Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
Jinbin Yng,Yn Xu,b,c,∗
a Department of Chemical Engineering,Graduate School of Engineering,Osaka Prefecture University,Sakai,Osaka 599-8570,Japan
b Japan Science and Technology Agency (JST),PRESTO,Kawaguchi,Saitama,332-0012,Japan
c NanoSquare Research Institute,Research Center for the 21st Century,Organization for Research Promotion,Osaka Prefecture University,Sakai,Osaka 599-8570,Japan
Keywords:Sub-single cellular matter Single cell Single molecule Extracellular vesicles Nanochannel Femtoliter Attoliter Digital Information sciences
ABSTRACT In the field of cell studies,there is a burgeoning trend to further downscale the investigation from a single-cell level to a sub-single-cell level.Subcellular matter is the basic content in cells and correlates with cell heterogeneity.Sub-single cellular studies focus on the subcellular matter in single cells and aim to understand the details and heterogeneity of individual cells in terms of the subcellular matter or even at the single component/vesicle/molecule level.Hence,sub-single cellular studies can provide deeper insights into fundamental cell biology and the development of new diagnostic and therapeutic technologies and applications.Nonetheless,the contents of a single cell are not only ultra-small in volume but also extremely complex in composition,far exceeding the capabilities of most tools used in current cell studies.We believe that nanofluidics holds great potential in providing ideal tools for sub-single cellular studies,not only because of their capability to handle femtoliter/attoliter-scale samples,but also because of their possibility to manipulate and analyze subcellular matters at the single component/vesicle/molecule level in a high-throughput manner.In this review,we summarize the efforts in the field of nanofluidics for sub-single cellular studies,focusing on nascent progress and critical technologies that have the potential to overcome the technical bottlenecks.Some challenges and future opportunities to integrate with information sciences are also discussed.
1.Introduction
People can learn the large,general “whole” from the small,specific “part”.In nature,if organisms are considered as the “whole”,the cells are then the “part”.Cells are the building blocks for tissues and organs and serve as the basic functional units of organisms.Various heterogeneous cells render organisms diverse.Hence,studying cells is a reliable pathway for investigating different organic lives,leading to a further understanding of the origin and development of organisms,which finally contributes to analytical sciences,modern biology,drug discovery and medicine [1–6].
The evolution of cell studies and their related strategies are shown in Fig.1.In the past,cell studies,attributed to optical microscopic technologies,enabled the classification of cells and the understanding of cellular structures and behaviors at the population level.Hence,it is possible to study the morphology and functional differences of different cell types at the microscale level[7–10].With the development of chemical,biological,and analytical technologies,the contents of cells,which are the so-called“subcellular matter,” could be investigated using strategies such as enzyme-linked immunosorbent assay and western blotting analysis.Subcellular matter,including many types of biomolecules (such as proteins and nucleic acids),subcellular components (such as membranes,cytoskeleton,genetic materials and organelles),and vesicles (within or outside a cell),are the basic materials and information carriers of cell structures,functions,processes,and activities.As a development of cell studies at the population level,subcellular studies have permitted the investigation of the contents of cells and the understanding of cells at a much smaller scale than before,revealing the content composition of different cells at the population level [11–14].However,in those traditional cellular and subcellular studies,their investigation targets are a large group of cells.They are usually limited to obtaining homogeneous results,which means it is difficult to recognize the difference between individual cells.To advance,single-cell studies have been highly developed,permitting the analysis of cells one by one owing to the advances in single-cell manipulation and detection technologies such as microfluidics,flow cytometry,fluorescence imaging,and label-free detection [15,16].In this way,the results obtained are not the average,continuous signals at the cell population level but discrete signals for the individual objects.Thus,information on the heterogeneity of individual cells in terms of a whole-cell can be obtained [17–20].Nevertheless,the information obtained from current studies is insufficient to fully understand and elucidate the heterogeneity of individual cells in terms of subcellular matter.In other words,the heterogeneity inside a single cell is still largely unclear.
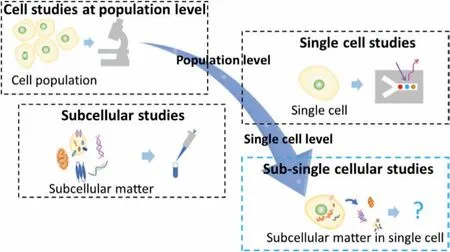
Fig.1.Schematic of evolution of cell studies and relevant strategies.
