Exploiting styrene-maleic acid copolymer grafting chromatographic stationary phase materials for separation of membrane lipids
Mengying Liang,Delu Liu,Yangyang Nie,Yanli Liu,Xiaoqiang Qiao
College of Pharmaceutical Sciences,Hebei Province Key Laboratory of Analytical Science &Technology,Key Laboratory of Medicinal Chemistry and Molecular Diagnosis,Ministry of Education,Hebei University,Baoding 071002,China
Keywords:Styrene-maleic acid copolymer Mixed-mode Stationary phase Chromatographic separation Lipids
ABSTRACT High performance liquid chromatography-mass spectrometry is one of the most commonly used strategies for lipid analysis.The development of versatile chromatographic stationary phases to meet the increasing demands for separation of complex lipids is very important.Styrene-maleic acid (SMA) copolymer is an amphiphilic polymer,which has been proven to have the ability to solubilize lipid molecules of various structures.In this study,styrene-maleic anhydride copolymer coated silica was first prepared by the thiol-ene click reaction.With L-cysteine hydrochloride or dodecanol as the post-modification reagents,Sil-SMA-amino acid and Sil-SMA-dodecanol stationary phase materials were further successfully fabricated via nucleophilic ring-opening reaction.The Fourier-transform infrared,thermogravimetric analysis,and elemental analysis results confirmed the two stationary phase materials were successfully prepared.Furthermore,both the Sil-SMA-dodecanol column and the Sil-SMA-amino acid column possessed reversed-phase/hydrophilic interaction/ion exchange mixed-mode retention mechanisms.The column efficiency of the Sil-SMA-derivatives columns reached 77,300 N/m.Based on the mixed-mode retention characteristics,the Sil-SMA-derivatives columns achieved both the lipid classes and species separation via a single column.The Sil-SMA-amino acid column was further successfully used to separate lipid extract from gastric cancer cell membrane.All these results demonstrated that the SMA-based stationary phase materials have a good potential for use in lipid separation.
As the boundary of the cell,the cell membrane is abundant in various finely sculpted proteins and lipids.The membrane lipids fulfill multiple biological functions,such as serving as selective barriers to cells,providing fuel for energy-demanding cellular activities,regulating the structure and activity of membrane proteins,and acting as messengers or transforming into bioactive molecules for working in a variety of physiological and pathological processes[1–3].Severe imbalance of membrane lipids homeostasis is closely associated with metabolic diseases,immunological diseases,neurodegenerative diseases and cancers [4].A recent study demonstrated that membrane lipids remodeling occurred in red blood cells from COVID-19 patients [5].Some other studies have shown that bioactive lipids,such as anti-inflammatory lipoxin A4 and sphingolipid derivatives,may be beneficial in the prevention and treatment of COVID-19 [6,7].Besides,the study of membrane lipids is important for its own sake.Tens of thousands of membrane lipids not only differ greatly in polarity and abundance,but also include complex isomer forms,such assn-position isomers,double bond position isomers,double bond stereochemical isomers and enantiomers,which pose huge challenges for the multi-level structural identification of membrane lipids [8–11].
As an important approach for lipid separation and identification,high performance liquid chromatography-mass spectrometry(HPLC-MS) has been widely used.Through HPLC separation,lipid molecules can be separated according to classes or species,which can efficiently reduce matrix complexity and ion suppression effects during subsequent MS detection [12],thereby greatly expanding the identification coverage of lipid molecules.Moreover,the chromatographic separation process greatly reduces the limitations of MS on the identification of lipid isomers [13,14].The realization of high efficiency and robust separation of complex lipids largely depends on the types and performances of the chromatographic stationary phase materials [15–17].For example,traditional reversed-phase liquid chromatography (RPLC) columns (C18or C8)can achieve intra-class lipid separation based on hydrophobic differences deriving from their fatty acyl chain moieties [18,19].Normal phase liquid chromatography (NPLC) or hydrophilic interaction liquid chromatography (HILIC) columns generally use different mobile phase systems to separate lipids based on their polar headgroups.Despite a little lower resolution than NPLC,HILIC performs better in terms of reproducibility and mass spectrum compatibility [20,21].In general,chromatographic stationary phases suitable for lipid separation are relatively limited.Further development of specifically designed stationary phase materials targeting for lipid separation could meet the increasing demand for in-depth separation and identification of complex lipid samples.
