Construction of Expression Vector for Porcine Gastrin-releasing Peptide Fusion Protein
Zhiyu MA Jie ZHANG Junpei GUO Zhuo MA Chang YU Ying ZHANG Jinlong ZHANG


Abstract[Objectives] This study was conducted to obtain porcine gastrin-releasing peptide (GRP) fusion protein. [Methods]The constructed pET32a(+)-GRP plasmid was transformed into Escherichia coli BL21(DE3) competent cells to obtain the pET32a(+)-GRP-BL21(DE3) fusion protein expression strain, which was induced with 0.5 mM IPTG at 25 ℃ and 150 r/min for 12 h, and the His-tagged GRP fusion protein was detected by SDS-Page gel electrophoresis and Western Blot. [Results] After optimizing the IPTG-induced expression conditions, it was confirmed that the porcine GRP fusion protein was obtained, and the porcine GRP fusion protein was soluble, stable and highly active. [Conclusions]This study lays a foundation for the subsequent preparation of anti-pig GRP antibodies.
Key wordsGastrin-releasing peptide; Vector construction; Induced expression; Fusion protein; Pig
Gastrin-releasing peptide (GRP) is a neuropeptide isolated and purified from porcine gastric tissue in 1979. Both GRP and neuromedin B (NMB) are members of the mammalian bombesin (BN)-like peptide family[1-2]. The high-affinity receptor of GRP is the gastrin-releasing peptide receptor (GRPR), which has seven transmembrane domains and is a member of the G protein-coupled receptor family[3-4]. In vivo, GRP activates G protein-coupled receptor signaling pathways by binding to its receptors to play a variety of biological roles, such as stimulating smooth muscle contraction, regulating hormone synthesis and secretion, promoting normal or tumor cell growth, participating in regulating the body's immune function, regulating food intake, body temperature and circadian rhythm, and mediating behavioral responses (such as stress, memory)[3,5-6].
Since the discovery of GRP, researchers at home and abroad have conducted a lot of research on the expression and distribution of GRP and its receptors in humans, rats, mice and other animals in vivo, and found that GRP and its receptors are widely expressed in the central nervous system and peripheral tissues and organs[7-9]. At present, no specific antibody against porcine GRP has been found in the market. The amino acid sequences of human, rat and mouse GRP proteins share low homology with the amino acid sequence of porcine GRP (respectively: 68%, 70%, and 68%)[10], and the antibodies that detect human, rat and mouse GRP proteins cannot be used to detect porcine GRP protein. Pigs are the main economic livestock in China and one of the main sources of animal protein for people in China, and also serve as a kind of important model animal. Ensuring the health of pigs and improving the production performance and reproductive ability of pigs is an important responsibility of animal husbandry and veterinary work, and also provides important reference value for further research on human diseases. Therefore, this study intended to obtain porcine GRP fusion protein by constructing a porcine GRP fusion protein expression vector, which lays a foundation for the subsequent preparation of porcine GRP polyclonal antibodies.
Materials and Methods
Experimental materials
Main instrumentsThe main instruments used in the experiment included: constant temperature oscillation incubator (Benchmark, USA); ultra-low temperature refrigerator (Thermo Fisher, USA); ordinary constant temperature incubator (Shanghai Cimo Medical Instrument Co., Ltd, China); low temperature high-speed centrifuge (Sigma Aldrich Co., Ltd.); gel imaging system (Shanghai Tanon Technology Co., Ltd., China); autoclave (TOMY, Japan); ultrasonic breaker (Shanghai Yuejin Medical Instruments Co., Ltd., China); PCR instrument; protein electrophoresis instrument (US BIO-RAD company).