It is believed that complete elucidation of the nature of the heterogeneity of cells depends on the development of cell studies at the sub-single-cell level,which is defined and referred to here as sub-single cellular studies.Sub-single cellular studies are considered as an evolution of the field of cell studies.Sub-single cellular studies comprise a variety of investigations on subcellular matter in a single cell,aiming to understand the details and heterogeneity of individual cells in terms of subcellular matters or even at the single component/vesicle/molecule level.In contrast to single-cell studies,sub-single cellular studies primarily focus on subcellular matters,including intracellular,intercellular,and extracellular matters.In addition,unlike conventional subcellular studies that concentrate on subcellular matters from cell populations,sub-single cellular studies focus on subcellular matters from individual cells and aim to investigate single cells at the molecular level or even at the single molecule level.As they provide extensive information regarding individual cells,these subcellular matters would be promising biomarkers for future biological analysis,offering a new path to elucidate the heterogeneity of cells and understand biology in a much deeper and more precise manner.However,exploration at the sub-single-cell level is still rare and remains a significant challenge because of the lack of proper strategies and tools.
Subcellular matter from single cells can be considered as nanoscale objects in samples with ultra-small volumes (generally at the picoliter (10−12L) order for a single mammalian cell)and extremely complicated compositions.Nanoscale objects usually perform active Brownian motion in a liquid sample,making it difficult to observe and detect at a single object level.Thus,measures to restrict and suppress the Brownian motion of objects are required.In the meantime,to handle picoliter-order samples,tools with feature volumes in the order below picoliter for liquid handling are also necessary.Moreover,because of the complicated composition of samples,it is difficult to reveal the heterogeneity at the single component/vesicle/molecule level using conventional approaches.Conventional approaches for population-level analysis are primarily based on concentration,which means considering many subcellular matters as an entirety (compared to an individual).Hence,approaches based on the number of molecules and particles are required,allowing the individual analysis of the subcellular matter.These are obstacles to sub-single cellular studies that need to be overcome.
Nanofluidics is a study of the understanding and application of fluids confined in nanoscale structures.Nanofluidics is usually considered a realm that evolved from microfluidics but is not a mere extension of microfluidics [21–24].New physical phenomena and effects (e.g.,non-linear transport mechanisms [25–33]and altered liquid properties [34–38]) that are not observed in bulk or at microscales start to emerge and dominate at nanoscales,opening up an unchartered research territory for exploring new scientific insights and applications of fluids [39–48].Such new phenomena and unusual effects have been gradually unveiled and exploited using silicon/silicon nitride-based nanofunnels [49],nanotubes [50],nanopores [51],nanopipettes [52],nonporous polymer membranes [53],2D material membranes [54],and recently chip-based nanofluidic devices [55].Chip-based nanofluidic devices (referred to as “nanofluidic devices” in this review) are planar,transparent,lithography-based devices with nanoscale sizeregulated nanochannels [56–59].Owing to their ease of coupling with a variety of microscopes,analysis/measurement instruments,functional equipment,and new advanced technologies,chip-based nanofluidic devices have great potential for diverse applications in fields of chemistry [60],physics [61],biology [62],diagnostics [63],medicine [64],photonics [65],energy [66],and information science[67],among others.
The use of nanofluidic devices not only allows for precise and parallel (high-throughput) handling of samples with volumes ranging from picoliter to zeptoliter (10−21L) order,but also offers confined nanospaces where Brownian motions of nanoscale objects can be effectively restricted,being favorable for manipulation,observation,detection,and analysis of single nanoscale objects.In addition,nanochannel structures of nanofluidic devices can be used to mimic a variety of subcellular environments.Such features would be very helpful in obtaining authentic and fresh,detailed biological information on subcellular matters,thereby revealing unknown cellular phenomena and unusual biological effects occurring in the nanoscale compartments inside a single cell.These advantages meet the requirements of sub-single cellular studies,allowing for the sampling,handling,isolation,capture,immobilization,arraying,observation,detection,and analysis of subcellular matter even at a single object level.Furthermore,these advantages would give rise to a way to perform cell studies in a revolutionized manner based on the number of target molecules and particles rather than conventional methods based on concentration,which is not enough to precisely describe and fully understand subcellular phenomena and processes in a single cell.
In addition,recently,some state-of-the-art technologies and methodologies such as nanopipettes and super resolution fluorescence microscopy have been applied to the handling of contents of a single cell and imaging of subcellular matter in a single cell[68,69].However,their further applications in sub-single cellular studies are greatly restricted by critical issues such as low throughput,difficulties in quantification,and high complexity in operation.Further integrating those technologies with nanofluidic devices will not only resolve these critical issues but also provide opportunities to build new systems allowing for much more comprehensive,more precise,and more detailed sub-single cellular studies in a simple,quantitative,and high throughput way.Therefore,the use of nanofluidic devices is a promising and key strategy for advancing sub-single cellular studies (Fig.2).