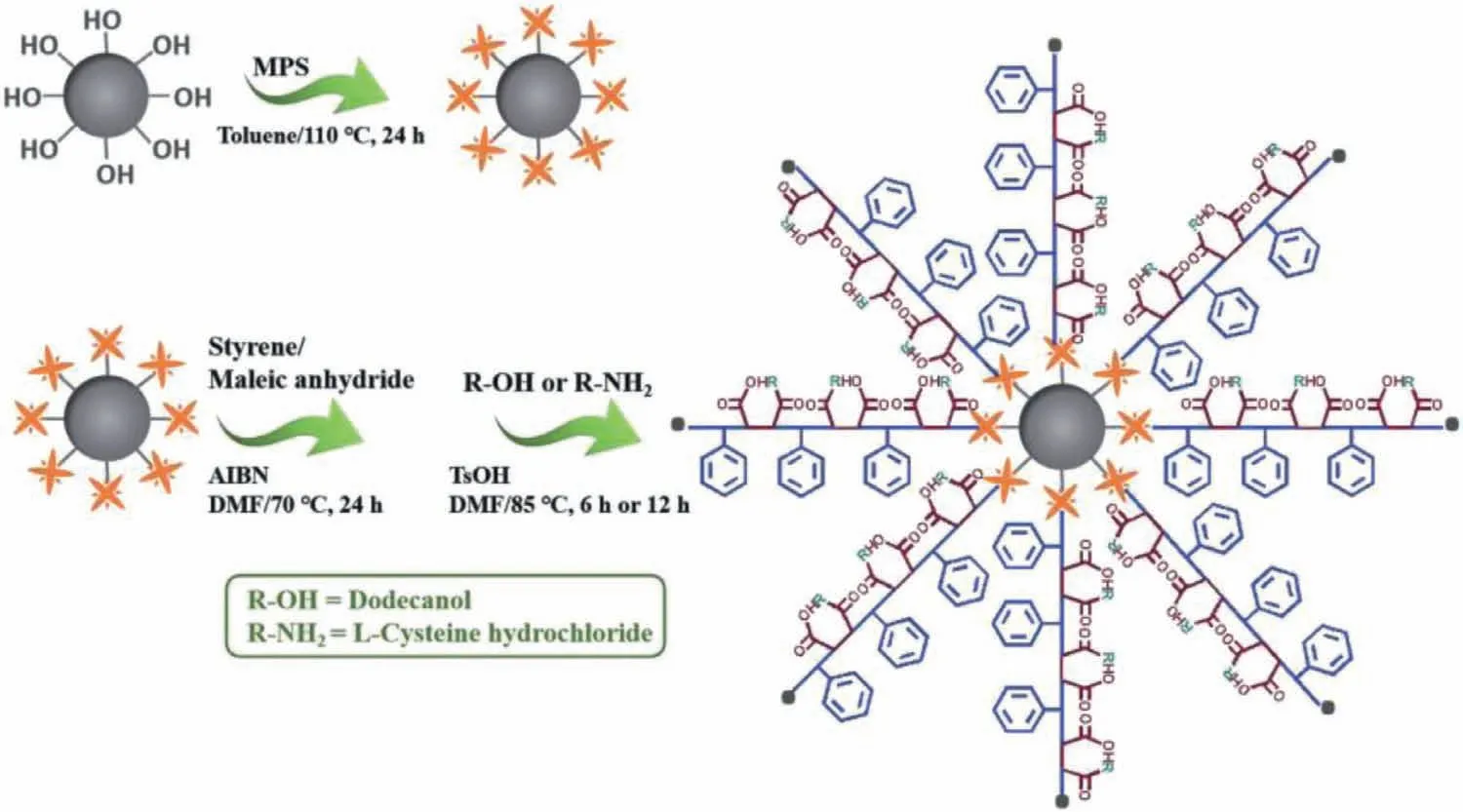
Fig.1.Synthesis of Sil-SMA-derivatives chromatographic stationary phases.
