Trimester-specific urinary metabolome alterations associated with gestational diabetes mellitus: A study in different pregnancy stages
Hongzhi Zho,Yunyun Zheng,Lin Zhu,Li Xing,Shunqing Xu,Zongwei Ci,∗
a State Key Laboratory of Environmental and Biological Analysis,Department of Chemistry,Hong Kong Baptist University,Hong Kong,China
b Ministry of Education Key Laboratory of Pollution Processes and Environmental Criteria,College of Environmental Science and Engineering,Nankai University,Tianjin 300350,China
c Key Laboratory of Environment and Health,Ministry of Education &Ministry of Environmental Protection,and State Key Laboratory of Environmental Health,School of Public Health,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430074,China
Keywords:Gestational diabetes mellitus Non-targeted metabolomics Dynamic metabolic profile Maternal urine High-resolution mass spectrometry
ABSTRACT Gestational diabetes mellitus (GDM),a frequently-occurring disease during pregnancy,may cause some adverse healthy outcome of both mother and offspring.However,the knowledge about metabolite alterations during the pathogenesis and development process is limited.Here,a large longitudinal nontargeted metabolomics study of 195 pregnant women (64 women with subsequently developed GDM and 131 healthy controls) was conducted.Each participant provided urine samples at three timepoints during early,middle and late pregnancy,respectively.The metabolic profiles of 585 urine samples (195 × 3) were measured by using ultra-high performance liquid chromatography coupled with Orbitrap high-resolution mass spectrometry.Among the 56 identified metabolites,the levels of eight metabolites increased and three ones decreased in the first trimester,the concentration of one metabolite increased and those of 20 decreased in the second trimester,as well as the levels of five metabolites increased and two decreased in the third trimester.After false discovery rate correction,the levels of valine and 5-acetamidovalerate in GDM group significantly increased in the first trimester,the levels of 1-methylguanine and 1,3-dihydro-(2H)-indol-2-one significantly decreased in the second trimester and three metabolites (threonine,OH-octanedioyl-carnitine and pimelylcarnitine) increased and N-acetyltryptophan decreased in the third trimester,respectively.Six metabolites,such as pantothenic acid and threonine,had significant interaction effects between gestational stage (different trimester) and group (GDM or control).The differential metabolites were involved in “tryptophan metabolism”,“purine metabolism”,“valine,leucine and isoleucine degradation” and other pathways.The findings may provide insights into further pathogenesis study of GDM.
Gestational diabetes mellitus (GDM) is a world-wide high risk complication during pregnancy and the prevalence of GDM increased these years [1].GDM may cause adverse health consequences for mothers and offspring.The women who suffered with GDM had more health risk,such as type 2 diabetes [2],hypertension [2],and cardiovascular diseases [3].The children of GDM mothers often have an increased risk of glucose intolerance and obesity [4].The increasing incidence of GDM poses a serious problem to public health worldwide and needs to be drawn attention to.However,the knowledge about its pathogenesis and development process is very insufficient.
Mass spectrometry (MS)-based metabolomics is a promising technology to investigate low-molecular-weight metabolite profiles in organism [5–8].It is an important method of pathogenesis and pathophysiology research and can reveal the metabolite signatures and the alterations in metabolic pathways related to diseases [9].Non-targeted metabolomics provides a snapshot of relative concentrations of all measurable analytes within a biological sample [10–12].Some metabolomics studies on GDM have been reported,certain prenol lipids,sterol lipids,fatty acyls,sphingolipids,and glycerophospholipids were found to be different in the second trimester serum samples of 30 GDM women and 30 controls [13].Another metabolomics study showed 11 metabolites were linked to gestational diabetes mellitus [14].In our previous study [15],the “purine metabolism”,“urea cycle”,“fatty acidβ-oxidation” and“TCA cycle” pathways were found to be altered in serum samples from GDM group.
