Non-woven cotton fabric based intimately coupling of photocatalysis and biodegradation system for efficient removal of Cu(II) complex in water
Yuxun Ye,Peng Yng,Yuwei Deng,Yezhi Yng,Kun Zhng,Yueying Wng,Wenjing Shng,Qing Li,Lei Sun,Fei Pn,∗,Dongsheng Xi,∗
a School of Environmental Engineering,Wuhan Textile University,Wuhan 430073,China
b Engineering Research Center for Clean Production of Textile Dyeing and Printing,Ministry of Education,Wuhan 430073,China
Keywords:Cu complex Photocatalysis Biological treatment Non-woven cotton fabric
ABSTRACT The efficient remediation of heavy metal complexes in water has become a difficult and challenging task owing to their high stability and strong mobility.In this study,a novel strategy was employed for highly efficient removal of Cu-citrate by using intimately coupled photocatalysis and biodegradation (ICPB) system with non-woven cotton fabric as a carrier.Experimental results showed that the ICPB system caused 94% Cu removal,which was higher than those of single photocatalysis.After 5 cycles,Cu removal efficiency could still reach 78% within 5 h.The existence of 0–40 mg/L citrate had negligible influence,whereas the presence of 60–100 mg/L citrate exhibited a limited adverse effect on Cu removal (∼70%).The decomplexation of Cu-citrate was realized via the function of free radicals and microorganisms.Two main processes,such as bio-adsorption of Cu2+ by microorganisms,deposition of Cu0 on the surface of material,played important role in Cu removal from aqueous solution.The dominant microorganisms in the system were Proteobacteria,Actinobacteria,Bacteroidetes,Chloroflexi,Chlorophyta,Planctomycetes,and Verrucomicrobia.Furthermore,the performance of ICPB system was also validated through treatment of other heavy metal complexes.This study provided a feasible strategy for the decontamination of heavy metal complexes in wastewater.
Heavy metals (HMs) are typical pollutants in water,thorough purification of HMs has become an important topic for human beings because of the toxic and carcinogenic effect posed for livings[1].Traditional methods including precipitation,coagulation,membrane separation have evidenced significant removal efficiency toward heavy metals in the free states [2].However,free metals are not the main components in real wastewaters treated by such traditional methods [3–5].The existed organic ligands (e.g.,citric acid,oxalic acid,and EDTA) will be complexed with HMs to form stable heavy metal complexes through the connection of coordinate bond[3,4].Consequently,the removal efficiency of heavy metals is hindered,further resulting in increased mobility of metals to the environment [6,7].The released metal complexes cannot be degraded naturally and thus migrate through air,water,soil,further polluting drinking water and food chain [8].Therefore,it is essential to remove heavy metal complexes before being proceeded to the receiving water for alleviating the potential risks to the environment and human beings.
A variety of methods,such as coagulation/flocculation [9],adsorption [10],and advanced oxidation processes (AOPs) [11,12],have been developed to deal with heavy metal complexes.Due to the stable structure of metal complexes,adsorption and coagulation were ineffective in removing such pollutants in most cases[13].Although AOPs with active radical production were proved to maintain good performance toward metal complexes,the high dependence on outer energy (e.g.,UV,electricity) had some influences on the real application for wastewater treatment plants [14].Meanwhile,the removal of heavy metal complexes by a single AOP was limited due to the accumulation of excessive intermediates[15].Considering economy and practicality,biological treatment had been widely used in wastewater treatment [16,17].Biodegradation process is an eco-friendly technique that uses microbes to break down the stable structure and result in complete mineralization of organic ligands [18].However,it is still faced with low sensitivity and low removal rate of heavy metal complexes [19].Thus,more efforts need to be paid for strengthening of single biological treatment.Hunting for an effective process for removing metal complexes with negligible residual metals is still urgent recently.
