Study on Tissue Culture and Rapid Propagation of Tilia amurensis
Yinhua WANG Yuguang KONG2 Yingjun HE Liping YAN Dejun WU
AbstractTo explore the establishment of a tissue culture and rapid propagation system of Tilia amurensis, the effects of basic medium and concentrations and ratios of plant growth regulators on tissue culture and rapid propagation of T. amurensis were studied. The results showed that 1/2MS medium was the most suitable proliferation medium, and the proliferation coefficient could reach 13.5 after adding 0.05 mg/L 6-BA and 0.03 mg/L IBA; MS medium was the most suitable medium for strong plantlets and rooting, and the best medium for strong plantlets was MS+0.1 mg/L 6-BA+0.1 mg/L IBA+0.03 mg/L GA3, with which the average plantlet height reached 5.15 cm; and the best rooting medium was MS+1.0 mg/L6-BA+0.05 mg/L NAA, with which the rooting rate was 93.3% and the number of roots was 5.7 roots.
Key wordsTilia amurensis; Tissue culture; Medium for strong plantlets; Medium for rooting; Vegetative propagation; Rapid propagation
Tilia amurensis is a precious broad-leaved wood species, and was listed as a national class II precious protected plant in 1999[1]. T. amurensis trees have a beautiful appearance and excellent texture. T. amurensis has high ornamental value and is an excellent wood species. Meanwhile, it is also the main nectar source plant in Northeast China. The quality of linden tree honey is excellent and ranks first in export honey[2]. In addition, the flowers and roots of T. amurensis can also be used as medicines[3]. Over the years, due to people's excessive logging and lack of corresponding renewal protection measures, the number of this precious tree species has decreased sharply. In order to protect this precious resource, it is urgent to carry out research on efficient breeding methods.
At present, the propagation of T. amurensis is still mainly based on seedlings, but the seeds of T. amurensis must undergo certain special treatments to germinate smoothly[4-6], which takes a long time and easily causes uneven emergence, which limits the cultivation of T. amurensis seedlings[7]. Therefore, scholars have carried out research on the vegetative reproduction of T. amurensis[8-10]. Cutting propagation is widely favored due to its simple operation and fast propagation speed, but cutting propagation is easily restricted by seasons and cutting environment, so it has certain limitations, and it is difficult to meet the needs of T. amurensis seedling production.
Tissue culture is a vegetative propagation technology developed based on the totipotency of plant cells, and has been widely used in the cultivation of woody plants. Scholars have also carried out research on the tissue culture and rapid propagation of Tilia plants. Lyu[11] analyzed the organization of several Tilia species such as T. amurensis, Tilia mandshurica, and Tilia mongolica from the aspects of explant disinfection, basic medium selection, concentrations of plant growth regulators, rooting culture, and seedling training and transplanting. The cultivation technology has been systematically studied, and the corresponding regeneration system has been established. Wang et al.[12] used the terminal buds and lateral buds of T. amurensis at four different tree ages as materials to screen the basic medium and the types and concentrations of plant growth regulators for the tissue culture of T. amurensis. They believed that any mediums can be used for tissue culture of T. amurensis as long as appropriate exogenous hormones are selected. Zhang et al.[13] conducted a tissue culture study on the proliferation pathway of T. amurensis axillary buds, and pointed out that the basic medium suitable for T. amurensis growth was WPM medium. At present, the tissue culture technology of T. amurensis is immature, and the research results have not reached a relatively consistent conclusion, and a mature technical system has not yet been formed. In this study, a series of studies were carried out on aseptic seedling culture, proliferation culture and rooting culture of T. amurensis on the basis of the existing ones, so as to provide certain technical support for its factory plantlet raising.
Materials and Methods
Experimental materials
The experimental material was T. amurensis seeds, and collected from Quancheng Park, Jinan City. The mother tree was 20 years old, and the seeds had a 1 000-seed weight of 44.3 g.
Experimental methods
Aseptic seedling culture① Culture substrate treatment: The MS medium was replaced by ordinary river sand, and the river sand was mixed with water to keep the humidity at about 65%. The wet sand was divided into culture flasks for autoclaving, and sterilized at 121 ℃ and 0.12 MPa/cm for 20 min.
