profound impact on the nitrogen cycle process of forest ecosystems[6-7]. Eucalyptus is the main tree species in the fast-growing and high-yield forest bases in South China[8]. At present, the planting area of Eucalyptus exceeds 4.5 million hm2, and it has become an important part of China's wood resources, and has played an active role in wood production, economic construction and ecological construction[9]. Due to the rapid growth of Eucalyptus, it needs to absorb a large amount of nitrogen and other nutrients from the soil, while its litter is relatively less and decomposes slowly, resulting in the reduction of the biogeochemical cycle function of Eucalyptus plantations, and the destruction of the balance of nitrogen and other nutrients. Coupled with unreasonable forest management measures such as short-term management and multi-generation continuous planting, the trend of forest productivity decline is further exacerbated. In recent years, domestic and foreign scholars have carried out a lot of research work on soil nitrogen and its mineralization in Eucalyptus plantations[10-13], but the effects of litter on soil nitrogen mineralization in Eucalyptus plantations are rarely reported so far. In this study, with a Eucalyptus plantation in Fusui County, Guangxi as the research object, the effects of litter on the transformation of soil nitrogen in the Eucalyptus plantation were revealed by the PVC tube closed-top in-situ incubation method through comparative analysis of soil nitrogen mineralization after changing litter input (removal, addition and retention). This study provides a theoretical basis for nutrient management of Eucalyptus plantations, especially nitrogen management.
Materials and Methods
General situation of the study area
The study area is located in Guangxi State-owned Dongmen Forest Farm (107°14′-108°00E, 22°17′-22°30′N) in Dongmen Town, Fusui County, Guangxi, which belongs to the south subtropical climate type. The annual average temperature, extreme maximum temperature and extreme minimum temperature are 21.2-22.3, 38.0-41.1, and -4.0-1.9 ℃, respectively. The annual accumulated temperature over 10 ℃ is 7 190-7 762 ℃. The annual average rainfall reaches 1 100-1 300 mm, and the relative humidity is higher than 74%. The annual sunshine hours range from 1 634 to 1 739 h. The test plots are located in the Leika branch of the farm, with an altitude of 82-95 m, a southeast slope of 8-10°. The Eucalyptus (E. urophylla×E. grandis) plantation was 5 years old, and the stand reserved density was 1 370 plants/hm2. The average tree height was 17.4 m, and the diameter at breast height was 13.2 cm. The understory plants are mainly Litsea glutinosa, Rhus chinensis, Litsea pungens, Miscanthus floridulus, Mallotus repandus and Eupatorium odoratum. The coverage was 75%. The thickness of the litter layer was about 1.7 cm. The soil of the test site is latosolic red soil, and the parent material (parent rock) is sand shale. The soil thickness was 80-120 cm; the soil (0-15 cm) bulk density was (1.10±0.05) g/cm3; the pH value was 4.36±0.03; and the organic matter and total nitrogen contents were (27.14±0.83) and (0.81±0.04) g/kg, respectively.
Study methods
Plot settingAt the end of June 2017, a 5-year-old E. urophylla×E. grandis plantation was selected as the research object. Three fixed quadrats with an area of 400 m2 (20 m×20 m) were set up, and 9 plots with an area of 2.25 m2 (1.5 m×1.5 m) were evenly set in each quadrat for following treatments: ① retaining litter (control check), ② removing litter, and ③ adding litter. In the treatment of removing litter, all the litter on the surface of 2.25 m2 (1.5 m×1.5 m) was first removed, and then a 1.5 m × 1.5 m nylon collection net with a height of 0.5 m was placed on the surface of the plot to collect litter and prevent it from entering the surface of the plot. For the treatment of adding litter, the litter collected in the treatment ② was first evenly spread in a 2.25 m2 plot, and then the litter collected in the litter removal treatment ② was evenly spread into the plot every month. Three replicates were set for each treatment, and there were a total of 27 plots. From July 1, 2017 to the end of June 2018, litter was collected at the end of each month and brought back to the laboratory, and dried at 80 ℃ to constant mass, and the amount of litter was calculated. Meanwhile, the growth status of forest trees in the sample plots was investigated, and soil samples were collected and their main physical and chemical properties were determined.