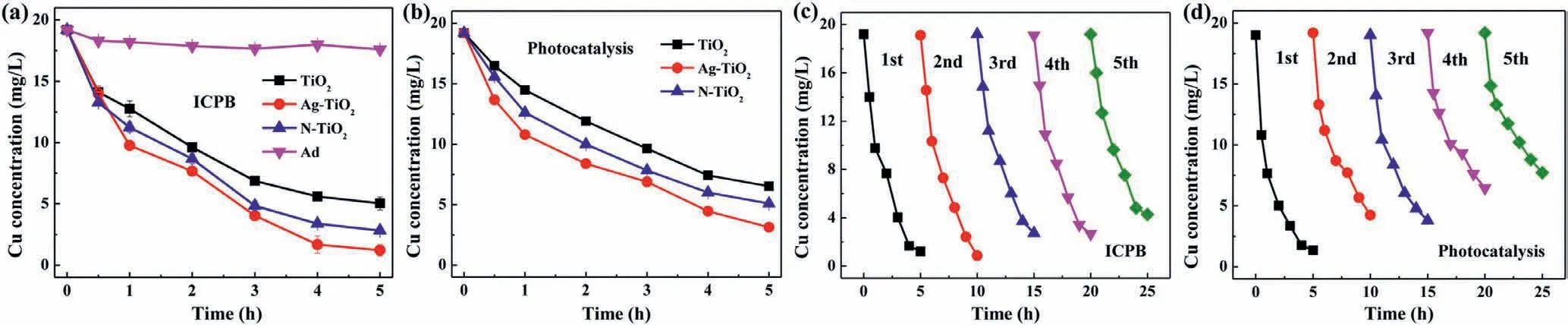
Fig.1.(a) Cu-citrate removal in different ICPB systems,(b) Cu-citrate removal by photocatalysis process with different catalysts,cyclic performance of (c) ICPB system,(d)single photocatalysis process for Cu-citrate removal ([Cu]0=0.3 mmol/L,pH0 6.0).
As inspired by the discussion above,it is expected to integrate AOPs and biological treatment for dealing with heavy metal complexes.Adopting such coupled system can not only maximize the overall performance,but also minimize the existed shortcomings of a single process.Recently,intimately coupled photocatalysis and biological degradation (ICPB) system have attracted the attention of researchers [20].As a novel water treatment process,the ICPB combines the advantages of AOPs and biological process,which exhibits better pollutant removal performance and shows great potential in wastewater treatment [21].For instance,Xionget al.proposed a novel ICPB system with Ag-doped TiO2and microbes,and about 85% of tetracycline was degraded in 2 h [22].Our previous study proposed non-woven cotton fabric based ICPB system,which combined the advantages of good wettability and convenience belonged to the fabric [23].This study also proved that the ICPB with non-woven cotton fabric has good performance toward tetracycline and chemical oxygen demand (COD),in which,87% degradation rate of tetracycline (TC) and 50% of COD were simultaneously achieved [23].Other recent studies have proved the performance of many pollutants,including antibiotics [24],nitrogen [25],phenol [26],etc.The key advantages of ICPB system are more thorough degradation of organics,which are beneficial for HMs removal from solution [27].Hence,it is presumed that satisfactory results of HM complex removal could be obtained with the assistance of the ICPB process.Until now,the feasibility of HM complexes remediation mediated by the ICPB has rarely been documented,and related researches have not been carried out.More researches should be done to fill the knowledge gap on the performance and mechanism of ICPB in decomplexation of HM complexes.
To verify the assumption,we synthesized a flexible composite consisted of titanium-based photocatalysts and activated sludge to deal with a metal complex containing wastewater accompanied with UV light.The typical metal complex,Cu-citrate,was selected as the model contaminant in this study.Cu complex removal by single photocatalysis and ICPB were compared,and the mechanisms of the process were explored.The diversity of microbes was identified by high-throughput sequencing.Different water matrixes were also obtained for further evaluation of Cu complex removal performance through the ICPB process mediated with flexible composite material.