② Seed treatment: The seeds were added into an aseptic bottle, into which concentrated sulfuric acid (the amount of concentrated sulfuric acid was appropriate to not cover the seeds) was poured. The seeds were treated for 10-20 min with continuous stirring using a glass rod. The concentrated sulfuric acid was poured out, and the seeds were rinsed with sterile water several times until residual concentrated sulfuric acid was washed away. The seeds were soaked in 0.1% mercuric chloride solution for 5 min, and washed with sterile water for 5 times. They were then soaked in 500 mg/L aseptic GA3 solution for 6 h (the seed treatment process was carried out on a clean bench).
③ Acquisition of sterile materials: On a clean bench, the treated seeds were put into the wet sand described in "Aseptic seedling culture", with about 20 seeds per bottle. Then, the culture bottles were placed in a 0-4 ℃ environment for cultivation (with or without light conditions). At about 20 d, more than 90% of the seeds had cracked (some seeds had germinated radicles). Meanwhile, the culture flasks were transferred to a tissue culture room [daytime temperature (25±2] ℃, night temperature (18±2) ℃, and the seeds would germinate in 3-5 d.
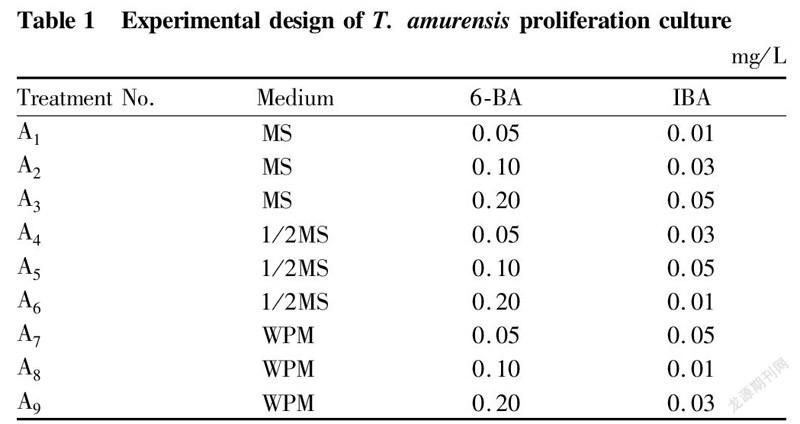
Proliferation cultureThe basic mediums, plant growth regulators and their concentrations were studied by orthogonal experiment method. The basic media were MS, 1/2MS and WPM, and the plant growth regulators were 6-BA and IBA. The obtained aseptic seedlings were cut into 1-2 cm stem segments with buds, which were inoculated to each medium, with 3 bottles for each treatment, 5 stem segments per bottle. The average proliferation coefficient was calculated after 30 d of cultivation [Equation (1)]. The experimental design is shown in Table 1.
Proliferation coefficient = Number of effective plantlets formed per bottle/Number of inoculated plantlets(1)
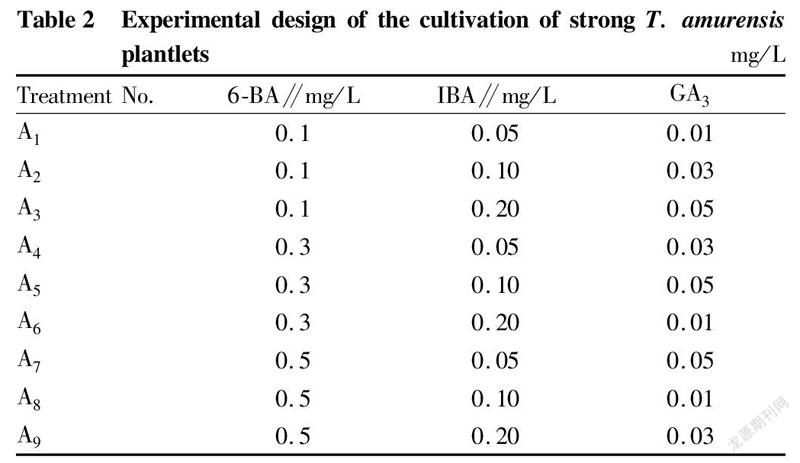
Cultivation of strong plantletsMS was used as the basic medium, and three plant growth regulators, IBA, 6-BA and GA3 (Table 2) were selected. Each medium was inoculated with 3 bottles, and each bottle was inoculated with 5 stem segments. The average plantlet height was calculated after 30 d of culture.