Tongeet al.first researched the membrane-solubilizing effect of amphiphilic styrene-maleic acid (SMA) copolymer which can transform lipid bilayers into stable polymer/lipid assemblies (nano-sized bilayer disks) [22,23].In the first study to make use of the benefits of this discovery,Knowleset al.utilized SMA copolymer to extract membrane proteins.Proteins PagP and bacteriorhodopsin can integrate into the SMA/lipid particles (SMALPs,also referred to as "native nanodiscs") with improved solubility [24].Since then,SMALPs technology has become a promising tool for membrane research,as it can simultaneously and non-selectively extract lipids and proteins from biological membranes [25].A series of SMA derivatives(such as SMA-ED,SMAd-A,SMA-EA,SMA-SH,SMA-Glu,SMI,and SMA-MA/EtA/PA) have been developed by post-modification of the anhydride groups in the styrene-maleic anhydride (SMAnh) copolymer,which exhibited enhanced pH and divalent cations tolerance and expanded application range [26–31].Furthermore,membrane lipids co-extracted in SMALPs have also been analyzed [32,33].SMA copolymer exhibited excellent solubilization ability to lipids of various headgroups,alkyl chains and configurations [34,35].
This study takes advantage of the excellent solubilization properties of SMA copolymers.They were first exploited as the chromatographic stationary phase materials to separate membrane lipids.For fabrication of the chromatographic materials,styrene and maleic anhydride werein-situpolymerized and grafted on the surface of spherical silicaviathe one-pot method.As a proofof-concept demonstration,L-cysteine hydrochloride and dodecanol were further utilized to post-modify the material (Fig.1).The performances of the Sil-SMA-dodecanol and Sil-SMA-amino acid stationary phases thus obtained were researched and they were further exploited to separate phospholipid standards and gastric cancer cell membrane lipid extracts.
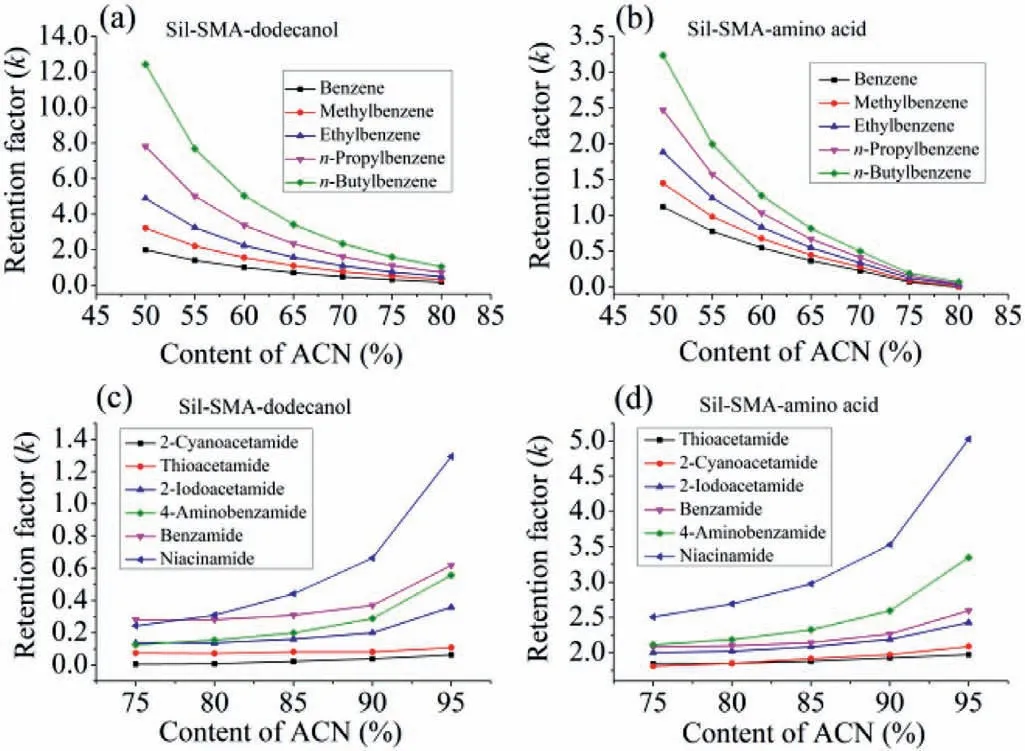
Fig.2.Effect of ACN content in mobile phase on chromatographic retention for hydrophobic alkylbenzenes by Sil-SMA-dodecanol column (a) and Sil-SMA-amino acid column (b),for hydrophilic amides by Sil-SMA-dodecanol column (c) and Sil-SMAamino acid column (d).Chromatographic conditions: mobile phase,ACN/H2O;flow rate,1.0 mL/min;UV detection wavelength,214 nm.