To find out the most suitable photocatalyst for treating Cucitrate,three kinds of flexible materials were selected.The removal efficiency of Cu-citrate by adsorption (Ad) and the ICPB systems within 5 h were compared.Approximately 17.6 mg/L Cu was left with non-woven cotton fabric (NCF) only,indicating negligible adsorption of Cu-citrate brought by NCF.With the ICPB systems,the residual concentration of Cu by TiO2@NCF,N-TiO2@NCF,and Ag-TiO2@NCF were 5.0 mg/L,2.8 mg/L,and 1.2 mg/L,respectively(Fig.1a),suggesting that the ICPB process was efficient for the decomplexation of Cu complex.Ag-TiO2@NCF showed better performance than others,which may be ascribed to the deposition of Ag in TiO2that enhanced photocatalysis performance through expansion of photo responding range and strengthen the electron separation characteristics [28].For comparison,decomplexation experiments of Cu-citrate by photocatalysis only with prepared flexible materials were carried out,and poor performances were obtained in terms of total Cu elimination.Single photocatalysis systems,i.e.,TiO2,N-TiO2,Ag-TiO2,resulted in 6.5 mg/L,5.1 mg/L and 3.1 mg/L Cu left in solution (Fig.1b).More efficient removal of Cu by the ICPB system,demonstrating microorganisms played a certain role in the removal of Cu(II) complex.
Cycling experiments were conducted and the results were displayed in Figs.1c and d.For photocatalysis only,about 83.9%,78.1%,72.4%,66.7%,and 60% removal efficiency were obtained after 1–5 cycles,respectively.In comparison,the ICPB system remarkably enhanced Cu removal,with the Cu removal efficiency maintained at more than 78.1% even after 5 cycles.These results indicated the ICPB system was strengthened associated with biological processes.Moreover,the XRD results proved that the crystal structure of fresh and used materials did not change (Fig.S2 in Supporting information).The diffraction peaks at 25.3°,37.7°,48.0°,53.8° were corresponded to (101),(004),(200),(105) lattice plane that belonged to anatase type TiO2[29].This revealed Ag-TiO2was stable enough to further attribute to the satisfactory removal effi-ciency of Cu-citrate.In view of the removal efficiency of Cu,Ag-TiO2@NCF was selected in further experiments.
Considering the operation process and complex water matrices,the effect of various factors (e.g.,loading rate,initial pH,citrate concentration) on removal efficiency of Cu-citrate were evaluated.With the increase of loading rate of Ag-TiO2,residual Cu concentration decreased gradually (Fig.2a).When the loading rate was 25%,the residual Cu was only which may be ascribed to the enhancement of photoactivity brought by loaded material.Previous studies showed that electrons and holes were generated through irradiation of photocatalysts,and higher loading of Ag-TiO2result in generation of more radicals [30].Based on the experimental results,when the loading rate was 25%,the residual Cu was only which were adopted as the optimum loading amount of Ag-TiO2in the further experiments.
Experiments at pH 3.0,6.0,7.0 and 10.0 were conducted,and the results were displayed in Fig.2b.At pH 6.0 and 7.0,Cu concentration in solution were 1.2 mg/L and 2.6 mg/L,respectively,which was lower than that of pH 3.0 (∼3.8 mg/L) and 10.0 (∼4.2 mg/L).According to previous studies,at acidic conditions,the poor performance of ICPB process for removing Cu-citrate was ascribed to the electrical repulsion as well as consumption of electrons by H+[31,32].Further elevating pH to 10.0 resulted in a decline in Cu removal,which was due to the weak activity of microbes in such circumstances [33].As a result,pH 6.0 was selected as the optimum condition in view of efficient processing.