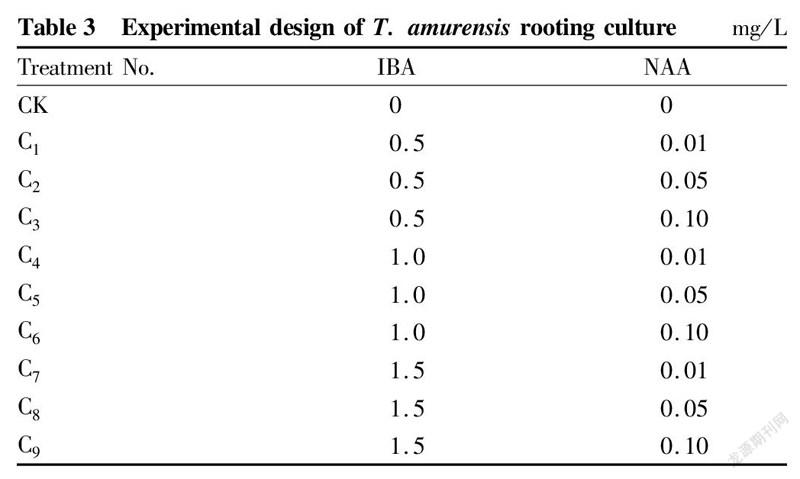
Rooting cultureWhen the test-tube plantlets were about 3 cm high, they were inoculated on MS medium, and different concentrations of IBA and NAA were added (Table 3). Each medium was inoculated with 3 bottles, 5 plants in each bottle. The rooting situation was observed and recorded after culturing for 20 d.
Statistical analysisAnalysis of variance and multiple comparisons were performed using SPSS 20.0 software, and graphs were drawn in Excel 2010
Results and Analysis
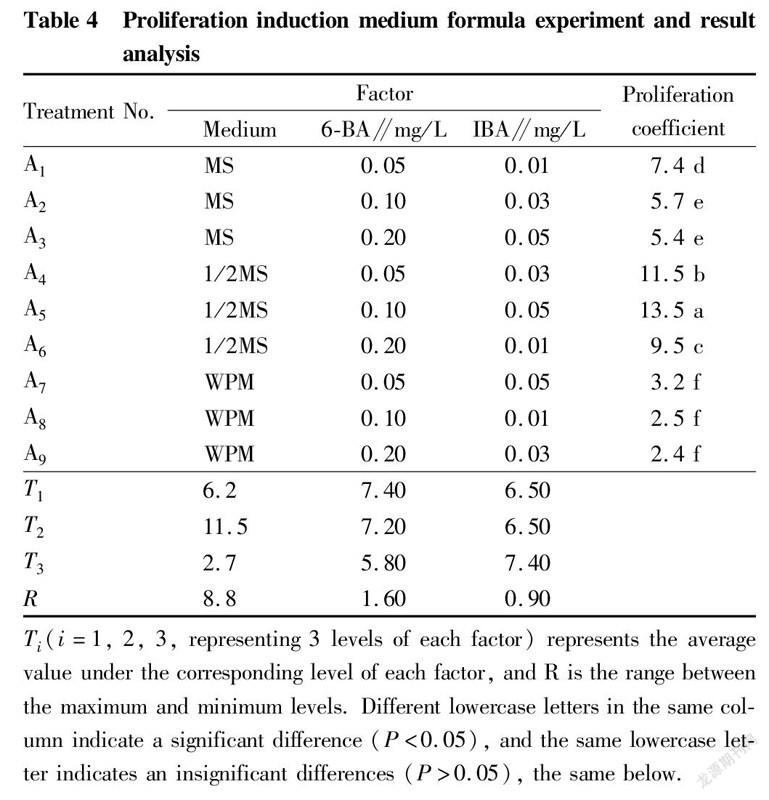
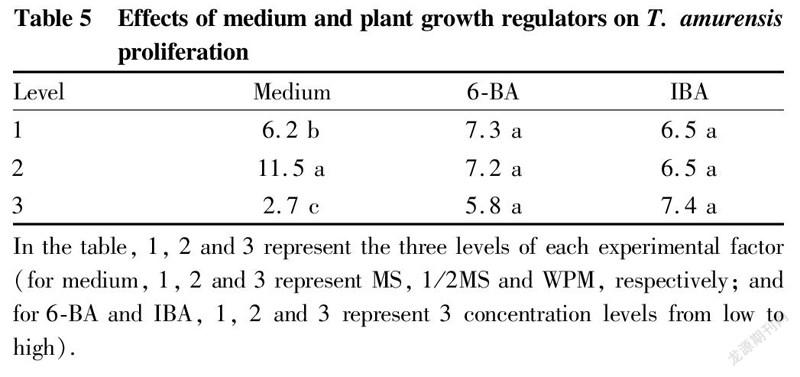
Effects of different mediums on the proliferation of test-tube T. amurensis plantlets
The proliferation of test-tube T. amurensis seedlings under various treatments is shown in Table 4-5. Among the 9 different treatments, the differences in proliferation coefficient reached a significant level (P<0.05). Among them, the proliferation coefficients of the three treatments (A, A and A6) in which the basic medium was 1/2MS were the most ideal. A5 showed the highest coefficient of 13.5, followed by treatment A4 with a proliferation coefficient of 11.5, and A6 treatment with a proliferation coefficient of 9.5. The proliferation coefficients of the three treatments (A, A and A) with MS as the basic medium were medium, and the proliferation coefficients of the three treatments (A, A and A9) with WPM as the basic medium were all lower. For the 3 different basic media, the average proliferation coefficient of test-tube plantlets on MS medium was 6.2, the average proliferation coefficient on 1/2MS medium was 11.5, and the average proliferation coefficient on WPM medium was the lowest, only 2.7. The range analysis of various factors showed that the range of the basic medium was the largest (R=8.8), which indicated that the type of medium had the greatest effect on the proliferation and culture of T. amurensis. The