The Sil-SMA-dodecanol and Sil-SMA-amino acid stationary phases were first characterized by Fourier-transform infrared (FTIR).For Sil-SMAnh,the absorption peaks at 1495 cm−1and 1450 cm−1are attributed to the skeleton stretching vibration of the benzene ring,and the absorption peak at 700 cm−1represents the bending vibration of C–H of the benzene ring.The absorption peaks at 1860 cm−1and 1780 cm−1belong to the asymmetric and symmetric stretching vibration of C=O in maleic anhydride,respectively.For Sil-SMA-dodecanol and Sil-SMA-amino acid stationary phases,the weakening of the peak intensity at 1780 cm−1and the appearance of the peak at 1730 cm−1indicates that the anhydride groups were esterified or amidated (Fig.S1 in Supporting information).These results demonstrate the successful preparation of the Sil-SMA-dodecanol and Sil-SMA-amino acid stationary phase materials.The successful preparation of the Sil-SMA-derivatives stationary phase materials was further proved by thermogravimetric analysis (Fig.S2 in Supporting information) and elemental analysis(Table S1 in Supporting information).
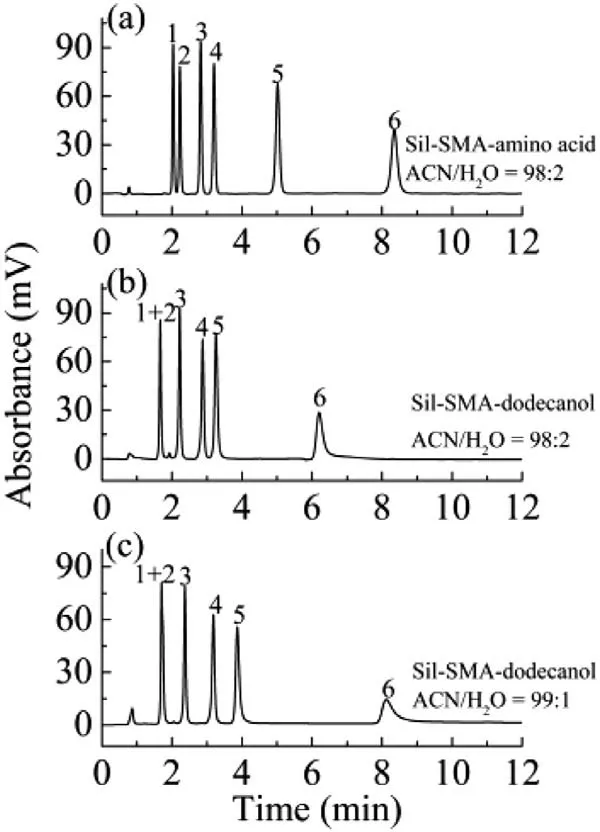
Fig.3.Chromatograms of amides separated by Sil-SMA-amino acid column (a) and Sil-SMA-dodecanol column (b and c).Chromatographic conditions: mobile phase,ACN/H2O (98:2,v/v) for (a,b),ACN/H2O (99:1,v/v) for (c);flow rate,1.0 mL/min;UV detection wavelength,214 nm.Analytes: (1) thioacetamide,(2) 2-cyanoacetamide,(3) 2-iodoacetamide,(4) benzamide,(5) 4-aminobenzamide,(6) niacinamide.