Experimental methodsOn July 1, 2018, three in-situ incubation sites were selected in each test plot, and the soil nitrogen mineralization test and measurement were carried out by the PVC tube closed-top in-situ incubation method. First, the litter on the surface was removed, and then two PVC tubes with an inner diameter of 5 cm and a length of 18 cm were vertically driven into the 15 cm soil layer. One of the tubes was taken out and put into a ziplock bag and brought back to the laboratory. The fresh soil samples were used for the determination of the initial values of moisture, NH-N and NO-N mass fractions, and the organic matter and total nitrogen content were determined with dry soil. The top of the other one was sealed with plastic film, and the bottom was sealed with gauze, and both were fixed with rubber bands and put back in place. After 30 d of in-situ incubation, it was taken out, put in a ziplock bag and brought back to the laboratory, for the determination of soil moisture, NH-N and NO-N content.
Soil sample determination methodsThe soil moisture, organic carbon and total nitrogen contents were determined by the drying method, potassium dichromate-sulfuric acid volumetric method and Kjeldahl method, respectively[14]. For soil NH-N and NO-N contents, the samples were first extracted with 2 mol/L KCl solution, and the extracts were determined for NH-N by indophenol blue colorimetry, and for NO-N by the phenol disulfonic acid method[14].
Calculating methods and data processing
The relevant numerical calculation of net soil nitrogen mineralization quantity and net nitrogen mineralization rate was carried out according to following formulas:
M=M--M
V=M/t
M=M-M
M=M-M
In the formulas: M is the net soil nitrogen mineralization quantity, and M and M are the inorganic nitrogen contents before and after incubation, respectively; M is the net soil ammonification quantity, and M and M are the NH-N contents before and after incubation, respectively; M is the net soil nitrification quantity, and M and M are the NO-N contents before and after incubation, respectively, mg/kg; VN is the net soil nitrogen mineralization rate, mg/(kg·d); and t is the incubation days, d.
SPSS 22.0 software was used for data processing and analysis. One-way ANOVA was used to analyze the differences in soil NH-N and NO-N contents, as well as net soil nitrogen mineralization quantity and net nitrogen mineralization rate between different litter treatments. Differences between treatments were further tested for multiple comparisons.
Results and Analysis
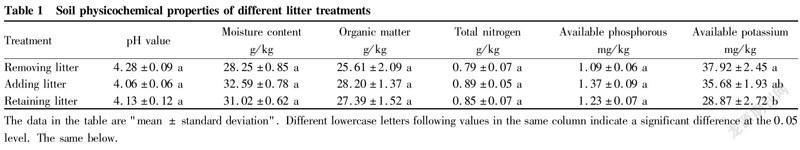
Soil physical and chemical properties of different litter treatments
The results showed (Table 1, Fig. 1) that after 12 months of addition and removal of litter in the Eucalyptus plantation, the soil physical and chemical properties of the treatments were different to varying degrees. Except for the soil pH value showing an order of removing litter>retaining litter>adding litter, soil moisture and main nutrient contents showed opposite trends, and soil inorganic nitrogen content among them exhibited the most significant difference, followed by available potassium content. Compared with the treatment of retaining litter (CK), the soil inorganic nitrogen content of the litter removal treatment decreased by 35.73%, while the soil inorganic nitrogen content of the litter addition treatment increased by 24.95%, and the differences of inorganic nitrogen content between the treatments all reached a significant level (P<0.05). Specifically, the contents of NH-N and NO-N in the soil with the addition of litter were 25.78% and 16.86% higher than those in the litter retaining treatment, respectively, while the contents of NH-N and NO-N in the soil without litter decreased by 27.59% and 14.53% than those in the litter retaining treatment, respectively, showing a trend of increasing with the increase of litter volume. The content of NH-N in the soil inorganic nitrogen composition of different litter treatments accounted for more than 88.70%, which was significantly higher than that of NO-N, indicating that NH-N was the main form of soil inorganic nitrogen in the Eucalyptus plantation.