analysis of variance showed that the effect of medium type on the proliferation coefficient reached a significant level, while 6-BA and IBA had no significant effects on the proliferation coefficient within the concentration ranges set in this study (Table 5). The optimal level combination of the three factors was treatment A5 (1/2MS+0.1 mg/L 6-BA+0.05 mg/L IBA), which had the highest proliferation coefficient and was the best formula for the proliferation culture of T. amurensis, followed by treatments A and A.
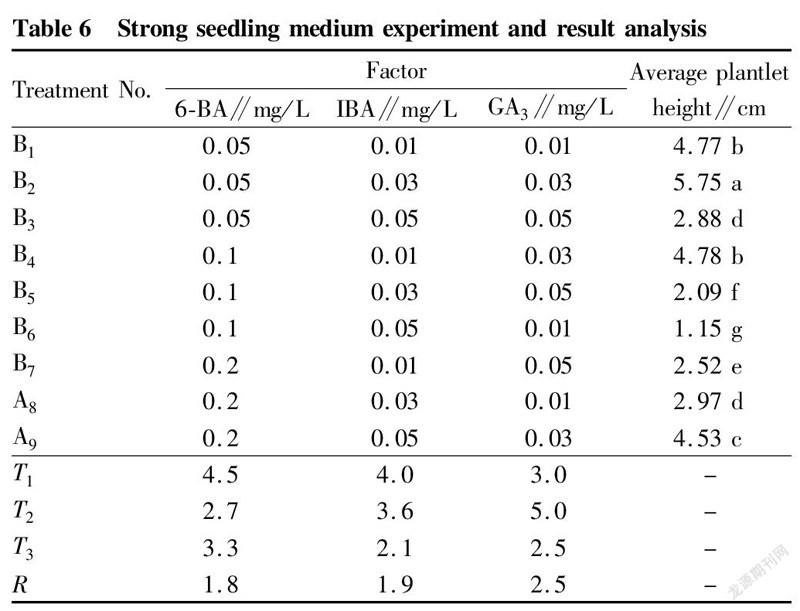
Effects of plant growth regulators on the strengthening of test-tube T. amurensis plantlets
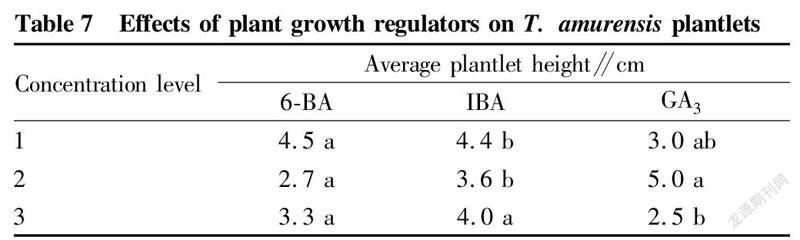
In this study, it was found that in the proliferation culture stage, the medium only added with 6-BA and IBA greatly improved the proliferation effect of T. amurensis, but the buds grew poorly, did not draw stems, and were short, so different concentrations of GA were added to the formula to promote the elongation and growth of buds. The results (Table 6) showed that after adding GA3, the elongation and growth effect of T. amurensis buds was significant. The growth condition of treatment B was the best, and the test-tube plantlets grew fast, had large and green leaves and reached 5.75 cm after 30 d of culture. Treatments B4 and B1 were the second, and the average seedling heights were 4.78 and 4.77 cm, respectively. The range analysis showed that among the three growth regulators, the factor with the largest range was GA, which had the greatest impact on the height of test-tube T. amurensis plantlets. Further analysis of variance was performed, and the results (Table 7) showed that both GA and IBA had significant effects on plantlet height, while 6-BA had no significant effect on seedling height. The best combination of the 3 factors was B, which was 0.05 mg/L 6-BA+0.03 mg/L IBA+0.03 mg/L GA.