This promising strategy will bring a new and exciting direction in both cell research and nanofluidics,despite only a few exploratory studies associated with sub-single cellular studies using nanofluidics reported in the past few years.The primary purpose of this review is to provide a bird’s eye view of this exploratory field,focusing on its current status,critical technical issues,and future perspectives,rather than a complete review of the whole field.First,the nascent progress in this field is highlighted,followed by a discussion on the technical bottlenecks that impede sub-single cellular studies using nanofluidics.Some recently developed critical nanofluidic technologies that have the potential to overcome these technical bottlenecks are highlighted in terms of fabrication,sampling,handling,arraying,and detection.Finally,perspectives including challenges and opportunities in this field are described,followed by an outlook on a future trend possibly initiated by the fusion of nanofluidic sub-single cellular studies and information sciences.
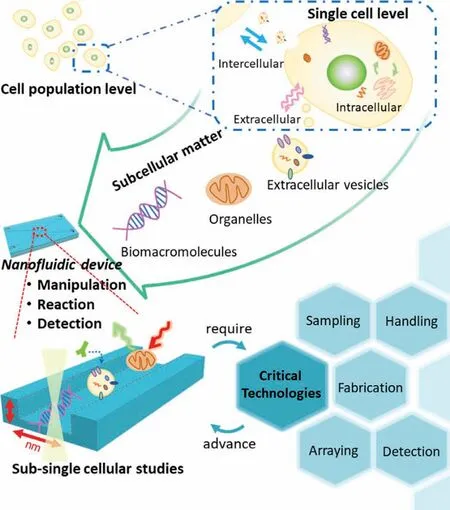
Fig.2.Conceptual drawing of sub-single cellular studies enabled by a nanofluidic strategy.
2.Nascent progress
Currently,although the field is still in the nascent stage,a few explorations have been made.Herein,we highlight some of them.
Kantet al.reported a nanofluidic method for single cell lysis and nucleic acid extraction (Fig.3a) [70].A single cell was trapped by the structure on one side of the focus ion beam (FIB)-milled nanochannel,which functioned as a connector between two microchannels,and the lysis solution was introduced from the opposite side (Figs.3b and c).Finally,the released sub-single cellular matters,including nucleic acids,were extracted through the nanochannel,which only permitted the passage of nanoscale objects.This is a simple demonstration of single-cell-level sampling using a nanofluidic device with a bare nanochannel.
As a step forward,Linet al.utilized lipid-modified nanochannels for on-chip sampling from a single living cell in a micro space[71].In their study,the cell membrane was fused with a lipidmodified nanochannel.The micro-nano interfacial structure and the biocompatible nanochannel used in their study allowed the extraction of subcellular matter from living single cells and subsequent analysis.During the sampling processes,while nothing was observed in the nanochannel without the lipid bilayer,approximately 39 fL samples were observed in the nanochannel with the lipid bilayer.
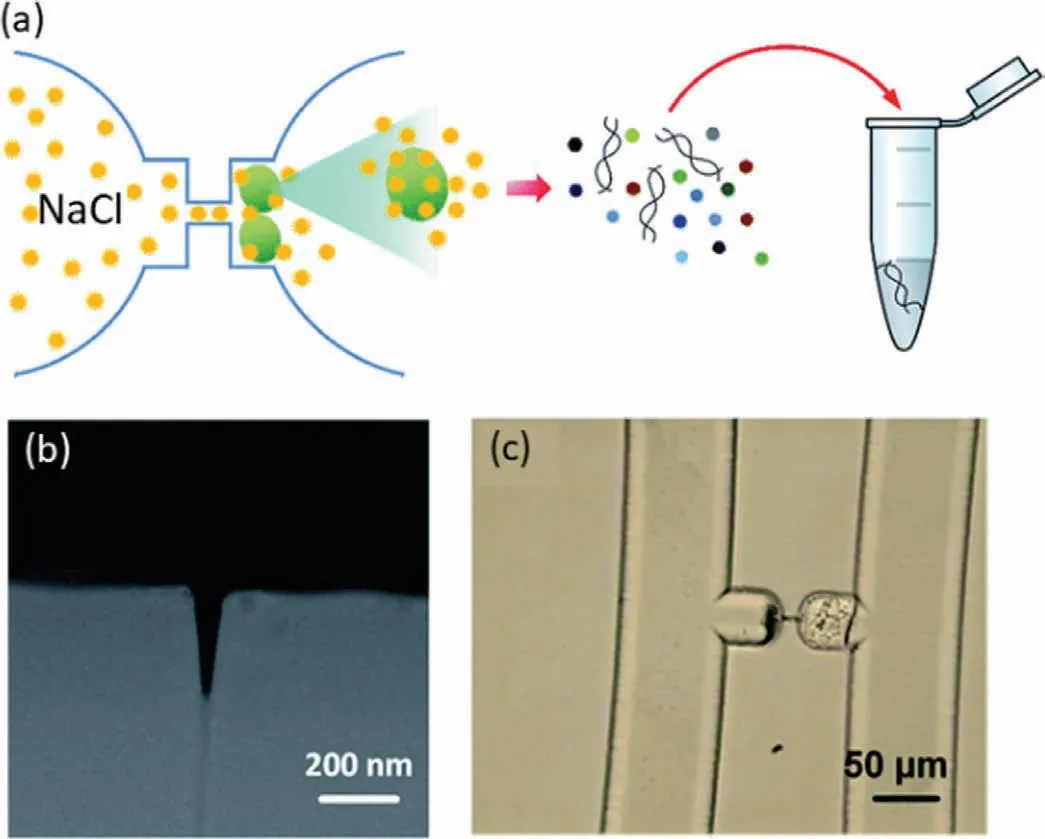
Fig.3.Single cell-level sampling technology using nanofluidic device.(a) Schematic of cell trapping,lysis and matter extraction using nanofluidic device.(b) SEM image of the FIB-milled nanochannel.(c) Optical image of the trapped cells after the lysis.Reproduced with permission [70].Copyright 2015,the Royal Society of Chemistry.
