Freezing the conductance of platinum(II) complexes by quantum interference effect
Sai-Sai Yan,Jin-Yun Wang,Zi-You Pan,Da-Sheng Zheng,Qian-Chong Zhang,c,∗,Zhong-Ning Chen,c,∗
a Fujian Normal University,Fuzhou 350007,China
b State Key Laboratory of Structural Chemistry,Fujian Institute of Research on the Structure of Matter,Chinese Academy of Sciences,Fuzhou 350002,China
c Fujian Science &Technology Innovation Laboratory for Optoelectronic Information of China,Fuzhou 350108,China
Keywords:Platinum(II) complex Electronic effect Single-molecule conductance Quantum interference Magic ratio rule
ABSTRACT Understanding the impact of substituents on the quantum interference effect at single molecule scale is of great importance for the design of molecular devices.In this work,three platinum(II) complexes with–H,–NH2 and –NO2 groups on conductive backbones were designed and synthesized.Single-molecule conductance,which was measured using scanning tunnelling microscope break junction (STM-BJ) technique,demonstrated a conductance freeze phenomenon under the variation of substituents.Theoretical study revealed that,despite the electronic effect of the substituents shifting the energy level of molecular orbital,the quantum interference effect vanished the influence of electronic effect on the conductance and eventually leaded to the conductance freeze.
Quantum interference effect (QIE) is so important for charge transport at molecular scale that understanding the structural factor to manipulate QIE is of fundamental importance to the design of molecular devices [1–4].Although theσ-bond based QIE has been discovered [4],the relevant investigations on aromatic systems are still conducted vigorously,which limit the current QIE investigation to organic molecules.Metal-organic complex,which is a potential material for molecular device as important as the organic structures [5–7],in which the metal center (e.g.,Ru and Pt)participates in the conjugation system through dπ–pπconjugation and influences the energy levels of whole complex [5–7],offers a new candidate for understanding the QIE in nanoscale structures.Especially,the platinum complexes exhibit unique features by the Pt(II) taking part in the conjugation system with a restrained ratio [8].However,to our knowledge,the experimental investigation of quantum effect in metal-organic complexes has been rare conducted.
‘Magic ratio rule’(MRR) is a recent developed method to theoretically explain the QIE in aromatic systems [9–11].Compared with traditional graphic methods [12,13],MRR not only provides a quantitative ratio [10,14,15]to compare the conductance of corresponding molecules,but also provides the influence of electronic effect of substituents (called ‘pendant’group) on the interferometer group to the QIE on conductive backbone [14,15],from which the influence of electronic effect on the conductance could be enhanced or vanished by QIE.This implies a chance to investigate the QIE in metal-organic molecular devices by introducing electrondonating and electron-withdrawing substituents on the conductive backbone,of which the conductance is modulated by the combination of electronic effect and QIE.
Here we report the design of three platinum(II) complexes(1,1-NH and 1-NO,Fig.1) with Pt(II) center bonded to 4-(methylthiol)phenylethynyl as the conductive backbone.Electrondonating-NH2(1-NH) or electron-withdrawing-NO2(1-NO) was introduced to act as the ‘pendant’group interacting with QIE [14].The single-molecule conductance was measured by scanning tunneling microscope break junction (STM-BJ) technique [16–20]and demonstrated a conductance freeze in all three complexes.Theoretical study revealed that the influence of electronic effect,which was introduced by ‘pendant’group,was validated by the varied energy level of molecular orbital.However,the QIE analysis according to the description of MRR confirmed the vanishing of the influence of electronic effect on conductance,eventually leading to the conductance freeze.
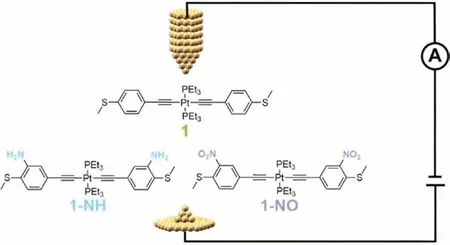
Fig.1.Molecular structures of complexes 1,1-NH and 1-NO,of which the singlemolecular conductance was measured by STM-BJ technique.