Urine collection is a non-invasive way to monitor the metabolites.In the previous report [16],the difference in maternal urine between 24 GDM and 11 controls in the third trimester was investigated by ultra-high performance liquid chromatography (UHPLC) coupled with MS for non-targeted metabolomics,indicating that the concentrations of 14 metabolites were altered due to GDM physiopathology.Ethylmalonate,pyruvate,3-hydroxyisovalerate and 2-hydroxyisobutyrate were higher in urine samples of GDM women than non-GDM controls [17,18].
Herein,a MS-based non-targeted metabolomics study of pregnant women who provided urine samples in the early,middle and late pregnancy,respectively,in order to obtain a better understanding of GDM progress.The trimester-specific alterations of the endogenous metabolites and GDM-related pathways were investigated.
The Ethics Committees of Tongji Medical College,Huazhong University of Science and Technology approved the study protocol.All the participants were from our birth cohort [24]and were recruited during January 2014-March 2015 in Wuhan,China.None of the participants had abnormal kidney function.All the pregnancy women were follow-up until delivery and each woman provided fasting morning urine samples three times,in early,middle and late pregnancy,respectively.The pregnant women were defined as GDM group based on the oral glucose tolerance test (OGTT) test at 24-28 gestational weeks.Diagnostic criteria for GDM were: Fasting≥5.1 mmol/L,or 1 h glucose level ≥10.0 mmol/L,or 2 h glucose level ≥8.5 mmol/L.The women diagnosed with GDM were selected from the cohort and the normal controls were selected from the cohort considering maternal age and gestational weeks at urine collection.A total of 585 urine samples (195 × 3) from 64 GDM mothers and 131 healthy controls were used in this metabolomics study.
The metabolic profiles of 585 urine samples were obtained by Ultimate 3000 UHPLC system (Dionex,Sunnyvale,CA,USA) coupled with Q ExactiveTMFocus hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Scientific,Bremen,Germany) as described previously with some modifications [15].One hundred μL of methanol was added to 100 μL of urine samples,with 2.5 μg/mL of 4-chloro-phenylalanine as internal standard.
A reversed-phase column Waters ACQUITY UPLC HSS T3 column(100 mm × 2.1 mm,1.8 μm) was employed for separation.The mobile phases and elution gradient were the same as our previous study [25].The experiment was operated in positive electrospray ionization mode and the MS parameters were exactly the same as in our previous study [25].
For statistical analysis,the output file was performed by XCMS software and processed by R language.The results were normalized by total ion current (TIC) method to take into account of the urine dilution variations.The signal drift correction of data was conducted by using statTarget [26].The multivariate statistical analysis method orthogonal partial least squares discriminant analysis (OPLS-DA) was applied in the metabolic profiling analysis.Mann-Whitney U tests were employed to compare the results between GDM and control groups.Benjamini-Hochberg procedure was used for false discovery rate (FDR) correction [27]to take into account of the multiple comparisons.Normal distribution evaluated by using Kolmogorov-Smirnov test.For comparison of the levels of differential metabolites in the repeated measurements,the general linear model for repeated measurement was used.Reliability analysis,which were expressed as intraclass correlation coefficient (ICC),Spearman correlation,and other statistical analysis were performed on IBM SPSS Statistics Version 20.Longitudinal changes in the metabolic profiles were estimated in a subgroup of women in GDM group and those in control group.