Considering that the Sil-SMA-dodecanol and Sil-SMA-amino acid stationary phase materials contain versatile groups,both the two packed columns can provide multiple interactions for the analytes.For example,the hydrophobic alkylbenzenes demonstrated RPLC retention mechanism on both the two columns (Figs.2a and b).However,compared with the Sil-SMA-amino acid column,the Sil-SMA-dodecanol column exhibited stronger retention under the same elution condition,derived from the stronger hydrophobicity of the introduced dodecanol groups.For the hydrophilic amides,both the two columns indicated HILIC retention characteristic while the Sil-SMA-amino acid column exhibited stronger hydrophilicity (Figs.2c and d).Moreover,ion exchange retention mechanism was also observed from both the two columns (Fig.S3 in Supporting information).The suitability of the columns was also investigated by separation of alkylbenzenes and amides for the Sil-SMA-dodecanol and Sil-SMA-amino acid columns,respectively (Fig.S4 in Supporting information).After continuous injection for 100 runs,similar chromatographic profiling was observed,except that the retention time of niacinamide slightly lagged.
In order to test the chromatographic performances of the Sil-SMA-derivatives columns,different kinds of samples were selected.For example,for the hydrophilic amides,6 amides were efficiently separated with sharp peaks on the Sil-SMA-amino acid column using acetonitrile (ACN)/H2O (98:2,v/v) as the mobile phase (Fig.3a).The tailing factors of the 6 peaks were between 0.95–1.12.The maximum theoretical plate number was up to 77,300 N/m.Under the same conditions,the retention ability of these amides on the Sil-SMA-dodecanol column was weaker than that on the Sil-SMA-amino acid column,and the peaks of thioacetamide and 2-cyanoacetamide were completely stacked together (Fig.3b).When further decreasing the proportion of water in the mobile phase to 1%,the resolution of thioacetamide and 2-cyanoacetamide on the Sil-SMA-dodecanol column did not improve (Fig.3c).
More hydrophobic phenols were further used to test the chromatographic properties of the Sil-SMA-derivatives columns.From Fig.S5 (Supporting information),we can see the 6 phenols were fully separated on Sil-SMA-amino acid column within 14 min,with the tailing factors of 0.93–1.05.The highest theoretical plate number reached 52,400 N/m.Under the same conditions,only 5 compounds were eluted on the Sil-SMA-dodecanol column on the Sil-SMA-dodecanol column even though the separation time was prolonged to 20 min.For the weakly polar and basic anilines,the two columns also indicated different separation selectivity (Fig.S6 in Supporting information).
RPLC and HILIC are the most commonly used chromatographic modes for lipids separation.In HILIC mode,the lipids are often separated according to the properties of their polar headgroups which define the lipid classes.In RPLC mode,lipids are usually separated based on lipophilicity differences caused by the various lengths,geometries and double bonds numbers of acyl-or alkyl-chains which define the lipid species.Usually,it is difficult to achieve both classes and species separationviaa single HPLC column.Herein,we exploited the separation potential of the Sil-SMA-derivatives columns for both the lipid classes and species.As shown in Fig.4a,with Sil-SMA-amino acid column as an example,the phosphatidylcholine (PC) standards of lysoPC,DMPC,DPPC,DOPC,and DSPC were eluted and separated according to their hydrophobicity within 20 min using methanol/ACN/water(80:10:10,v/v/v) as the mobile phase.All the samples showed good peak shapes without obvious tailing phenomena.As shown in Fig.4b,the phosphatidyl ethanolamine (PE) standards of DPPE,DOPE,and DSPE were also efficiently separated with the mobile phase of methanol/ACN/water (40:40:20,v/v/v).DPPG,DPPS,DPPE,and DPPC possess identical acyl chains but belong to different lipid classes.As shown in Fig.4c,withn-hexane/isopropanol/H2O/glacial acetic acid/triethylamine (5:81:14:1.5:0.08,v/v/v/v/v) as mobile phase A and methanol as mobile phase B,the different classes of phospholipids were also efficiently separated.Obviously,on basis of the mixed-mode retention characteristics,the Sil-SMAderivatives columns can simultaneously achieve separation of both lipid classes and species,showing good application potential for the comprehensive analysis of lipid molecules in biological samples.