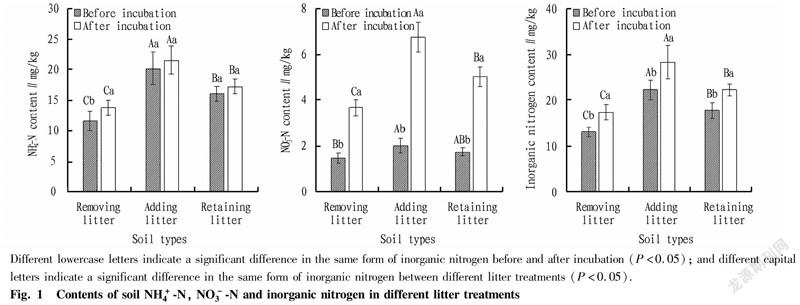
Changes of soil inorganic nitrogen content after different litter treatments
It can be seen from Fig. 1 that after 30 d of in-situ incubation, the soil inorganic nitrogen contents of the little removal, retention and addition treatments increased from 13.07, 17.74 and 22.16 mg/kg before incubation to 17.31, 22.20 and 28.27 mg/kg, respectively. The soil inorganic nitrogen content showed the same change trend as before incubation. Specifically, NO-N increased from 1.47, 1.72 and 2.01 mg/kg before incubation to 3.66, 5.02 and 6.73 mg/kg, respectively, and the differences before and afterincubation in various treatments all reached a significant level (P<0.05); and the soil NH-N contents increased from 11.60, 20.15 and 16.02 mg/kg before incubation to 13.65, 21.54 and 17.18 mg/kg, respectively, and there were no significant differences in soil NH-N content before and after incubation in various treatments (P>0.05). After incubation, the contents of NH-N in soil inorganic nitrogen were also significantly higher than those of NO-N in various treatments, indicating that NH-N was still the main form of soil inorganic nitrogen in the Eucalyptus plantation after incubation.
Net soil nitrogen mineralization quantities and net nitrogen mineralization rates under different litter treatments
It can be seen from Table 2 that after 30 d of incubation, the net soil nitrogen mineralization quantities of the litter removing, adding and retaining treatments in the Eucalyptus plantation were 4.24, 6.11 and 4.46 mg/kg, respectively. Specifically, the net ammonification quantities were 2.05, 1.39 and 1.16 mg/kg, respectively, and the net nitrification quantities were 2.19, 4.72 and 3.30 mg/kg, respectively. The net soil nitrogen mineralization rates were 0.125, 0.180 and 0.141 mg/(kg·d), respectively. Specifically, the net ammonification rates were 0.068, 0.046 and 0.039 mg/(kg·d), and the net nitrification rates were 0.056, 0.134 and 0.107 mg/(kg·d) , respectively. The net soil nitrogen mineralization quantity and net nitrogen ammonification rate were both the highest with the litter adding treatment, followed by the litter retaining treatment, and the litter removing treatment showed the lowest values. There were significant differences between the litter adding and removing treatments in net soil nitrogen mineralization quantity and net nitrogen ammonification rate (P<0.05). The net nitrification quantity and net nitrification rate in the soil were higher than the net ammonification quantity and net ammonification rate of the soil in various litter treatments, indicating that the soil nitrification effect was stronger and the ammonification effect was weaker in various litter treatments, that is, the soil ammonium nitrogen was converted to nitrate nitrogen.
Conclusions and Discussion
Litter is the connecting reservoir of nitrogen cycle in the soil-plant system in forest ecosystems, and has an important impact on the dynamics and transformation of soil nitrogen[15-16]. Zhang et al.[17] and Wang et al.[18] conducted research on Chinese fir plantations and showed that adding litter would increase soil nitrogen content, while litter removal would result in a decrease in soil nitrogen content. Nadelhoffer et al.[19] and Fisk et al.[20] carried out experiments in Harvard Forest and White Mountain National Forest, respectively, and the results showed that the net soil nitrogen mineralization quantity and net nitrification quantity did not show significant differences due to litter addition and removal. Wang et al.[21] and Kong et al.[22] conducted studies on subtropical coniferous forests and temperate deciduous forests, respectively, and also showed that litter input had no significant effect on soil nitrogen content, while root turnover had a stronger effect on soil nitrogen. There are also studies showing that after the addition of litter, because the surface litter hindered the contact between the soil and the atmosphere, the ventilation and water permeability of the soil was weakened, and the microbial activities and other reactions in the soil were affected, resulting in the nitrogen transformation process in the soil slowed down in a certain period of time[23-24]. Meanwhile, due to the addition of litter, the soluble organic matter in the soil also increased, which promoted the absorption and utilization of nitrogen in the soil by plants and caused an increase in nitrogen consumption in the soil, resulting in a decrease in soil nitrogen content[16].