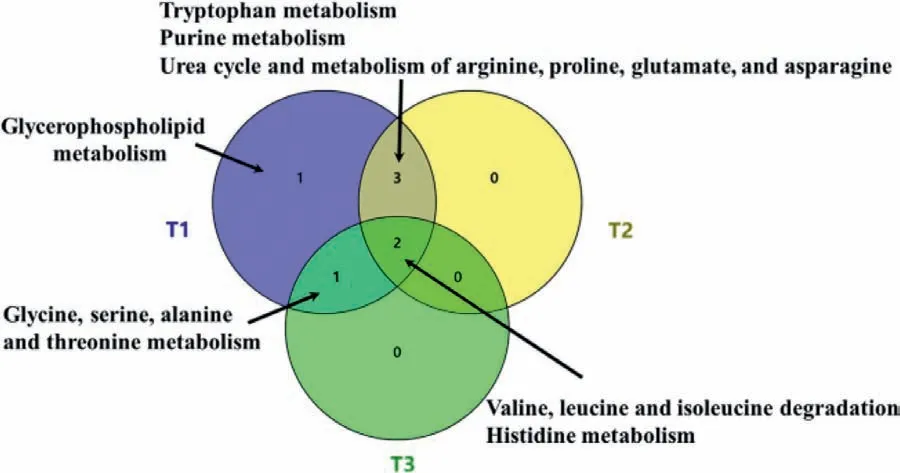
Fig.1.The overlap of altered pathways in different trimesters
The differential features were identified by comparing the accuratem/zvalues and MS/MS fragment patterns to HMDB,metlin and mzCloud databases [15].Part of the identified metabolites were verified by standards commercially available.The pathway network analysis was conducted on Cytoscape 3.5.1 with MetScape.
One after another, the church members shared their wishes, large and small. Margie was the last and the youngest to speak. As she looked out at the congregation, she spoke8 confidently, “I would like for my grandma to have church. She cannot walk, and she and my grandpa have to stay at home. They miss coming so much. So that is what I wish for. And please don’t tell them, for it needs to be a surprise.”
The demographic characteristics were summarized in Table 1,no significant difference of the time-point of gestational weeks was observed among between GDM and control groups.The body mass index (BMI) before pregnancy,which was calculated as weight in kilograms divided by the square of the height in meter was significantly higher (Mann-Whitney U testP <0.05) in GDM group(22.67 ± 3.02) than that in control (21.05 ± 2.70).All the participants did not consume alcohol and smoke during pregnancy.
As shown in Fig.S1 (Supporting information),profiles of GDM and control groups were separated in all three different trimester samples,indicating that there are many differences of the endogenous metabolites in the urine of women in GDM group and control groups almost during the whole pregnancy (T1,T2 and T3),even though the women in T1 were before the GDM onset.
Finally,58 metabolites were identified.Among them,18 of them were confirmed by commercial standards.The identification information,such as fragment ions,molecular formula and HMDB ID,was summarized in Table S1 (Supporting information).The list included 14 acylcarnitines,6 purine derivatives,and other compounds.As shown in Table S2 (Supporting information),levels of 8,1,and 5 metabolites were elevated (fold change>1.2 andP <0.05) in the profile comparison of GDM-T1vs.CON-T1,GDM-T2vs.CON-T2,GDM-T3vs.CON-T3,while levels of 3,20,and 2 metabolites were declined (fold change<0.8 andP <0.05).After FDR correction (Table S2),levels of 5 metabolites (choline,valine,5-acetamidovalerate,uric acid and 5′-methylthioadenosine) in GDM group were significantly increased (fold change>1.2 and FDR<0.1) in the first trimester,while one compound level decreased(fold change<0.8 and FDR<0.1).The level of one metabolite increased and 20 metabolites significantly decreased in the second trimester.The concentrations of five metabolites (such as valine,threonine and pimelylcarnitine) increased andN-acetyltryptophan decreased in the third trimester (FDR<0.1).Venn diagram shows the number of differential metabolites detected in the urine in different trimesters (Fig.S2 in Supporting information).