Besides the sampling,the trapping,restriction,and control of sub-single cellular matters can also be achieved using nanochannels.Zandet al.demonstrated a nanofluidic platform for trapping isolated mitochondria [72].Soft lithography-based polydimethylsiloxane nanofluidic channels with trapezoidal cross-sections functioned as capturers were used to trap the nanoscale mitochondria in their study.As a result,subsequent analysis can be easily achieved using microscopy.Yuet al.developed a dynamic and tunable confinement strategy for DNA linearization [73].By controlling the elastomeric collapse,nanochannels with confining dimensions down to 20 nm can be created and tuned dynamically,and the DNA molecule can be uncoiled and threaded into the as-formed nanochannel gradually.
It is noteworthy that nanofluidic devices also have the potential to deliver matter into single cells.Boukanyet al.presented a nanofluidic-based electroporation (NEP) strategy as a delivery technology for single cells [74,75].Utilizing a nanochannel between two microchannels,the substances (e.g.,nanoparticles and oligonucleotides) could be delivered to a living cell after applying an electric field (Fig.4a).Lipoplex nanoparticles containing FAM-labeled oligodeoxynucleotides were delivered to A549 cells using the NEP method,suggesting that this strategy can modify and investigate single cells conveniently and efficiently that would markedly advance the sub-single cellular studies (Fig.4b).
3.Critical technologies
The above-highlighted studies demonstrated some significant attempts to develop sub-single cellular studies.However,subsingle cellular studies are still in a nascent period because of the following bottlenecks which currently restrict the development of sub-single cellular studies using nanofluidic devices.First,current technologies for fabricating nanochannel structures are still lacking,resulting in difficulties to fabricate nanofluidic devices with special functional nanochannel structures suitable for sub-single cellular studies.Second,the strict condition,especially the extremely high temperatures (above 1000 °C) and the high vacuum,required in the bonding of glass nanofluidic devices has greatly impeded the further integration of functional components required by sub-single cellular studies.Third,mechanisms of regulation of nanofluidic flows are still lacking,and new mechanisms with high precision,accuracy,and flexibility need to be developed to meet the requirement in sampling sub-single cellular matter.Fourth,development of the mechanisms and methodologies for isolating,capturing,arraying,trapping and manipulating sub-single cellular matter in nanochannels is urgent but greatly challenging.Fifth,although a variety of state-of-the-art methods and technologies of single-particle measurement and single-molecule detection have been developed recently,how to fuse them with nanofluidic devices is a critical issue.Sixth,most nanofluidic devices only permit single use,which will impede their practical applications in subsingle cellular studies.Hence,new mechanisms and novel technologies are required to conquer these bottlenecks.In this section,some selected critical technologies,most of which have not been applied to sub-single cellular studies but are greatly promising for overcoming these bottlenecks,are introduced.