In this study, both removal and addition of litter had different degrees of effect on soil physical and chemical properties, especially the more significant effect on soil NH-N content. Compared with the litter retaining treatment, the content of NH-N in the soil with the removal of litter decreased by 38.10%, while the content of NH-N in the soil with the addition of litter increased by 25.78%; and the content of NO--N showed the same trend, respectively, and compared with the litter retaining treatment, it decreased by 17.01% and increased by 16.86%, respectively. Both of them showed an order of adding litter > retaining litter > removing litter, and the differences of soil NH-N content between different litter treatments were significant. The result is consistent with the research results obtained by Jean de Dieu Nzi-la et al.[25] in the Andrews forest in the United States and Holub et al.[26] in the Sikfokut forest of Hungary and forests of Congo, and Deng et al.[27] in the study on a camphor tree plantation in Changsha City, Hunan Province. The inorganic nitrogen in the soil of various treatments was mainly NH-N, and its content accounted for more than 88.75% of the inorganic nitrogen content, which is related to the acidic soil in the study area. The acidic environment has an inhibitory effect on the growth of nitrifying bacteria[9], and NH-N has a positive charge and is easily adsorbed by negatively charged clay minerals and organisms without being easily leached and lost, while negatively charged NO-N is not easily adsorbed by soil colloids and is easily lost by rainwater, resulting in lower soil NO-3-N content.
After 30 d of cultivation, the content of NO-N in the soil of different treatments increased significantly, and the increase amounts ranked as adding litter (4.72 mg/kg) > retaining litter (3.30 mg/kg) > removing litter (2.19 mg/kg); soil NH-N content also showed an increasing trend, and the increase amounts ranked as removing litter (2.05 mg/kg) > control (1.39 mg/kg) > adding litter (1.16 mg/kg), which was exactly opposite to the change of NO-N content; and the contents of inorganic nitrogen showed an order of adding litter (6.11 mg/kg) > retaining litter (4.46 mg/kg) > removing litter (4.24 mg/kg). It indicated that the input of litter promoted soil nitrogen mineralization and significantly enhanced nitrification, but had a weak effect on ammonification. Studies have shown that there is a very close relationship between net soil nitrogen mineralization rate and soil microbial activity[28]. Higher soil microbial activity helps to improve the net nitrogen mineralization rate of the soil, and the level of soil microbial activity is closely related to soil organic carbon and total nitrogen content. Compared with the soil of other regional forests[29], the net nitrogen mineralization quantities and net nitrogen mineralization rates of the soil in different letter treatments in this study were lower, which might be related to the climatic conditions and tree growth during incubation. From July to August in the study area, the temperature is high, the rainfall is high, the soil moisture content is high, and the effective nutrients in the soil are released quickly. However, at this time, the trees grow vigorously, and need to absorb a large amount of inorganic nitrogen from the soil to meet their demand for nitrogen nutrition[9], so the inorganic nitrogen in the soil is mainly consumptive[30], and the net nitrogen mineralization rate in the soil is also low. In this study, the net nitrogen mineralization rates of soil ranked as litter addition > litter retention > litter removal, and the value was not significantly reduced by adding litter, which is different from the results of some studies[18, 23-24], which might be related to the climatic conditions, soil characteristics and the absorption and utilization of inorganic nitrogen forms by forest trees (Eucalyptus) in the study area.