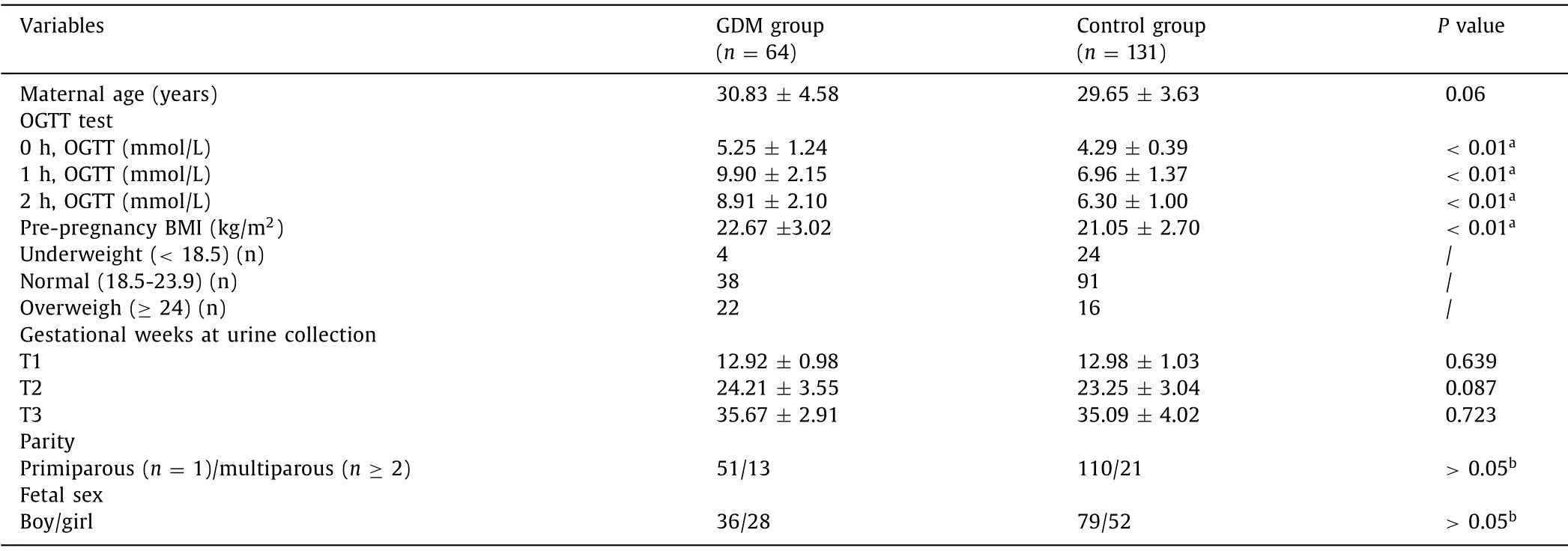
Table 1 Demographic characteristics and clinical informationof the GDM women and healthy control group.
As shown in Fig.1 and Figs.S3a-c (Supporting information),the altered pathways associated with GDM in different stages during pregnancy.The most GDM altered pathways were in the first trimester and the least ones were in the third trimester.The “valine,leucine and isoleucine degradation” pathway was altered in all three trimesters,indicating that this pathway was disturbed by GDM during almost all the pregnant progress.The pathways “tryptophan metabolism”,“purine metabolism”,and “urea cycle and metabolism of arginine,proline,glutamate,and asparagine” were overlapped in the comparison of GDM-T1vs.CON-T1 and GDM-T2vs.CON-T2.There was an overlap of "histidine metabolism" pathway in “GDM-T2vs.CON-T2” and “GDM-T3vs.CON-T3” as well as “glycine,serine,alanine and threonine metabolism” pathway in“GDM-T1vs.CON-T1” and “GDM-T3vs.CON-T3”.
The Spearman rank-correlation analysis of early pregnancy levels of differential metabolites and baseline characteristics were summarized in Tables S3 and S4 (Supporting information).Two(choline and valine) of 13 first trimester differential metabolites were statistically significantly and weekly corrected with maternal age (P <0.001) with Spearman correlation coefficient (r) 0.186 to 0.248.Eight of 13 differential metabolites from first trimester,such as choline and valine,were statistically significantly and weekly corrected with pre-pregnancy BMI (P <0.001),predominantly comprising choline and valine and others,with Spearman rank-correlation coefficient (r) ranged from −0.194 (N1,N12-diacetylspermine) to 0.306 (valine).