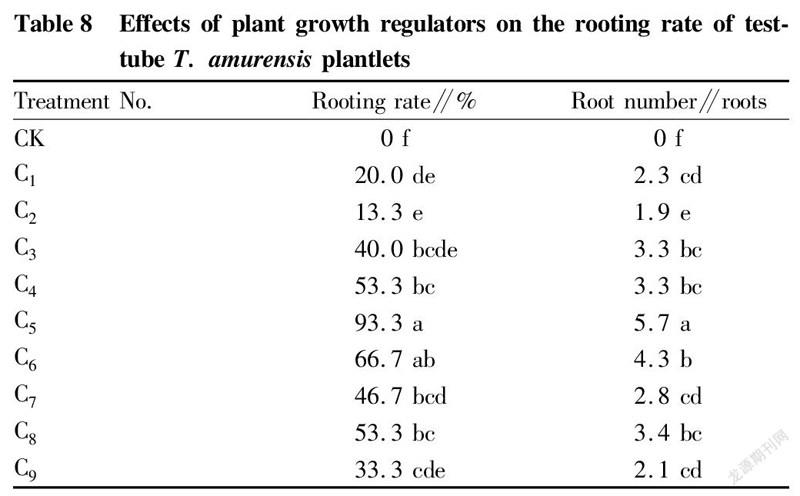
Effects of plant growth regulators on rooting of test-tube T. amurensis plantlets
When the test-tube plantlets grew to 2-3 cm, they were transferred to 1/2MS medium supplemented with different concentrations of plant growth regulators, and the 1/2MS medium without growth regulators was used as a control. The results (Table 8) showed that in the combinations of different concentrations of IBA and NAA, the differences in the rooting rate of test-tube T. amurensis plantlets reached a significant level (P<0.05). Among the 9 treatments, treatment C5 showed the largest rooting rate, reaching 93.3%, and the largest root number, with an average root value of 5.7 roots, and the rooting rate of the control was 0.
Conclusions and Discussion
Choosing an appropriate medium is the first step in doing a good job in tissue culture. Different plant species have different adapted mediums. In the research of tissue culture of Pholidota chinensis Lindl., the most suitable medium for seed germination and protocorm-induced proliferation is MS medium, and the most suitable medium for its pseudobulb induction is 1/2MS medium[14]. The most suitable medium for Rhododendron dauricum L. is WPM medium, and almost the explants cultured on MS medium will all die[15]. The basic medium suitable for Catalpa bungei propagation culture is DKW medium[16]. In the tissue culture work of Tilia plants, people have also carried out research and screening on the basic mediums. Liu[17] pointed out in his/her ue on the tissue culture technology of Tilia miqueliana Maxim. that MS medium was suitable for the adventitious bud induction and proliferation culture of T. miqueliana, which is consistent with the research conclusion of Tang et al.[18]. In the process of researching the tissue culture technology of T. amurensis, WPM was considered to be a more suitable medium for the induction and proliferation of T. amurensis axillary buds[11-13]. However, in this study, it was found that 1/2MS medium was more suitable for the proliferation and culture of test-tube T. amurensis plantlets, and WPM medium had the worst proliferation effect. Therefore, 1/2MS medium was selected as the basic medium for the proliferation and culture of T. amurensis, while MS medium was more suitable for the cultivation of strong T. amurensis plantlets.