Because the net nitrogen mineralization of forest ecosystems is affected by many factors, its nitrogen mineralization process is also complicated[30-31]. Restricted by actual conditions, this study is only an investigation and analysis of different litter treatments after 1 year. The results of the study have certain limitations, and further positioning monitoring and research need to be carried out in the future. However, from the perspective of Eucalyptus plantation management, retaining and adding litter can not only increase the soil organic pool and nutrient pool of the woodland, but also help to improve the nitrogen mineralization capacity of woodland soil, promote the growth and development of trees, and maintain the lasting productivity of Eucalyptus plantation forests.
References
[1] HUI HJ, GUO HX. N mineralization and nitrification in a primary Lithocarpus xylocarpus forest and degraded vegetation in the Ailao Mountain, Yunnan Province[J]. Acta Botanica Sinica, 2004, 46(2): 194-201.
[2] STEFANO M, AMILCARE P. Soil carbon and nitrogen mineralization: Theory and models across scales[J]. Soil Biology & Biochemistry, 2009(41): 1355-1379.
[3] XIONG Y, ZENG H, XIA H, et al. Interactions between leaf litter and soil organic matter on carbon and nitrogen mineralization in six forest litter-soil systems[J]. Plant & Soil, 2014, 379(1/2): 217-229.
[4] RUAN CY, LIU XF, LYU MK, et al. Effects of litter carbon, nitrogen and enzyme activity in soil under Chinese fir[J]. Acta Pedologica Sinica, 2020, 57(4): 954- 962. (in Chinese).
[5] PAN SH, CHENG YQ, DU H, et al. Litter decomposition and DOC release during forest succession in Greater Khingan Mountains[J]. Journal of Southwest Forestry University, 2019, 39(5): 75-83. (in Chinese).
[6] ZHOU WJ, SHA LQ, SCHAEFER DA, et al. Direct effects of litter decomposition on soil dissolved organic carbon and nitrogen in a tropical rainforest[J]. Soil Biology and Biochemistry, 2015(81): 255-258.
[7] CóRDOVA SC, OLK DC, DIETZEL RN, et al. Plant litter quality affects the accumulation rate, composition, and stability of mineral-associated soil organic matter[J]. Soil Biology and Biochemistry, 2018(125): 115-124.
[8] HUANG Z, WANG LH, CHEN Z, et al. Study on reproductive growth characteristics and seed germination of Eucalyptus dunnii in Sichuan Province[J]. Agricultural Biotechnology, 2018, 7(3): 207-211.
[9] HUANG ZG, HE B, XIE MY, et al. Seasonal dynamic characteristic of soil nitrogen in Eucalyptus plantations under successive rotation[J]. Journal of Northeast Forestry University, 2020, 48(9): 88-94. (in Chinese).
[10] FERNNDEZ C, VEGA JA , BAR S, et al. Nitrogen mineralization after clearcutting and residue managementin a second rotation Eucalyptus globulus Labill. stand in Galicia (NW Spain)[J]. Annals of Forest Science, 2009, 66(8): 807-807.
[11] LYU XY, HE B, WU YF, et al. The soil organic carbon and nitrogen storage and the evolution characteristics of Eucalyptus plantations under successive rotation[J]. Chinese Journal of Tropical Crops, 2017, 38(10): 1874- 1880. (in Chinese).
[12] DEVIM EMM, LEONARDUS VS, SAMUEL VVB, et al. Soil nutrient stocks are maintained over multiple rotations in Brazilian Eucalyptus plantations[J]. Forest Ecology and Management, 2019(448): 364-375.
[13] LPEZ-POMA R, PIVELLO VR, DE BRITO GS, et al. Impact of the conversion of Brazilian woodland savanna (cerrado) to pasture and Eucalyptus plantations on soil nitrogen mineralization[J]. Science of the Total Environment, 2020(704): 135397.1-15397.12.
[14] LU RK. Soil and agricultural chemistry analysis[M]. Beijing: China Agriculture Science and Technique Press, 1999: 146-271. (in Chinese).
[15] ZHOU WJ, SHA LQ, SCHAEFER DA, et al. Direct effects of litter decomposition on soil dissolved organic carbon and nitrogen in a tropical rainforest[J]. Soil Biology and Biochemistry, 2015(81): 255-258.