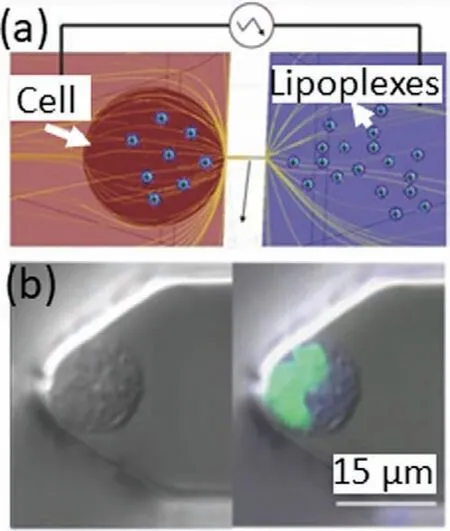
Fig.4.Delivery technology for single cell using nanofluidics.(a) Schematic of nanochannel electroporation (NEP) method for the injection of lipoplex nanoparticles into cell.(b) Confocal microscopy images for the confirmation of lipoplex nanoparticles injection by NEP.Reproduced with permission [75].Copyright 2014,Wiley-VCH.
3.1.Fabrication of functional nanochannels
Owing to sizes and volumes of nanochannels,nanofluidic devices have the potential to handle sub-picoliter volumetric samples and manipulate nanoscale objects,which match the requirements of sub-single cellular studies.The fabrication of nanochannels was a challenge.Microfabrication technologies such as photolithography and soft lithography,which are widely employed in the fabrication of microfluidic devices,have exhibited limited capability to fabricate nanochannels due to the diffraction limit of light.Owing to the highly developed nanofabrication technologies of microelectronics,nanochannel fabrication on solid,planar substrates has been achieved in the past decades [76–81].Among them,electron beam (EB) lithography [82],FIB [83],and nanoimprint (NIP)[84]become major methods to fabricate nanochannels.
Some strategies have been developed for functionalizing nanochannels.Surface modification of wall of nanochannels with functional materials is an effective way.For example,lipid-bilayermodified nanochannels with biological activity and selectivity have been proposed [85–87].The use of such modified nanochannels allows for better handling,detection,and analysis of sub-single cellular matters [88–90].
Rather than the modification of entire surface of nanochannels,the integration of functional components at the desired places of nanochannels is usually needed but quite difficult.Xuet al.has developed a technology enabling to site-specifically integrate a variety of functional components inside tiny nanochannels.The technology is called as nano-in-nano integration [91,92].Nanoscale pillars and metallic arrays can be fabricated inside channels with high precision and accuracy.Such nano-in-nano components hold the potential for sampling,handling,filtering,restriction,immobilization,and detection of sub-single cellular matter in the future.
3.2.Sampling at sub-picoliter order
Sampling of subcellular matter from a single cell is a challenge because the volume of the sample is extremely small,generally at sub-picoliter order.Simple nanochannels without additional functional structures or components are difficult to handle such small sample.Nakaoet al.developed and integrated a femtoliter volumetric pipette and flask into a nanofluidic device to bridge this gap[93].By utilizing surface tension and air pressure control,a welldesigned nanochannel consisting of an 11 fL volumetric pipette and a 50 fL flask successfully generated and transported femtoliterscale droplets after a series of processes (Fig.5a).As shown in the optical microscopic images in Fig.5b,water was first filled in the fL volumetric pipette channel controlled by surface tension.Thereafter,air pressures from the vertical channel and channel outlet were applied precisely to cut and form an fL droplet.Finally,the fL droplet in the pipette channel was transported into the fL flask via the removal of air pressure from the channel outlet.Such capabilities well meet the requirement of sub-single cellular study.Hence,the technology is very promising for handling subcellular matter from a single cell in the future.
3.3.Handling of sub-picoliter liquids
In sub-single cellular studies using nanofluidic devices,samples and reagents must be well handled in ultra-small nanochannels for subsequent restriction,detection,and analysis of the subcellular matter.Regular fluidic handling and manipulation processes such as transportation,mixing,and separation in nanofluidic devices are necessary.However,they are still challenging because of not only the ultra-small volumes of the sub-single cellular samples but also the highly closed nanofluidic spaces.Valves are usually used to regulate fluids [94,95],but the integration of valves in nanochannels has been a challenge.Recently,passive valves for nanofluidic control have been developed but they usually only permit single-use [96,97].More recently,Xuet al.have developed soft matter-regulated active nanovalves locally self-assembled in femtoliter nanochannels by using their nano-in-nano integration technology [98].The use of the active valves made it possible to actively regulate femtoliter-order fluids for the first time.Some other nanofluidic active valves based on glass deformation [99]and laser-induced nanobubbles [100]have been demonstrated by Kitamoriet al.We believe that these progress in the handling of sub-picoliter liquids would finally contribute to sub-single cellular studies.