Cytokinins and auxins are often used in combination in plant tissue culture work. Among them, 6-BA can significantly promote the formation of shoots, and it is more conducive to proliferation when used in combination with low concentrations of auxins[19]. Adding certain concentrations of 6-BA, NAA and IAA to modified MS medium can make the proliferation coefficient of Cotinus coggygria vat. ‘Zixia’ up to 8.97[20]. The synergistic effect of 6-BA with KT and IBA is most conducive to the proliferation and culture of Tetrathyrium subcordatum Benth.[21]. The combination of 6-BA and NAA can also achieve good proliferation effects in Leucanthemella linearis (Matsum.) Tzvel. and Salix suchowensis Cheng[22-23]. In this study, adding 0.05-0.20 mg/L 6-BA to 1/2M medium, together with 0.01-0.05 mg/L IBA, could effectively promote the proliferation and growth of test-tube T. amurensis plantlets, and the proliferation coefficient could reach 13.5.
Gibberellin (GA3) is often used for the proliferation and growth of tissue culture plantlets[24], and its physiological role is to promote cell elongation, thereby causing stem elongation and plant heightening[18]. Used in combination with cytokinins and auxins, it can effectively promote the elongation and growth of tissue culture plantlets[25]. In this study, GA3 played a very important role in the tissue culture of T. amurensis. When only 6-BA and IBA were added to the medium, the test-tube T. amurensis plantlets only proliferated and did not grow taller, but after adding an appropriate concentration of GA3, the elongation and growth of the test-tube plantlets could be significantly promoted, so that high-quality test-tube plantlets of T. amurensis were successfully obtained, which is consistent with the conclusion drawn by Tang et al.[18] in the study of T. miqueliana.
In this study, with T. amurensis seeds as the material, a technical system of aseptic plantlet culture, proliferation culture, strong plantlet culture and rooting culture was initially established. Specifically, the most suitable growth medium was 1/2MS+0.05 mg/L 6-BA+0.03 mg/L IBA, with which the proliferation coefficient was 13.5; the best strong plantlet medium was MS+0.1 mg/L 6-BA+0.1 mg/L IBA+0.03 mg/L GA3, with which the average plantlet height reached 5.15 cm; and the best rooting medium was MS+1.0 mg/L 6-BA+0.05 mg/L NAA, with which the rooting rate reached 93.3% and the number of roots was 5.7 roots. However, in this study, we have not found a suitable medium that can satisfy both the proliferation and growth of T. amurensis. It is the direction of continuing research in the next step, for establishing a mature T. amurensis tissue culture technology system as soon as possible.
References
[1] State Forestry Bureau, Ministry of Agriculture. List of national key protected wild plants in China (first group)[Z]. Beijing, 1999. (in Chinese).
[2] ZHUGE R, TANG Y. Morphological evolution and biogeography of Tilia[J]. Journal of Southwest Forestry College, 1995, 15(4): 1-14. (in Chinese).
[3] SHI FH, LU F, SHEN YB, et al. Research advances on Tiliaceae[J]. China Forestry Science and Technology, 2006, 20(1): 12-15. (in Chinese).
[4] ZHENG JH, LIN SJ, ZHANG YM, et al. Effects of NaOH treatment on breaking dormancy and physiological and chemistry characteristic of Tilia amurensis Rupr. seeds[J]. Chinese Agricultural Science Bulletin, 2014, 30(16): 35-40. (in Chinese).
[5] YANG LX, WANG HN, FAN J, et al. Seed treatments to overcome dormancy of Tilia amurensis[J]. Journal of Beijing Forestry University, 2011, 33(6): 130-134. (in Chinese).
[6] WANG HN. Study on dormancy mechanism and dormancy release technology of Tilia amurensis seeds[D]. Harbin: Northeast Forestry University, 2012. (in Chinese).
[7] LUO LF, WANG FX, ZHAO KT, et al. Study on fast germinating and seedlings of Tilia amurensis Rupr. seeds[J]. Journal of Northeast Forestry University, 1990, 18(2): 103-108. (in Chinese).