[16] KANERVA S, SMOLANDER A. Microbial activities in forest floor layers under silver birch, Norway spruce and Scots pine[J]. Soil Biology and Biochemistry, 2007(39): 1459-1467.
[17] ZHANG X, FAN YX, LUO X, et al. Effects of litter and root treatments on soil nitrogen content of Chinese fir plantation[J]. Journal of Subtropical Resources and Environment, 2014, 9(2): 39- 44. (in Chinese).
[18] WANG GJ, TIAN DL, YAN WD, et al. Effects of litter-fall addition and removal on the nitrogen mineralization in soils of Cunninghamia lanceolata plantation[J]. Journal of Central South University of Forestry & Technology, 2009, 29(3): 6-10. (in Chinese).
[19] NADELHOFFER KJ, BOONE RD, BOWDEN RD, et al. The DIRT experiment: Litter and root influences on forest soil organic matter stocks and function[C].∥Foster D, Aber J. (Ed s.). Forests in Time: The Environmental Consequences of 1000 Years of Chang e in New England. New Haven: Yale University Press, 2004: 300-315.
[20] FISK MC, FAHEY TJ. Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young northern hard wood forests[J]. Biogeochemistry, 2001(53): 20-1223.
[21] WANG QK, HE TX, WANG SL, et al. Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest[J]. Agricultural and Forest Meteorology,2013,15(9): 178-179.
[22] KONG Q, WANG CK, WANG XC. Effects of detritus removal on soil carbon, nitrogen and phosphorus stoichiometry and related factors in a temperate deciduous forest in the Maoershan Mountain, China[J]. Chinese Journal of Applied Ecology, 2018, 29(7): 2173-2182. (in Chinese).
[23] FONTAINE S, BAROT S, BARR P, et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply[J]. Nature, 2007, 450(7167): 277-280.
[24] KUZYAKOV Y. Priming effects: Interactions between living and dead organic matter[J]. Soil Biology and Biochemistry, 2010, 42(9): 1363-1371.
[25] DE DIEU NZILAJ, BOUILLET JP, LACLAU JP, et al. The effects of slash management on nutrient cycling and tree growth in Eucalyptus plantations in the Congo[J]. Forest Ecology and Management, 2002(171): 209-221.
[26] HOLUB SM, LAJTH AK, SPEARS JDH, et al. Organic matter manipulations have little effect on gross and net nitrogen transformations in two temperate forest mineral soils in the U SA and central Europe[ J]. Forest Ecology and Management, 2005(214): 320-330.
[27] DENG HP, WANG GJ, GENG G. Response of nitrogen mineralization to litter addition and exclusion in soils of Cinnamomum camphora plantation[J]. Journal of Beijing Forestry University, 2010, 32(3): 47-51. (in Chinese).
[28] CHEN F, ZHENG H, ZHANG K, et al. Soil microbial community structure and function responses to successive planting of Eucalyptus[J]. Journal of Environmental Sciences, 2013, 25(10): 2102-2111.
[29] XIAO RH, MAN XL, DING LZ. Soil nitrogen mineralization characteristics of the natural coniferous forest in Northern Daxing'an Mountains, Northeast China[J]. Acta Ecologica Sinica, 2019, 39(8): 2762-2771. (in Chinese).
[30] HUANG ZQ, WAN XH, HE ZM, et al. Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China[J].Soil Biology & Biochemistry, 2013(62): 68-75.
[31] MAN XL, DING LZ. Soil nitrogen mineralization characteristics of the natural coniferous forest in Northern Daxing'an Mountains, Northeast China[J]. Acta Ecologica Sinica, 2019, 39(8): 2762-2771.
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Report on the Breeding of Dahen 799 Broilers
- Evaluation and Analysis for Survey of Tea Production Quality Safety Management and Control
- Study on the Preparation of "Oil-tea" Instant Tea from the Compound Extract of Green Tea and Ginger
- Research Progress on Chemical Constituents and Pharmacological Effects of Zhuang Medicine Cocculus laurifolius DC.
- Study on Quality Standard of Lujing Yiqi Shengxue Pills
- Research on the Development of Guangxi Zhuang and Yao Ethnic Medicine Industry