The ICC values were calculated to show the variance among each time-point in case group,control group and all the participants,respectively.The results were shown in Table S5 (Supporting information).All the metabolites show poor reliability (ICC<0.5) [28],either in case or control group groups,indicating great metabolite level variations across pregnancy.Specifically,the ICC values of valine,threonine,uric acid,pantothenic acid,butyrylcarnitine and 2-methylbutyroylcarnitine in case group are higher than those in control group,indicating that the above metabolites have the high within-person variability.
The interaction between time-point and group is shown in Figs.S4-S9 (Supporting information).Five metabolites (N1,N12-diacetylspermine,threonine,pantothenic acid,hypusine,and Pro-Thr) had significant interaction effects (P <0.05) between gestational stage (different trimester) and group (GDM or control).
In this study,to understand GDM development and progression,the metabolic profiles of three different pregnancy stages of 64 women with GDM and 131 healthy controls were obtained by using MS-based non-targeted metabolomics strategy.The results showed that the GDM-associated metabolome was enriched with amino acids and metabolites involved in purine metabolism,confirming this metabolic process to be associated of GDM development.It seems that the altered pathways were different among different trimesters.
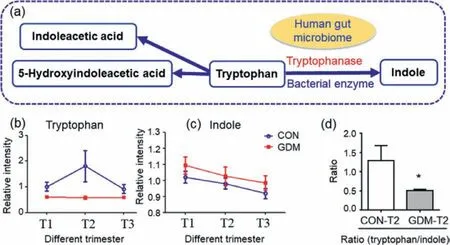
Fig.2.The alteration trend of the metabolites of tryptophan metabolism (Data are presented as the mean ± SEM).(a) Tryptophan metabolism.(b) Alteration trend of tryptophan level.(c) Alteration trend of indole level.(d) Ratio of tryptophan to indole (∗represents Mann-Whitney U test P < 0.05).
The “tryptophan metabolism” pathway was found to be altered in both the first and second trimesters,but not in the third trimester (Fig.1).Tryptophan is an essential amino acid for human and “tryptophan metabolism” pathway plays important roles in nutrition and health.In this study,tryptophan was down-regulated in all trimesters in participants with GDM.Indole had the trend of up-regulated trend in all trimesters in participants GDM,although not significant (P >0.05).In addition,the ratio of tryptophan to indole was significantly higher in GDM group than that in control group in the second trimester (Fig.2).It was conjectured that tryptophanase,which converts tryptophan to indole,was up-regulated in GDM group.Tryptophanase is a bacterial enzyme which can degrade tryptophan to indole.Tryptophanase is in the human gut microbiome and from intestinal bacteria such asEscherichia coli.The findings about tryptophan metabolism provide evidence for the hypothesis that the human gut microbiome may modulate metabolic health and it may play an important role in the etiology of GDM[29].
5-Hydroxyindoleacetic acid (5HA) is also a component of the tryptophan pathway.5HA is the main metabolite of serotonin (5-hydroxytryptamine),which participates in insulin secretion [30]and plays an important role in glucose homeostasis.In this study,the level of 5HA had the lower trend in the women who subsequently developed GDM (GDM-T1vs.CON-T1) and higher trend in GDM group in other two trimesters (GDM-T2 vs CON-T2 and GDM-T3vs.CON-T3).It might be explained that,in the middle and late pregnancy,the GDM women had impaired insulin secretion,which is strongly associated with GDM [31].The findings support the relationship between insulin secretion patterns and gestational diabetes risks [31]and the association between “tryptophan metabolism” pathway and GDM.
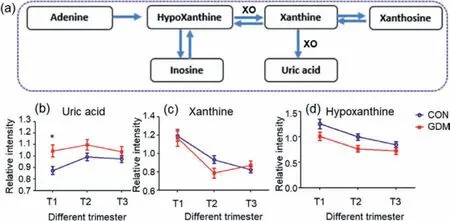
Fig.3.The alteration trend of the metabolites of purine metabolism (Data are presented as the mean ± SEM).(a) Purine metabolism.(b) The alteration trend of uric acid level (∗represents Mann-Whitney U test P < 0.05).(c) The alteration trend of xanthine level.(d) The alteration trend of hypoxanthine.Abbreviation: XO,xanthine oxidase.