[8] YANG SZ, CHEN ZC, WANG B. Grafting technique of Tilia amurensis (Rupr.)[J]. Journal of Northeast Forestry University, 1992(6): 22-27. (in Chinese).
[9] ZHANG YF, CHEN WS, LIU GY, et al. The hardwood cutting techniques of Tilia amurensis[J]. jilin Forestry Science and Technology, 2002(2): 12-13. (in Chinese).
[10] WANG HN, SHEN HL, YANG LX. Techniques of softwood cuttings propagation in Tilia amurensis[J]. Nonwood Forest Research, 2012, 30(3): 106-110. (in Chinese).
[11] LYU XS. Studies on tissue culture of Tilia[D]. Baoding: Agricultural University of Hebei, 2004. (in Chinese).
[12] WANG YB, JIE YM, CHEN R, et al. Tissue culture of Tilia amurensis Rupr[J]. Protection Forest Science and Technology, 2002(2): 37-38, 46. (in Chinese).
[13] ZHANG JY, LYU YD, LI J, et al. Analysis of factors affecting rapid propagation of Tilia amurensis through axillary bud proliferation pathway[J]. Acta Agriculturae Jiangxi, 2018, 30(11): 23-26. (in Chinese).
[14] WU YH, YU HX, LI J, et al. Effect of medium on germination of Pholidota chinensis Lindl seeds[J]. Agricultural Engineering, 2019, 9(3): 107-111. (in Chinese).
[15] DU FG, LYU WW, WANG H, et al. Preliminary study on the selection of basic culture medium and explants of Rhododendron dauricum[J]. Journal of Anhui Agricultural Sciences, 2019, 47(12): 136-138. (in Chinese).
[16] MENG L, LIU Y, HE GX, et al. Multiplication and rooting culture of Catalpa bungei ‘Zhaoxia’[J]. Journal of Northwest Forestry University, 2019, 34(1): 119-123,156. (in Chinese).
[17] LIU F. Study on the tissue culture of Tilia miqucliana[D]. Nanjing: Nanjing Forestry University, 2007. (in Chinese).
[18] TANG SJ, LI NW, TANG KG. Establishment of the rapid propagation system of Tilia miqueliana[J]. Jiangsu Agricultural Sciences, 2008(6): 87-89. (in Chinese).
[19] ZANG WJ, CHEN Y, LI Q, et al. Tissue culture differentiation and callus induction of different explant from Pennisetum americanum×P. purureum[J]. Pratacultural Science, 2015, 32(9): 1451-1456. (in Chinese).
[20] WU F, ZHAO BB, WANG H, et al. Hormones effects on tissue culture of Continus coggygria var. ‘Zixia’[J]. Hubei Agricultural Sciences, 2017, 56(15): 2942-2946, 2958. (in Chinese).
[21] MENG L, LIU Y, HE GX, et al. Multiplication and rooting culture of Catalpa bungei ‘Zhaoxia’[J]. Journal of Northwest Forestry University, 2019, 34(1): 119-123,156. (in Chinese).
[22] SHAO BJ, LIU WZ, XU JX, et al. Preliminary establishment of rapid propagation of Leucanthemella linearis by tissue culture[J]. Chinese Agricultural Science Bulletin, 2019, 35(2): 62-67. (in Chinese).
[23] SUN YL, DAI XG, LI XP, et al. Plant regeneration of Salix suchowensis through tissue culture[J]. Journal of Nanjing Forestry University: Natural Science Edition, 2019, 43(2): 31-37. (in Chinese).
[24] FU XJ. The research on callus induction and subculture of Scutellarin bartata D. Don.[D]. Nanjing: Nanjing Normal University, 2016. (in Chinese).
[25] LIU J. Study on subculture proliferation and liquid rooting of gerbera[D]. Shenyang: Shenyang Agricultural University, 2016. (in Chinese).
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Report on the Breeding of Dahen 799 Broilers
- Evaluation and Analysis for Survey of Tea Production Quality Safety Management and Control
- Study on the Preparation of "Oil-tea" Instant Tea from the Compound Extract of Green Tea and Ginger
- Research Progress on Chemical Constituents and Pharmacological Effects of Zhuang Medicine Cocculus laurifolius DC.
- Study on Quality Standard of Lujing Yiqi Shengxue Pills
- Research on the Development of Guangxi Zhuang and Yao Ethnic Medicine Industry