Main reagentsThe main reagents used in the experiment include: pET32a(+)-GRP recombinant plasmid (self-made); BCA protein detection kit, ampicillin sodium, IPTG, ultrasensitive chemiluminesent liquid (ECL) and His-tag protein purification kit (Shanghai Beyotime Biotechnology, China); protein prestained Marker and Anti-His Mouse Monoclonal Antibody (Beijing TransGen Biotech, China); 2×Taq Master Mix (Dye Plus) enzyme (Nanjing Vazyme Medical Technology Co., Ltd., Jiangsu, China); BL21 (DE3) competent cells (Wuhan Tiangen Biotechnology Co., Ltd.); DNA Marker and 10× Loading Buffer (Takara Bio Inc).
Acquisition of porcine GRP fusion protein
Acquisition of porcine GRP fusion protein expressing strain
The pET32a(+)-GRP recombinant plasmid was transformed into Escherichia coli BL21(DE3) competent cells to obtain the pET32a(+)-GRP-BL21(DE3) fusion protein-expressing strain, and the bacterial liquid was detected by RT-PCR.
IPTG-induced expressionAs an active inducer of β-galactosidase in bacteria, isopropyl-beta-D-thiogalactopyranoside (IPTG) can induce E. coli pET32a(+)-GRP-BL21(DE3) fusion protein-expressing strain to express porcine GRP recombinant fusion protein under specific conditions. Different induction temperatures (25 and 37 ℃), different concentrations of IPTG (0.1, 0.5, 1 and 1.5 mM) and different induction time (4, 6, 8, 12 and 24 h) were designed to screen the optimal induction conditions of IPTG.
SDS-Page gel electrophoresisThe expression of porcine GRP fusion protein induced by IPTG was detected by SDS-Page gel electrophoresis. Specifically, 5 ml of bacterial liquid was collected under each induction condition, and centrifuged to obtain bacterial precipitate, which was then re-suspended in PBS and added with protein loading buffer. The resultant liquid was heated at a high temperature (5-10 min in boiling water) for protein denaturation, and after centrifugation, the liquid was directly loaded for SDS-Page gel electrophoresis. After electrophoresis, the gel was stained with Coomassie brilliant blue for 2 h, and then it was washed several times with ddH2O until clear bands could be observed. According to the size and position of the porcine GRP fusion protein band, the expression of the fusion protein induced by IPTG was judged.
Purification and identification of porcine GRP fusion protein
Purification of fusion proteinsThe expression strain was induced and expressed under the optimal induction conditions of IPTG, and the obtained fusion protein was purified by a protein purification nickel column. Specifically, a 5 ml of bacterial liquid was taken and centrifuged to obtain the bacterial precipitate while discarding the supernatant. The precipitate was added with 100 μl of non-denaturing lysis solution (a mixture containing protease inhibitor cocktail) and re-suspended fully. Then, lysozyme was added to a final concentration of 1 mg/ml, and the obtained liquid was mixed gently and cooled in an ice bath for 30 min. Centrifugation was performed a 4 ℃ and 1 000 g for 10 min, and 10 μl of the supernatant was left for subsequent detection while the rest was collected to another new centrifuge tube. Next, 20 μl of well-mixed 50% His-tag Purification Resin was added to the tube, and the gel and lysate was equilibrated on a constant temperature shaker at 4 ℃ for 30 min to fully bind the His-tagged protein to it. After equilibration, centrifugation was performed to obtain gel precipitate, which was added with 40 μl of non-denaturing washing solution to re-suspend the gel, and the operation was repeated once. Then, 20 μl of non-denaturing elution was added to re-suspend the gel, obtaining a liquid, which was centrifuged at 1 000 g and 4 ℃ for 10 s to collect the supernatant, and the operation was repeated once. The collected supernatant was His-tagged porcine GRP fusion protein. The purification result of porcine GRP fusion protein was detected by SDS-Page gel electrophoresis and Coomassie brilliant blue staining, and the operation method was the same as above.