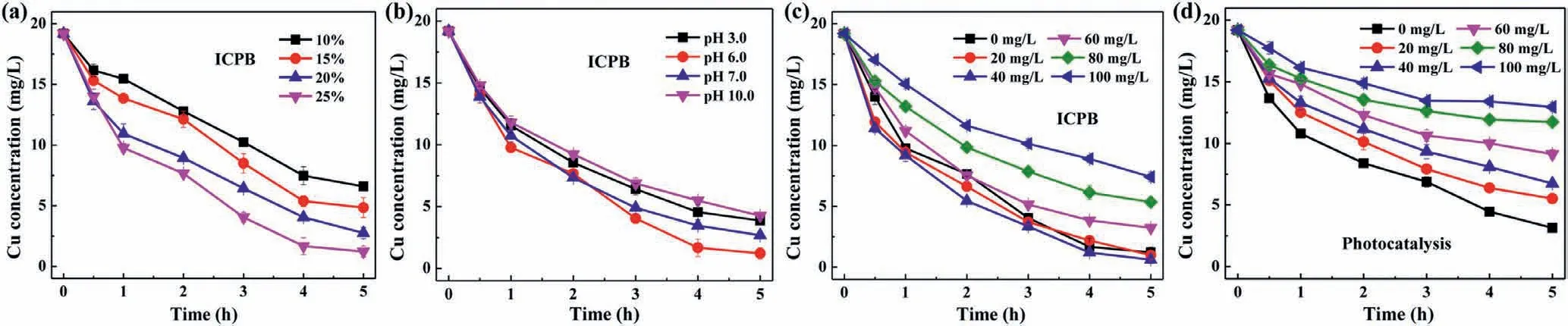
Fig.2.(a) Effect of loading rate,(b) effect of different initial pH,(c) effect of citrate on Cu removal by ICPB system,(d) effect of citrate on Cu removal by photocatalysis process ([Cu]0=0.3 mmol/L,pH0 6.0).
As a common organic substance in the water,citrate will affect water treatment process.In the ICPB system,it could be clearly found that removal efficiency of Cu-citrate increased slightly when 20 or 40 mg/L citrate was added (Fig.2c).The enhancement performance was due to the functions of microorganisms.A small amount of citrate could provide a nutrient source,which was beneficial to metabolism and utilization of microorganisms [34].If the concentration of citrate was more than 60 mg/L,a slightly inhibitory effect was found.The reason was that a large amount of citrate would consume the active radical,thus affected the decomplexation reactions of Cu-citrate [35].In a single photocatalysis process,due to the strong competition from citrate,significant depressing performance for Cu removal was observed(Fig.2d).These results demonstrated that the ICPB process had great potential in Cu complex removal especially in organic-rich wastewaters.
The preconditions for removal of Cu(II)-citrate depended on the happening of effective decomplexation reactions.The main active radicals were identified through EPR analysis.When DMPO was adopted as a spin-trapping agent,four significant peaks of DMPO-•OH with an intensity ratio of 1:2:2:1 were clearly observed,indicating the presence of•OH in the process (Fig.S3a in Supporting information).It is well known that irradiation of Ag-TiO2could generate positive holes and electrons.The formation of•OH depended on the combination of surface hydroxyl group and positive holes [29].In addition,O2•−had also been detected in the system(Fig.S3b in Supporting information),which was formed through reactions of oxygen and electrons.These results indicated the corresponding active radicals played a key role in decomplexation of Cu(II)-citrate.
In order to examine the removal performance of organic matter,residual TOC content in the solution was also determined.The removal rate of TOC with the ICPB treatment was ∼13% higher than that with photocatalysis within 5 h (Fig.S3c in Supporting information),proving more organics were degraded in the aid of microorganisms.Previous studies suggested that organic ligands in low concentration led to highly efficient removal of heavy metals [36].The results of TOC removal were consistent with those of Cu removal,which also confirmed the successful degradation of organic ligands.Direct degradation of citric acid had been studied earlier and the major products were acetone-dicarboxylic acid,acetone and oxalate [37].Therefore,the removal efficiency of Cu in the presence of possible intermediate ligands was evaluated.It could be clearly found that a high removal rate was obtained (Fig.S3d in Supporting information),suggesting the co-existed organic ligands had a negligible effect on Cu removal by the ICPB system.All the results indicated decomplexation process was a key and necessary procedure for Cu-citrate removal.