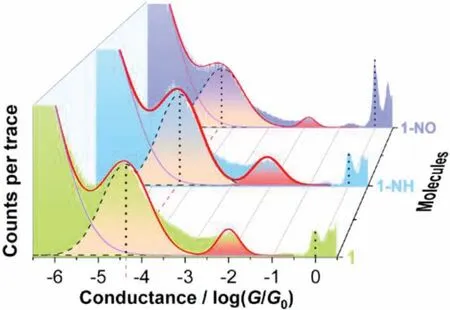
Fig.2.Conductance histogram for complexes 1,1-NH and 1-NO constructed from 3756,2185 and 2235 conductance-distance traces respectively.
Platinum(II) complexes 1,1-NH and 1-NO were synthesizedviamodified procedures according to previous reports [8].The single-molecule conductance was measured by STM-BJ technique(Fig.1 and Supporting information) [16–20].Fig.2 shows the histograms of complexes 1,1-NH and 1-NO,which are compiled by 3756,2185 and 2235 conductance-distance traces,respectively.By employing multimodal simulation,two peaks are clearly demonstrated in the 1D histograms of all three complexes.For each complex,the high conductance presented by a small peak around 10−2G0(G0is the quantum conductance equaling to 77500 nS) is attributed to the junctions formed by the gold electrodes connecting to one methylthiol group and the Pt(II) center [8,21].For complex 1,the main peak centered at 10−4.4G0matches well with the reported conductance [22–24]and represents the conductivity of the whole conductive backbone (from one methylthiol to another).For complex 1-NO with electron-withdrawing nitro group on conductive backbone,the conductance is intuitively expected to be varied from that of complex 1.However,the conductance of 1-NO is surprisingly identical to that of complex 1.More unexpectedly,not only the electron-withdrawing group substituted complex 1-NO,but also the electron-donating amino group substituted complex 1-NH demonstrates the unchanged conductance as that of complex 1.The frozen conductance implies that the electronic effect of the ‘pendant’groups exerts negligible influence on the charge transport at single-molecule conductance.
To validate the conductance and search the factor that the influence of electronic effect on conductance being vanished,the analyses of the junction length and 2D histogram were performed.
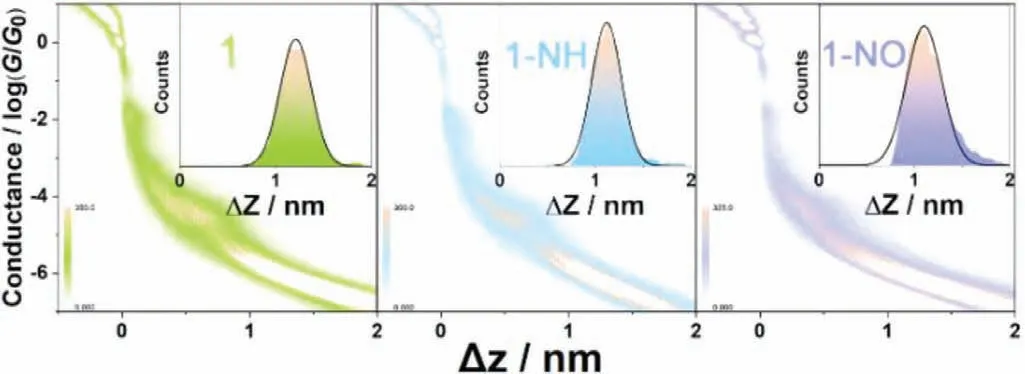
Fig.3.2D histograms for complexes 1,1-NH and 1-NO with the inset showing the corresponding statistical junction length.