For further test the separation performances for real samples,the Sil-SMA-amino acid column was further used for separation of lipids extracted from gastric cancer cells.Withn-hexane/isopropanol/H2O/glacial acetic acid/triethylamine(5:81:14:1.5:0.08,v/v/v/v/v)/methanol (45:55,v/L-cysteine hydrochloride and dodecanol were utilized to post-modify the stationary phase materials.The new Sil-SMA-dodecanol and Sil-SMA-amino acid columns indicated mixed-mode RPLC/HILIC/ion exchange separation characteristic and also exhibited different separation selectivity for both hydrophobic and hydrophilic compounds.The column efficiency of the Sil-SMA-derivatives columns reached 77,300 N/m.One of the important characteristics of the Sil-SMA-derivatives columns is that both the lipid classes and species can be separatedviaa single column.The Sil-SMA-amino acid column was successfully used to separate lipids extracted from gastric cancer cells.In subsequent research,versatile Sil-SMA-derivatives stationary phases,by connecting more possible post-modification reagents,will be further designed and fabricated,dedicated to improving the separation and identification capabilities of complex lipid samples.
In conclusion,SMA derivatives were first exploited as the coating materials for fabrication of HPLC staationary phases to separate membrane lipids.As a proof-of concept demonstration,L-cysteine hydrochloride and dodecanol were utilized to post-modify the stationary phase materials.The nwe Sil-SMA-dodecanol and Sil-SMAamino acid columns indicated mixed-mode RPLC/HILIC/ion exchange separatoin characteristic and alsoexhibited different separation selectivity for both hydrophobic and hydrophilic compounds.The column efficiency of the Sil-SMA-derivatives columns reached 77,300 N/m.One of the important characteristics of the Sil-SMAderivatives columns is that both the lipid classes and species can be separatedviaa single column.The Sil-SMA-amino acid column was successfully used to separate lipids extracted from gastric cancer cells.In subsequent research.versatile Sil-SMA-derivatives stationary phases,by connecting more possible post-modification reagents,will be further designed and fabricated,dedicated to improving the separation and identification capabilities of complex lipid samples.
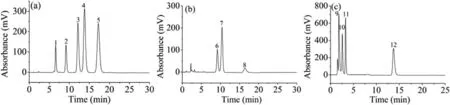
Fig.4.Chromatograms of phospholipid standards separated by Sil-SMA-amino acid column.Chromatographic conditions: mobile phase,methanol/ACN/H2O (80:10:10,v/v/v)for (a),methanol/ACN/H2O (40:40:20,v/v/v) for (b), n-hexane/isopropanol/H2O/glacial acetic acid/triethylamine (5:81:14:1.5:0.08,v/v/v/v/v)/methanol (45:55,v/v) for (c);flow rate,1.0 mL/min;ELSD detection.Analytes: (1) lysoPC,(2) DMPC,(3) DPPC,(4) DOPC,(5) DSPC,(6) DPPE,(7) DOPE,(8) DSPE,(9) DPPG,(10) DPPS,(11) DPPE,(12) DPPC.
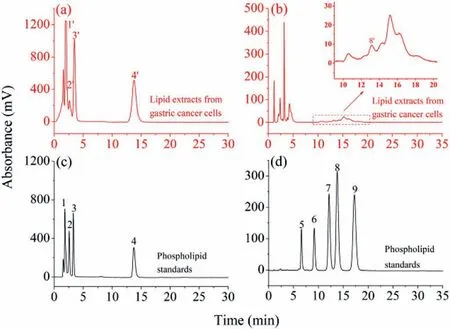
Fig.5.Chromatograms of lipid extracts from gastric cancer cells (a,b) and phospholipid standards (c,d) via Sil-SMA-amino acid column.Chromatographic conditions: mobile phase, n-hexane/isopropanol/H2O/glacial acetic acid/triethylamine(5:81:14:1.5:0.08,v/v/v/v/v)/methanol (45:55,v/v) for (a,c),methanol/ACN/H2O(80:10:10,v/v/v) for (b,d);flow rate,1.0 mL/min;ELSD detection.Analytes: (1)DPPG,(2) DPPS,(3) DPPE,(4) DPPC,(5) lysoPC,(6) DMPC,(7) DPPC,(8) DOPC,(9)DSPC.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the financial support from National Natural Science Foundation of China (No.21675039),Natural Science Foundation of Hebei Province (Nos.B2019201327 and H2019201170),Hundred Outstanding Innovative Talents in Universities of Hebei Province(No.SLRC2019016),Young Talent of Hebei Province,Natural Science Interdisciplinary Research Program of Hebei University (No.DXK201912).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.008.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