In order to confirm the removal mechanism of free Cu ions,the performances of adsorption and precipitation were compared in terms of residual Cu concentration.When the precipitation pH was 10.0,removal gap of Cu before and after precipitation is insignificant (lower than 0.5 mg/L),indicating Cu removal by precipitation is not the main route (Fig.S4a in Supporting information).By contrast,after 5 h,residual Cu in solution was still maintained at 19 mg/L with single photocatalysis.About 40% of Cu was removed through pH adjustment (Fig.S4b in Supporting information),suggesting Cu removal in photocatalysis was mainly dependent on precipitation process.
Apart from the process mentioned above,adsorption may be the main process for Cu removal.Negligible removal efficiency was observed for Cu-citrate,Cu-acetonate dicarboxylate,Cu-acetonate,and Cu-oxalate by adsorption mediated with non-woven cotton fabric (NCF) and photocatalyst (Fig.S4c in Supporting information).Nevertheless,adsorption brought by biofilms with various Cu(II) complex intermediates was quite different.About 15.7 mg/L,14.4 mg/L,and 12.7 mg/L Cu were removed,respectively,which indicated Cu(II) complexes were more easily absorbed by microorganisms after the destruction of stable structure.According to FTIR results,the significant peaks at 3402 cm−1,2927 cm−1,1649 cm−1,1035 cm−1were attributed to −OH,C−H,C=O and C−O groups (Fig.S4d in Supporting information).After reactions,the intensities of such peaks were declined,while other peaks located at 1451 cm−1,and 1245 cm−1were red-shifted to 1405 cm−1and 1233 cm−1.This demonstrated the capture of Cu compounds in the process was depended on the extracellular polymeric substances (EPS) containing carboxyl,hydroxyl,and amino groups that secreted by microorganisms [38].
To explore the main components of Cu on the surface of composite materials,XPS analysis was conducted (Fig.S5 in Supporting information).The main elements of composite material are Ti,O,Ag,and Cu.The significant peak located at 932.2 eV was identified as Cu0,suggesting free Cu ions were reduced to metallic Cu by electrons generated in TiO2-assisted photocatalysis [31].The other two peaks located at 934.0 eV and 935.4 eV corresponded to CuO and Cu(OH)2,respectively [39].The existence of divalent copper further proved that Cu was mainly removed through surface adsorption by flexible composite.