As shown in Fig.3,the 2D histograms of the three complexes exhibit similar clouds around the conductance of 10−4.4G0.For complexes 1 and 1-NH,the clouds show flat plateaus indicating the stable formation of molecular junctions,whereas for complex 1-NO,the slightly oblique plateau implies that the steric hindrance of nitro group reduces the stability of molecular junctions.The snapback (0.5 nm) [25–27]corrected junction length of complex 1 is 1.7 nm,which matches perfectly with the optimized S-S distance of the complex.Probably caused by the steric hindrance,the statistical junction lengths of the substituted complexes 1-NH and 1-NO are slightly shorter than that of complex 1,where the snap-back(0.5 nm) corrected junction lengths are both 1.6 nm.Noticeably,according to the previous studies [26,28],the conductance would be more than one-order lower than that of complex 1 if the junctions formed through the connection of gold electrodes and the‘pendant’amino groups in complex 1-NH [28].The possibly low conductance is out of the effective detection range of our equipment,which makes it impossible to be observed in the conductance traces.Thus,the statistical junction length indicates that the three frozen conductance peaks at 10−4.4G0represent the real electron transport characteristics through the conductive backbones of complexes 1,1-NH and 1-NO,respectively.
To get insight on the interesting conductance freeze in the structures with electron-donating and electron-withdrawing groups,theoretical simulation combining density functional theory(DFT) with the nonequilibrium Green’s function (NEGF) was employed [29–31].The transmission coefficient (T(E)) and molecular energy spectrum,which are commonly considered in the analysis of single-molecule conductance modulation,were computed in the QuantumATK program [20,22].The most striking feature of theT(E) curve is the sharp peak for molecule 1-NO at 0.36 eV.This peak probably indicates a Breit-Wigner type resonance caused by the electron-withdrawing effect of nitro group,in which the electron cloud is localized on the nitro group and the affiliated phenyl ring (Fig.S4 in Supporting information) [32].Although the peak manifesting a transmission coefficient peak with the similar height of a molecular orbital (e.g.,LUMO of molecule 1 at 1.26 eV),the resonance behavior does not support an orbital existing at this energy level.The reasons include: (1) the UV-vis spectra showing almost identical absorption peaks around 340 nm (Fig.4 inset) for all three complexes,which implies similar energy gaps between HOMO and LUMO for the three complexes;(2) the transmission peaks not only caused by the electron resonating with molecular orbital but also brought by the electron resonating with part of the structure (e.g.,Fano resonance,see Supporting information) [33].To further prove the resonance peak,aldehyde group,which also provides strongly electron-withdrawing effect,was employed as the ‘pendant’group to investigate the resonance of 1-CHO (one aldehyde group) and 1-diCHO (two aldehyde groups on each phenyl ring) by simulation (Supporting information).The simulatedT(E) plots of 1-CHO and 1-diCHO clearly demonstrate the Fano resonance near the energy level of LUMO (Fig.S3 in Supporting information).The electronic density distribution at the energy level of the resonance peak (dip) indicates the high localized clouds around the electronic-withdrawing group on one side of 1-CHO or on both sides of 1-diCHO,which shows almost the same distribution as complex 1-NO (Fig.S4).The localized distribution results in the Fano resonance in 1-CHO and 1-diCHO and a Breit-Wigner type resonance in 1-NO [32].Hence,the LUMO of 1-NO should be the orbital at the energy level of 1.16 eV.
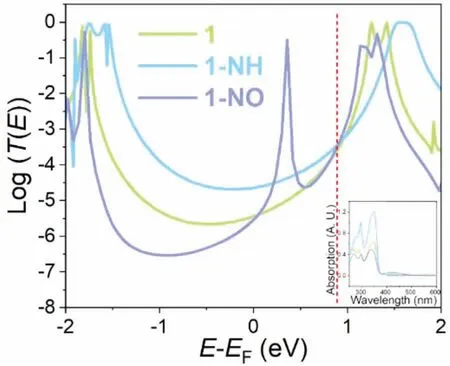
Fig.4.Transmission spectra for complexes 1,1-NH and 1-NO with the red dash line marking the possible EF and inset showing the UV-vis absorption spectrum.