Western BlotThe porcine GRP fusion protein induced and expressed in this experiment contained His-tag, which could be detected by Western Blot. Specifically, the purified protein was subjected to SDS-Page gel electrophoresis. Then, transfer was performed, and the membrane was sealed with 5% skimmed milk powder at room temperature for 2 h. With His-tagged mouse monoclonal antibody as the primary antibody, incubation was performed at 4 ℃ overnight, and the membrane was washed with TBST. The membrane was then incubated with goat anti-mouse enzyme-conjugated secondary antibody at room temperature for 1 h, and washed. Finally, it was incubated with an ECL chemiluminescent solution for 1 min, and the membrane was placed in a fluorescence imager for exposure, and photographed for recording.
Results and Analysis
IPTG-induced expression
The expression strain verified to be positive was amplified, and expression was induced with IPTG under different conditions. After screening, it was found that the fusion protein had a high expression level in the supernatant at 25 ℃. Under the same temperature, the induction effect of 0.5 mM IPTG was better. SDS-Page gel electrophoresis was used to detect the expression effect of the fusion protein induced by 0.5 mM IPTG for different times (Fig. 1). The results of Coomassie brilliant blue staining showed that the effect of induction was better for 12 h. It could be seen that the optimal induction conditions for IPTG were 0.5 mM IPTG at 25 ℃ for 12 h.
Purification of porcine GRP fusion protein
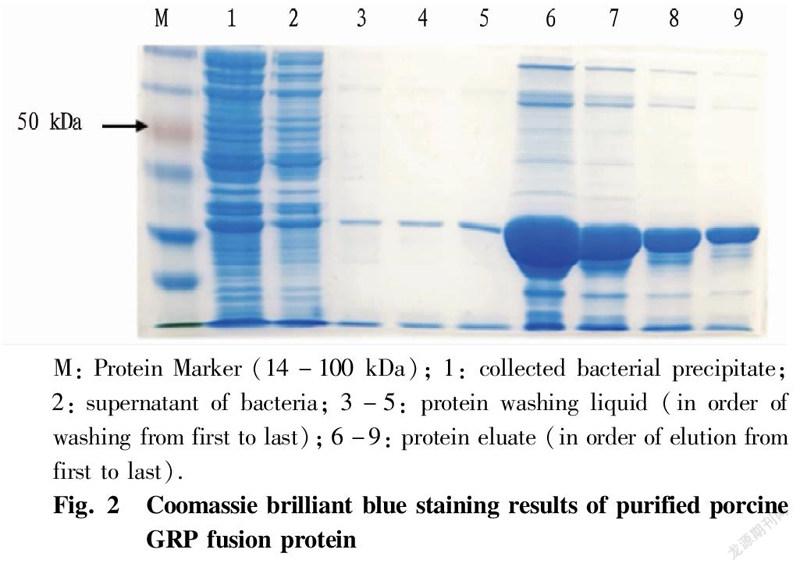
The porcine GRP fusion protein induced by IPTG was purified and detected by SDS-Page gel electrophoresis after purification (Fig. 2). The results of Coomassie brilliant blue staining showed that the concentration and purity of the purified protein were significantly improved. The analysis of the sequencing result of pET32a(+)-GRP plasmid showed that the constructed recombinant fusion protein of porcine GRP consists of 314 amino acids, contains 6 His-tags at the N-terminus, and has a predicted molecular weight of about 34 kDa.
Detection of His-tag of porcine GRP fusion protein
The purified fusion protein was further detected by the Western Blot method for the existence of His-tag (Fig. 3). The results showed that compared with the blank control, the purified fusion protein could be bound by the His-tagged antibody, indicating that the His-tagged porcine GRP fusion protein was successfully obtained.
Conclusions and Discussion
As a member of the mammalian bombesin-like peptide family, GRP has a wide distribution in animals[7]. Although GRP was originally discovered from the stomach of pig, the research on GRP mainly focuses on animals such as humans, rats, mice and chickens, and research in pigs needs to be supplemented. We successfully cloned porcine GRP gene in our previous study, and analyzed its gene and amino acid sequence. The homology comparison results showed that the porcine GRP amino acid shared low homology with human, rat and mouse GRP amino acid sequences, which was 68%, 70% and 68%, respectively[10]. Meanwhile, existing anti-GRP antibodies in the market cannot be used to detect porcine GRP protein. Therefore, obtaining porcine GRP protein is crucial for the preparation of anti-porcine GRP antibodies.