The surface changes of fresh and used materials were characterized by scanning electron microscopy (SEM) analysis.Significant microbes could be observed attaching to the surface of fresh materials (Fig.S6 in Supporting information).As the cycle number increased,large amounts of microbes also remained on the materials,suggesting the flexible material maintained certain reactivity.Furthermore,SEM mapping analysis of elements on the material was conducted.Apart from the raw elements (C,O,Ti and Ag),Cu element was clearly found on the surface of the material (Fig.S7 in Supporting information).This result further elucidated Cu removal was mainly dependent on the material,rather than on precipitation.
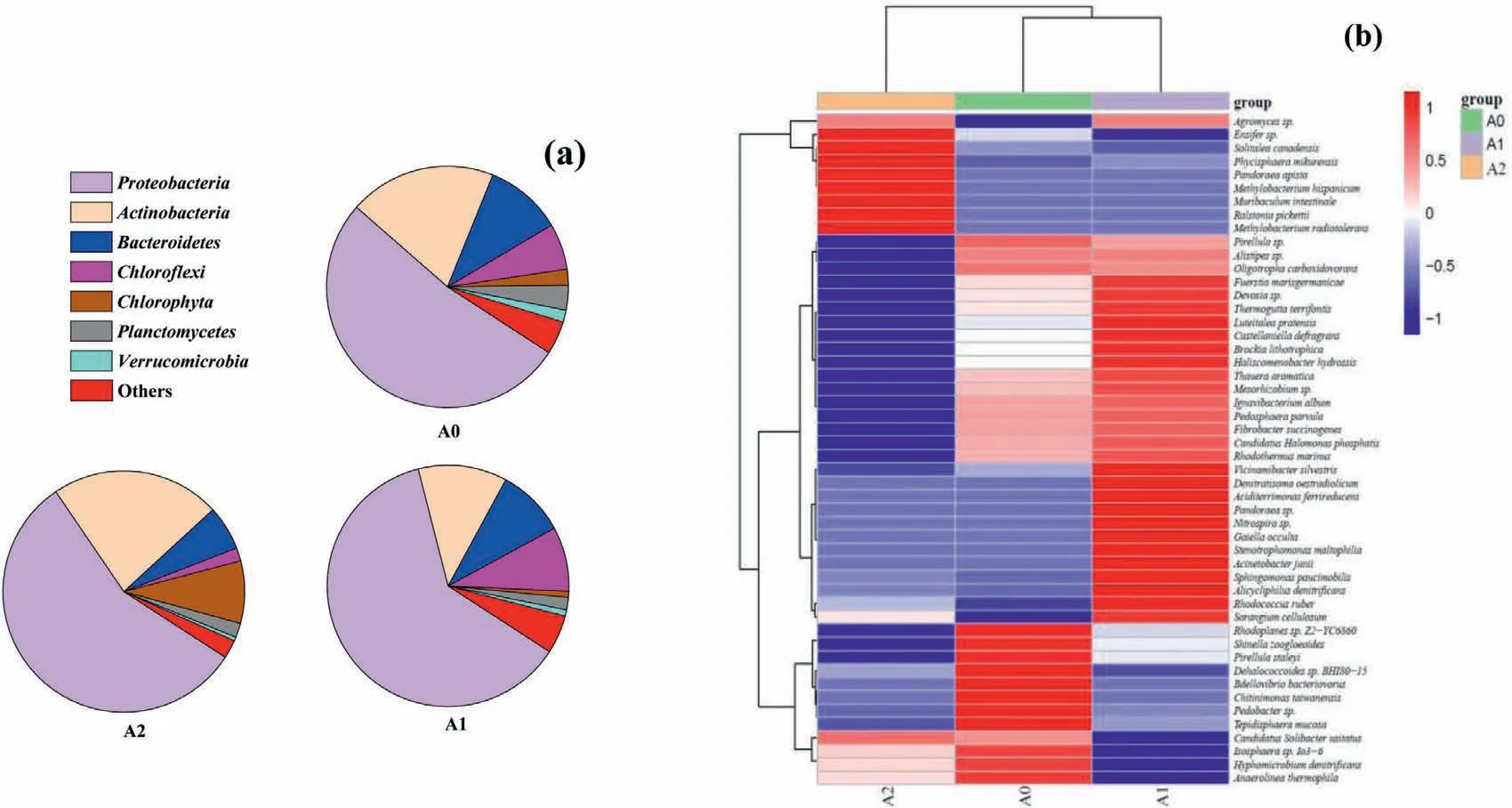
Fig.3.(a) Bacterial analysis of A0 (raw material),A1 (material after 2 cycles),A2 (material after 5 cycles) at phylum level.(b) Heat-map analysis of microbial community of flexible composite material after different cycles,A0 (raw material),A1 (material after 2 cycles),A2 (material after 5 cycles).
After gene sequencing on Illumina Miseq high-throughput sequencing platform,a total of 40,498 effective sequences were obtained from the activated sludge bacterial community in the three ICPB systems.The distribution of effective sequences of different bacterial communities in the system was shown in Table S1 (Supporting information).Among them,the Chao1 index,the Simpson index and the Shannon index were usually used as microbial diversity index.Specifically,the Chao1 index was often used to indicate the richness of biological community in organism,while the Shannon index and Simpson index were used to represent the diversity of biological community.The larger value implied a complex community with more richness.According to the data,the Chao1 index of A1 increased 49.4%,while A2 decreased 14.2%.This indicated the richness of biological community enhanced significantly at first,and then become bad due to the constant reactions in the coupled system.Simpson index and Shannon index showed a similar trend,suggesting microbial diversity decreased gradually.