The Fermi energy (EF) of the gold electrode,which locates in the HOMO-LUMO gap,unfortunately,fails to be accurately predicted by DFT method [32].To this end,some methods have been employed to correct theEF[34],among which fitting theT(E) according to the experimental result is the most convenient way [8,14,35,36].Thus,according to the measured conductance,theEFhere should be corrected to the energy about 0.92 eV,where theT(E) of all of the three complexes including 1,1-NH and 1-NO converge to an identical value,indicating the equivalent conductance as measured by experiment.
As shown in Fig.4,for complex 1 (green line),the peaks at-1.84,-1.74,1.26 and 1.42 eV indicate the orbital energy levels of HOMO-1,HOMO,LUMO and LUMO+1,respectively.Owing to electron-donating effect of the amino group,both HOMO and LUMO in complex 1-NH (blue line) shift to higher energy levels relative to those of complex 1.Opposite to 1-NH,the HOMO and LUMO for complex 1-NO (purple line) with electron-withdrawing nitro group,move to lower energy levels relative to those of molecule 1.The energy level shift of complexes 1-NH and 1-NO confirms the electronic effects of amino and nitro groups on the conductive backbones.According to the above correctedEF,all the three molecules exhibit LUMO dominated charge transport,of which the conductance should theoretically increase with the decrease of LUMO energy or decrease with the increase of LUMO energy.Thus,the conductance freeze of the three complexes implies that the energy effect of molecular orbital contributes little to the conductance.
Finally,we turn to the effect of quantum interference in this conjugation system.Since the phenyl ring being a well-known interferometer,by which the destructive quantum interference and constructive quantum interference are characterized in metaconnected structures and para-/ortho-connected structures [1],the‘pendant’group substituted phenyl group of phenylethynyl ligand is considered the interferometer to analysis the MRR [14].On the phenyl group,the combined constructive/destructive QIE of the three sites connecting ethynyl group,methylthiol group and ‘pendant’group determines the finally resultant conductance.As the MRR description [14],amino (1-NH) or nitro (1-NO) group plays the role of the ‘pendant’group that changes theπ-orbital energy of the connecting site with the electronic effect by the quantitative parameter ofε.The expression of Green’s function (GF),which determines the transmission coefficient and conductance,is simplified as Eq.1 when the energy of transporting electron equals toEF:

Whereiandjare the sites on phenyl ring connecting ethynyl and methylthiol,kis the site connecting the ‘pendant’group,Gij(0)is the GF of the molecule in the presence of ‘pendant’group,andgij(0) is the GF of the molecule without ‘pendant’group.Theg(0)is proportional to the magic number Mij.The magic number (M)equals to 0 when the two sites are the same or in meta-position to each other.Since ethynyl and ‘pendant’group are mutually located at the meta-position of phenyl ring,thegik(0) is zero for complexes 1-NH and 1-NO,respectively,which leads to the GF with the ‘pendant’group (1-NH and 1-NO) equaling to that without the‘pendant’group (1).This indicates that,for complexes 1-NH and 1-NO,the electronic effect of the amino and nitro group is vanished by the QIE and eventually results in the conductance freeze in the three complexes.
In conclusion,three conductive Pt(II) complexes with the same conductive backbones but different ‘pendant’substituents at phenyl rings were elaborately designed.The single-molecule conductance measured by STM-BJ technique shows an interesting conductance freeze at 10−4.4G0under the varied electronic effect of ‘pendant’group.Although the theoretical study confirms the distinct influence of electronic effect on molecular orbital energy level,the QIE described by MRR eliminates the influence of the electronic effect and results in the conductance freeze.This study not only confirms the metal-organic complex to be a functioning structural base for the QIE described by MRR,but also provides a structural awareness for modulating the conductance of molecular devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful for financial support from the National Natural Science Foundation of China (No.92061117),the Strategic Priority Research Program of the Chinese Academy of Sciences (No.XDB20000000) and Fujian Science &Technology Innovation Laboratory for Optoelectronic Information of China (No.2021ZR129).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.092.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