3.4.Molecular nanoarray in nanochannels for high-throughput analysis
For sub-single cellular studies using nanofluidics,array technologies will be significant and critical because high-throughput processing capability can be obtained by arraying.The aforementioned nano-in-nano integration technology has excellent potential to form functional arrays in nanofluidic channels.As an early exploration,Xuet al.reported a study that utilized gold nanoarrays in nanochannels fabricated using the nano-in-nano integration technology for high-throughput molecule arraying (Fig.6a) [92].Gold nanoarrays were precisely deposited in arrayed nanochannels via a multiple EB lithography,dry etching,and liftoff (Fig.6b).Further,fluorescein disulfide molecules were used for demonstration,and self-assembled monolayers of the fluorescein disulfide molecules were formed on the gold surface via disulfide-gold interactions,indicating the successful arraying of target molecules (Fig.6c).Such arraying technologies have the potential to be further used in arraying other subcellular matter such as proteins and nucleic acids from single cells.It can be envisioned that,as critical technologies,high-throughput arraying in nanofluidics will accelerate the development of sub-single cellular studies,approaching single-molecule-level studies.
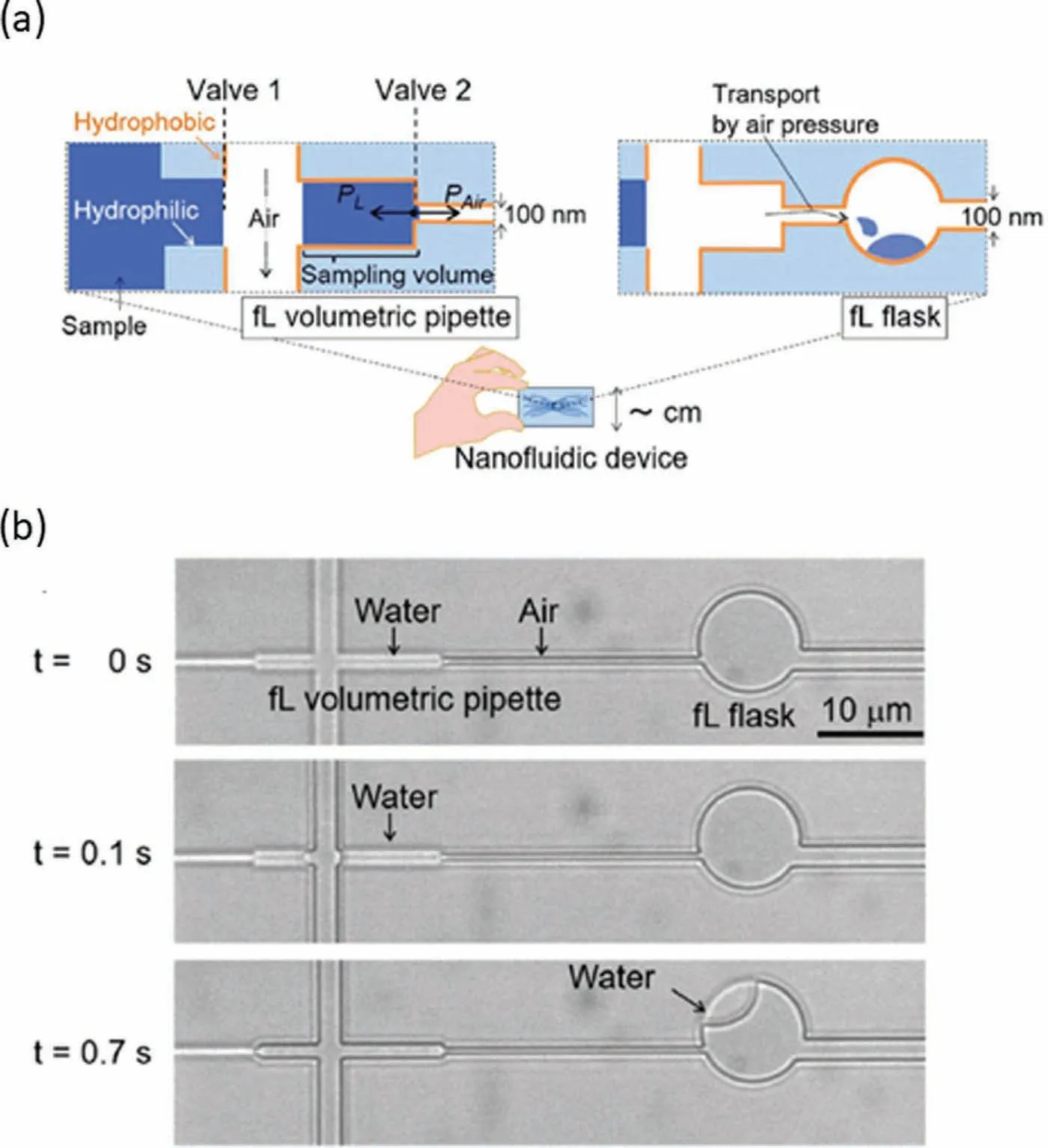
Fig.5.Potential nanofluidic sampling technology for sub-single cellular studies.(a) Schematic of femtoliter volumetric pipette and flask.(b) Images of the working demonstration of femtoliter volumetric pipette and flask.Reproduced with permission [93].Copyright 2020,the Royal Society of Chemistry.