The microbial communities of composite material at the phylum level during the treatment process were displayed in Fig.3a.The dominant bacteria on raw fabric wereProteobacteria(52.3%),Actinobacteria(19.7%),Bacteroidetes(10.3%),Chloroflexi(6.2%),Chlorophyta(2.1%),Planctomycetes(3.3%),andVerrucomicrobia(1.6%).After 2 cycles,the main microorganisms wereProteobacteria(61.8%),Actinobacteria(11.9%),Bacteroidetes(9.1%),Chloroflexi(8.6%),Chlorophyta(0.8%),Planctomycetes(1.7%),andVerrucomicrobia(0.8%).After 5 cycles,the main microorganisms changed,such asProteobacteria(56.3%),Actinobacteria(22.7%),Bacteroidetes(6.0%),Chloroflexi(1.7%),Chlorophyta(8.4%),Planctomycetes(1.9%),andVerrucomicrobia(0.5%).According to the phylum results,Proteobacteriaoccupied the main state of system and remained as high abundance during the cycling experiment.This suggestedProteobacteriaplayed an important role in degradation of organics as well as removal of heavy metals [17].The experiment results proved that theProteobacteriahad better adhesion performance than other microbes,and it would be better adapted to the Cucontaining wastewater.As the special bacteria responsible for Curesistant and Cu removal,Actinobacteriaaccounted for 19.7% in raw material,11.9% in the material after 2 cycles,and 22.7% in the material after 5 cycles.Previous studies indicated extracellular polymeric substances (EPS) produced byActinobacteriacould capture Cu ions through the formation of a coordinate bond [40].Nevertheless,the overall level of some bacteria,such asChloroflexi,Planctomycetes,andVerrucomicrobia,were kept at low abundance,which was ascribed to their dormancy property in the lab environment[41].It is worth noting that the content ofChlorophytaincreased significantly after 5 cycles,suggesting the treated water was suitable for algae growth.It was reported thatBacteroidetescould hydrolyze and degrade organic matter [17],thus the abundance decreased as the experiment proceeded.
Through the high-throughput sequencing platform,50 species that were higher than 0.2% were identified in the ICPB system,and the results were shown as a heat-map in Fig.3b.As the cycle experiments were carried out,the structure of bacteria inside the cotton changed significantly.Under the blank condition,the bacteria with high abundance were B39 to B50.The 12 kinds of bacteria played a major role in the system.The second group of bacteria was B10 to B38,and these 29 species were in high abundance after 2 cycles.The third group of bacteria was B01 to B09,and these 10 species are in high abundance after 5 cycles.The amounts of bacteria in blank were observed higher than that after 2 or 5 cycles,and the numbers exhibited the trend of increase first and then decrease,which were corresponded to the preliminary determination of Simpson and Shannon index.However,after 5 cycles,the level of species richness in system was not as high as that of blank experiment,which may be related to the presence of UV light,aeration and Cu complexes in the system.
According to the above analysis,the possible mechanisms of Cu complex removal were proposed (Scheme 1).The main mechanisms including the degradation reactions of Cu-citrate and removal process of Cu ions.In an aqueous solution,free Cu2+was liberated from Cu-citrate complex,and organic matters were simultaneously degraded to CO2and H2O.The whole processes were in the function of active radicals that were generated from photocatalysts under UV light irradiation as well as biological degradation.Then,free Cu(II) was mainly captured by microbes on the fabric through various processes,such as precipitation,ion exchange,and bio-adsorption.Microorganisms growing on the material provided sufficient functional sites for enhanced biological removal of Cu.Meanwhile,part of Cu2+was reduced to Cu0,which was due to the reduction behavior of photogenerated electrons.The whole process was finally attributed to the efficient remediation of Cu complex as well as recovery of Cu from water.