At present, the methods for obtaining porcine GRP protein mainly include artificial synthesis and genetic engineering expression systems. The artificially synthesized porcine GRP protein has the disadvantages of high production cost, poor polypeptide stability and activity and short storage time. Genetic engineering expression systems include yeast expression system and E. coli expression system, of which the yeast fermentation expression has the disadvantages of easy degradation and unstable expression, while E. coli expression has the characteristics of high expression level, easy control and low production cost[11]. To this end, we previously inserted the porcine GRP gene sequence into the prokaryotic expression vector pET32a(+) to construct a recombinant plasmid. In this study, the recombinant plasmid pET32a(+)-GRP was transformed into E. coli BL21(DE3) competent cells to obtain a fusion protein expression strain; and after optimizing the IPTG-induced expression conditions, the porcine GRP fusion protein was obtained by detection. The soluble, stable and highly active expression of porcine GRP fusion protein is realized, which lays a foundation for the subsequent preparation of anti-porcine GRP antibodies.
References
[1] MCDONALD TJ, NILSSON G, VAGNE M, et al. A gastrin releasing peptide from the porcine nonantral gastric tissue[J]. Gut, 1978(19): 767-774.
[2] MINAMINO N, KANGAWA K, MATSUO H. Neuromedin B: A novel bombesin-like peptide identified in porcine spinal cord[J]. Biochemical and biophysical research communications 1983(114): 541-548.
[3] GONZALEZ N, MOODY TW, IGARASHI H, et al. Bombesin-related peptides and their receptors: Recent advances in their role in physiology and disease states[J]. Curr Opin Endocrinol, 2008(15): 58-64.
[4] RAMOS-ALVAREZ I, MORENO P, MANTEY SA, et al. Insights into bombesin receptors and ligands: Highlighting recent advances[J]. Peptides, 2015(72): 128-144.
[5] CHOI Y, HEO SC, KIM YN, et al. Gastrin-releasing peptide (GRP) stimulates osteoclastogenesis in periodontitis[J]. Cells, 2020(10): 1-14.
[6] ROESLER R, KENT P, LUFT T, et al. Gastrin-releasing peptide receptor signaling in the integration of stress and memory[J]. Neurobiology of learning and memory 2014(112): 44-52.
[7] JENSEN RT, BATTEY JF, SPINDEL ER, et al. International union of pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states[J]. Pharmacol Rev, 2008(60): 1-42.
[8] MA Z, ZHANG Y, SU J, et al. Distribution of the pig gastrin-releasing peptide receptor and the effect of GRP on porcine Leydig cells[J]. Peptides, 2018(99): 142-152.
[9] SAYEGH AI. The role of bombesin and bombesin-related peptides in the short-term control of food intake[J]. Progress in molecular biology and translational science, 2013(114): 343-370.
[10] MA ZY. The distribution of NMB/GRP and its receptors in pigs and the mechanism of reproductive regulation in boars[D]. Nanjing: Nanjing Agricultural University, 2017. (in Chinese).
[11] XIE TB. Research progress on E. coli expression system[J]. Pharmaceutical Biotechnology, 2008(3): 77-82. (in Chinese).
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Report on the Breeding of Dahen 799 Broilers
- Evaluation and Analysis for Survey of Tea Production Quality Safety Management and Control
- Study on the Preparation of "Oil-tea" Instant Tea from the Compound Extract of Green Tea and Ginger
- Research Progress on Chemical Constituents and Pharmacological Effects of Zhuang Medicine Cocculus laurifolius DC.
- Study on Quality Standard of Lujing Yiqi Shengxue Pills
- Research on the Development of Guangxi Zhuang and Yao Ethnic Medicine Industry