3.5.In-situ detection in nanochannels
Some conventional detection technologies,such as scanning electron microscopy,atomic force microscopy,and X-ray photoelectron spectroscopy,which are widely applied in the analysis of morphology,structure,and components,are difficult to be directly applied to nanochannels due to their features of closeness.Hence,technologies allowing forin-situdetection of samples in nanochannels are urgently needed to apply nanofluidics to subsingle cellular studies [101–104].Recently,in-situdetection technology that utilizes gold nanotips fabricated inside a nanochannel by using the nano-in-nano integration technology has been reported [34].In this study,a pair of nanoelectrodes directly embedded inside a single nanochannel enabled thein-situprobing of femtoliter-order samples inside the nanochannel.In addition,Pungetmongkol and Yamamoto fabricated a nanofluidic device consisting of a nanochannel located between two gold electrodes for DNA detection based on impedance measurements [105].Different DNA samples at different concentrations were measured using the device,and the correlation between electrical impedance and concentration revealed successful single-molecule detection.The further development of these technologies in future may bringinsitudetection strategies allowing to obtain direct information with more details from the subcellular matter of single cells.
3.6.Others
Some peripheral technologies will also play an indispensable role in sub-single cellular studies.Peripheral technologies,such as low-temperature and room temperature chip bonding technology[106,107],detachable bonding technology [108],nanofluidic pumping technology [109],and chip regeneration technology [110],enable the practical use of nanofluidic devices.Although these technologies do not directly correlate with sub-cellular matter analysis,they are supplementary to major technologies,bridging the gap between nanofluidics and practical sub-cellular studies.
4.Future perspectives
While above-highlighted critical nanofluidic technologies possess great potentials to advance sub-single cellular studies in the future,there are still many limitations that need to be conquered.Some limitations can be resolved by further improving the performance of the nanofluidic technologies themselves.For example,the ability to fabricate nanochannel structures can be further improved to a much more delicate level by optimizing complicated processing parameters to meet the requirement of subsingle cellular studies [111].The ability to detect sub-single cellular matter may be improved by taking advantage of the unusual effects and new physics in confined nanospaces provided by nanofluidics.For instance,these effects may bring us efficient mechanism to collect [112]or enrich [113]sub-single cellular matter in nanochannels,which are very favorable for the detection of nanoscale sub-single cellular matter.Nevertheless,many other limitations of current critical nanofluidic technologies can not be resolved by only improving the performance of themselves,due to inherent restrictions stemming from their principles and/or complexity of fluid flows.These limitations may be broken by further combining nanofluidics with other advanced technologies and fields,such as optics,photonics,electronics,acoustics,and magnetics.Such combination could also offer a variety of additional abilities which the current critical nanofluidic technologies do not possess [114,115],thereby permitting much more comprehensive subsingle cellular studies in the future.
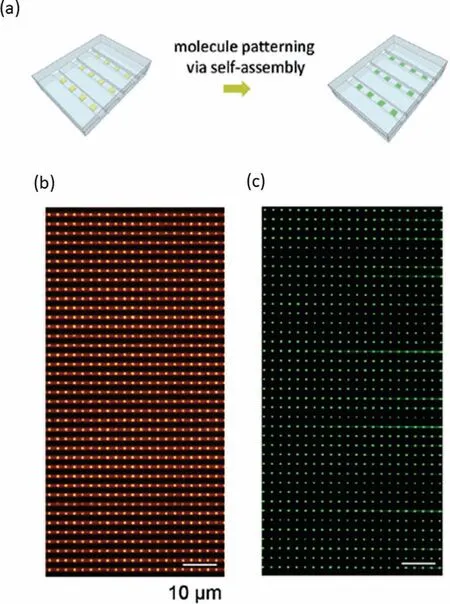
Fig.6.Promising high-throughput arraying technology for sub-single cellular matter.(a) Schematic of molecule patterning on the gold nanoarray via self-assembly.(b) Bright field image of gold nanoarray in nanochannels before molecule patterning.(c) Fluorescence image of nanoarrays with immobilized molecules.Reproduced with permission [92].Copyright 2015,the Royal Society of Chemistry.
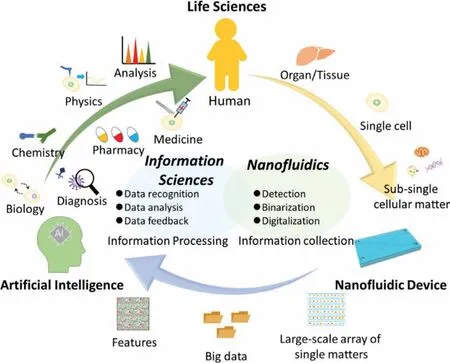
Fig.7.The conceptual workflow drawing of future perspective of nanofluidic subsingle cellular studies.
Conquering these limitations of the current critical technologies will allow us to develop integrated nanofluidic devices with ideal functions for precisely and accurately manipulating,detecting,and analyzing sub-single cellular matter at the single matter level.Such integrated nanofluidic devices promise to play as revolutionary tools to evolve cell research through evolving conventional cellular analysis based on the concept of concentrations of molecules and matters to new sub-single cellular analysis based on the concept of the number of molecules and matters.This would provide us a measure to bridge the life sciences with the information sciences as discussed as follows.
A human body usually consists of approximately 1012to 1013cells.Each cell is different,resulting in cell-to-cell heterogeneity,which is eventually ascribed to both static and dynamic differences in type,quantity,function,state,and interactions of subcellular matters in each individual cell.Hence,only if such sub-single cellular information is fully obtained,the cell-to-cell heterogeneity of the human body can be totally elucidated.However,the data to describe the information should be not only complex but also largescale.Obviously,most conventional tools for cell studies are impossible to handle such large-scale complex information.Advanced information sciences and technologies offer possibilities to conquer this challenge,but new biological tools which can be fused with them are necessary.From a perspective,we believe that nanofluidic device will be an ideal candidate to fill this gap (Fig.7).
Information sciences are a discipline on obtaining,managing,analyzing,and utilizing data from large-scale,complicated data by using tools such as supercomputers,big data,and artificial intelligence (AI).In the conceptual workflow shown in Fig.7,nanofluidics,which is good at handling and detecting subcellular matter,serves as “information collector.” Thereafter,sub-single cellular matters from a single cell are captured and arrayed at the single matter level.While the detected signal at the place of the array where a single matter exists can be expressed as “1”,the place of the array where no matter is detected can be expressed as “0”.Thereby,the number of a certain kind of subcellular matter can be converted to a digital data through the binarization.Such number is one of most basic and important quantities to describe the specific features of the single cell and should varies between cells in both static and dynamic conditions at certain spatiotemporal resolution,revealing the intrinsic nature of the heterogeneity between individual cells.The digitalization of such number will be indispensably necessary because to fully understand the heterogeneity,numbers of many kinds of molecules and subcellular matters from many cells will form large-scale data.While conventional data analytical methods are no longer effective for such large-scale data,tools such as super computers and big data will be applied.Hence,the digitalization of the basic quantities of subcellular molecules and matters through nanofluidics will be an ideal solution to subsingle cellular studies.The as-obtained big data will represent a variety of features containing a large amount of single cell feature information,which can be further processed,recognized,and analyzed using AI.As “data feedback,” information reflecting the biological origin could permit much deeper insight into the organism,or even a human level.Therefore,the use of nanofluidics will not only accelerate sub-single cellular studies but also contribute the fusion of life sciences and information sciences.Such fusion could revolutionize not only cell research but also many related areas,including basic sciences such as biology,chemistry,and physics,and as well as applied sciences such as diagnosis,medicine,and drug discovery.The fusion will significantly deepen our understanding of biology at multiple levels ranging from molecular,to cellular,organismal and human levels,and finally,contribute to the health and welfare of our human beings.
5.Conclusions
In summary,developments in nanofluidic devices now offer the potential to achieve cell studies at sub-single cellular level,evolving cell research to a new era.This will enable the essential elucidation of cell-to-cell heterogeneity which will finally serve for a broad range of applications in the future by further fusion with information sciences.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the JST,PRESTO (No.JPMJPR18H5),JSPS KAKENHI (Nos.JP21H04640,JP20H00497,JP19KK0129,JP18H01848,JP16K13653,JP26706010,JP26630403 and JP21J14595),MEXT KAKENHI (Nos.JP21H05231,JP19H04678,JP17H05468 and JP26107714) and the National Natural Science Foundation of China (NSFC,No.21628501).
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
- Recent progress on the smart membranes based on two-dimensional materials