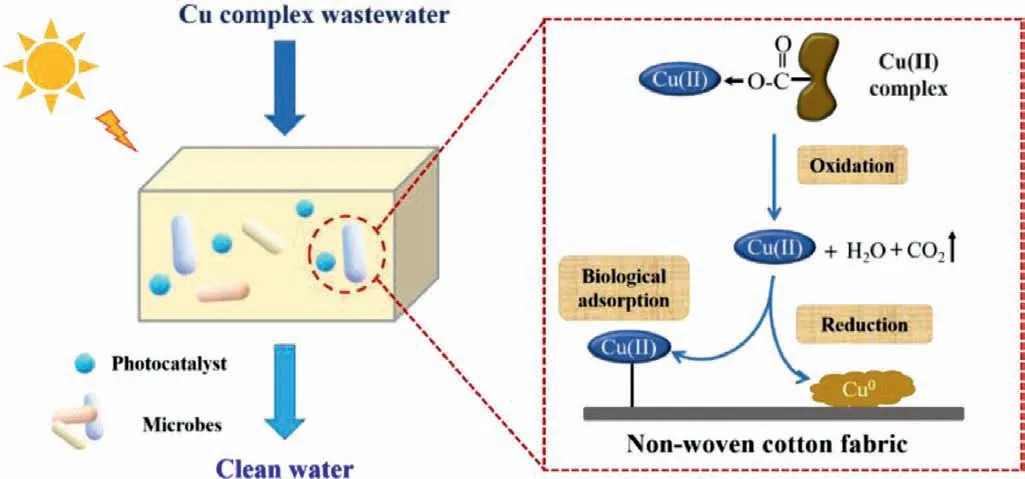
Scheme 1.Possible mechanisms for Cu complex decomplexation and Cu recovery by ICPB system.
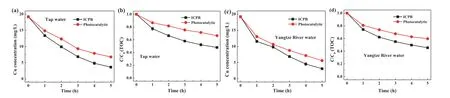
Fig.4.Treatment of authentic Cu-containing water by photocatalysis and ICPB processes in different water matrices: (a,b) tap water,(c,d) Yangtze River water([Cu]0=0.3 mmol/L,pH0 6.0).
In order to validate the wide application of ICPB,Cu-oxalate,Cu-tartrate,Cu-EDTA,Cd-citrate,and Pb-citrate were selected to further evaluate the performance toward heavy metal complexes.For Cu-EDTA,about 70% removal rate was observed after reaction for 5 h.The proposed ICPB system showed satisfactory performance toward Cu-oxalate and Cu-tartrate,for which,the removal efficiencies of Cu were both more than 80% during 5 h (Fig.S8 in Supporting information).As for Cd-citrate and Pb-citrate,only 1 h reaction time was required to achieve satisfactory metal removal efficiency (Fig.S9 in Supporting information).Moreover,tap water and Yangtze River water were sampled as real water background for treatment by ICPB system.As displayed in Fig.4,better performance of ICPB for treating Cu complex water was found than that of single photocatalysis considering removal efficiency of Cu and TOC.For tap water,TOC removal efficiency of the ICPB and photocatalysis were 55% and 41%,while Cu removal efficiency could be achieved at 81.3% and 65.1%.Similar trends were observed for Yangtze River water.The ICPB system could eliminate about 83.9%of Cu and 55% of TOC.The removal efficiency of Cu complex in Yangtze River water was partially inhibited,which may be caused by the consumption of active radical of coexisted natural organic matter (NOM).Such results demonstrated that ICPB is a promising strategy for removal of Cu complex from water.
Decomplexation of Cu complex and recovery of Cu element was achieved simultaneously in the proposed ICPB system.Cu complex removal by ICPB process was more efficient than that of single photocatalysis.The main mechanisms for Cu removal included:1) Cu complex was destroyed in the aid of active radical and microorganisms;2) free Cu ions were eliminated through biological adsorption;3) part of Cu2+was reduced to Cu0that deposited on the surface of the material.The satisfactory performance of ICPB process in dealing with various Cu complexes and authentic water further validates its potential application in real wastewater treatment.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the Central Government Guidance for Local Science and Technology Development Projects for Hubei Province,China (No.2019ZYYD068),National Natural Science Foundation of China (No.51908432),and Natural Science Foundation of Hubei Province (No.2018CFB397).The author would like to thank Shiyanjia Lab (www.shiyanjia.com) for the gene sequencing services.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.009.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