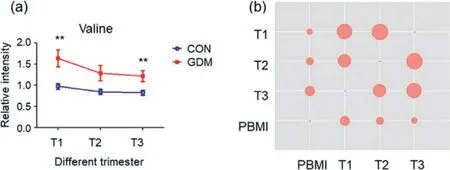
Fig.4.The alteration trend of the valine.(a) The alteration trend of valine level(Data are presented as the mean ± SEM;∗∗represents Mann-Whitney U test P <0.01).(b) Spearman correlation coefficients among valine levels and pre-pregnancy body mass index.
Uric acid,adenine,and hypoxanthine are purine compounds.In this study,uric acid level was up-regulated in GDM group.In our previous study of maternal serum [15],uric acid accumulated in women with subsequent GDM [15].In this study on urine samples,uric acid also had an elevated trend relative to control group during the whole pregnancy process and was significantly (P <0.01)elevated in GDM-T1vs.CON-T1(Fig.3).Thus,the GDM-related variation trend of uric acid in urine and serum samples was consistent.The findings confirmed that uric acid contributed to the occurrence and development of GDM.
Purine compounds provide organism with energy.Adenine and hypoxanthine are the hydrolytic products of adenosine monophosphate,an important energy-rich compound.In this study,the levels of adenine have increased trend,although not significant,in GDM group in all three trimesters (Table S2),indicating that the women in GDM group are likely to have more energy during the whole pregnancy.Thus,the results highlighted that “purine metabolism”pathway plays important role in the development of GDM.
The “valine,leucine and isoleucine degradation” pathway was altered in all the three trimesters (Fig.1).In this study (Fig.4 and Table S2),valine levels increased in GDM group compared with control group with fold change 1.69 (P <0.01 and FDR<0.1) for T1,fold change 1.51 (not significant withP >0.05) for T2 and fold change 1.48 (P <0.01 and FDR<0.1).In addition,valine showed positive correlation with pre-pregnancy BMI (spearmanr=0.306 for T1,0.248 for T2 and 0.200 for T3,P <0.05 for all),which was reported to independently increase the risk of GDM [32].The link of valine with BMI,a risk factor for GDM [33],was consistent with the study on general population in China [34]and women in Poland [35].Besides,valine level in the first trimester was correct with maternal age (Table S3 in Supporting information),also an important risk factor for GDM [36].Valine,leucine and isoleucine are termed branched-chain amino acids (BCAAs),and associated with insulin resistance [37].Thus,valine concentration was independently and positively associated with GDM through modifying insulin resistance and secretion.
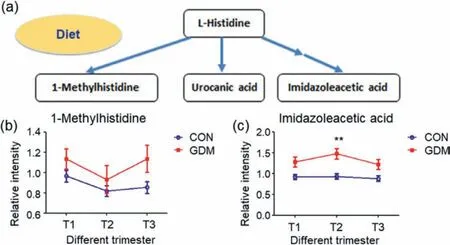
Fig.5.The alteration trend of the metabolites of histidine metabolism and other related pathways (Data are presented as the mean ± SEM).(a) Histidine metabolism.(b) The alteration trend of 1-methylhistidine.(c) The alteration trend of imidazoleacetic acid (∗∗represents Mann-Whitney U test P < 0.01).
On the other hand,valine,leucine and isoleucine are all essential amino acids and need to be ingested,and diet is an obvious factor that could alter amino acid levels.Valine is generated from protein breakdown [38].It was found that the level of valine increased significantly in the first trimester (Fig.5 and Table S2),indicating that dietary intervention is one of effective factors for reducing the incidence of GDM and should be initiated in early pregnancy [39].Taken together,the findings provided the evidence that the “valine,leucine and isoleucine degradation” pathway was altered in GDM.
The “histidine metabolism” pathway was also altered in all the three trimesters.Histidine is a precursor of histamine [40]and imidazoleacetic acid is a breakdown product of histamine.Histamine binds with histamine receptors and causes inflammation,which often accompanies GDM [41].Histidine was higher in GDM antepartum and postpartum [42].Histidine had a higher serum value in infants of GDM mothers than in control group [43].In this study,the levels of imidazoleacetic acid (Fig.5) were higher in GDM group in the second trimester and have increased trend in other two time-points (although not significant),which might be explained by inflammation in GDM patients.Urinary methylhistidine levels are elevated from consumption of soy-based products and meat,particularly chicken [44].The level of methylhistidine in GDM group also has increased trend in GDM group during the whole pregnancy,highlighting that diet plays an important role in GDM.To the best of our knowledge,this is the first time to report that “histidine metabolism” pathway was related to GDM.
Besides,the levels of more metabolites were different between GDM and control groups in the present study.For instance,the levels of threonine were higher in the comparison of GDM-T1vs.CON-T1 (P <0.05) and the third trimester (P <0.01 and FDR<0.1).Together with the previous report that significant elevated plasma level of threonine in GDM women [45],the findings indicated that threonine might be associated with GDM.
Hypusine,an amino acid in eukaryotic translation initiation factor 5A (eIF5A),contributes to the production of pro-inflammatory cytokines [46].Hypusine modification is important in the production of stress-responsive proteins in the pancreatic islet [47].It was hypothesized that eFI5A level might also be altered in GDM group,but it needs to be investigated in future study.
N1,N12-Diacetylspermine,an acetylated product of spermine,is a kind of polyamines,which are known to influence glucose metabolism [48].The significant decrease ofN1,N12-diacetylspermine level in early pregnancy (Tables S2,S5 and Fig.S4 in Supporting information) indicated that the polyamine flux and glucose metabolism were disturbed before GDM diagnosis.
The increase of choline in GDM group (Table S2) was consistent with results from the study in Portugal,in which urine samples were collected from pregnant women 14-25 gestational weeks[18].In addition,choline metabolism and dietary choline intake are potentially associated with insulin resistance and diabetes [49].Choline was increased after OGTT in healthy subjects [50].The levels of pantothenic acid increase in urine of GDM-T1 compared with control group,which was in line with the increase of the first trimester serum (compared with control group) in our previous study [15].
All in all,compared with our previous serum MS-based metabolomics study,we found that consistent changes were related to purine metabolites,acylcarnitines and BCAAs.The findings emphasized that the above-mentioned “purine metabolism”,“fatty acid oxidation” and other pathways were associated with GDM.
There are several strengths in this study.Firstly,three batches urine samples of different pregnancy stages were collected from each participant,either GDM case or healthy control.It provided the possibility to observe the longitudinal alterations of metabolites during the whole process of GDM.Additionally,MS with highresolution and sensitivity provides a highly reliable metabolomics platform and the large sample-size provides confidence and substantial statistical power.Thirdly,different statistical approaches were employed,such as OPLS-DA,Manu-Whitney U test with FDR correction.The results of several different statistical approaches were corroborated with each other,indicating that the results about the changes between different groups were credible.A limitation of this study is that all the participants in this study were ethnic Chinese,and the findings need to be validated in other populations.
In conclusion,the metabolic pathways associated with GDM during the different pregnancy stages were identified.The metabolic pathways associated with GDM were described,highlighting the role of metabolites of several pathways including“tryptophan metabolism”,“purine metabolism”,“valine,leucine and isoleucine degradation” and other pathways.The findings may improve the understanding of the metabolic pathways that may be affected by GDM throughout pregnancy and gain insights into the bioprocesses that may be associated with GDM.This study may be helpful for the screening of high-risk population of GDM and the study of pathogenesis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (Nos.42177412 and 21437002) and National Key Research and Development Program of China (Nos.2017YFC1600500 and 2019YFC1804602)
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.001.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
