Combining metal-microbe and microbe-microbe dual direct electron transfer on Fe(0)-cathode of bio-electrochemical system to enhance anaerobic digestion of cellulose wastewater
Yng Li,Ying M,Jingjing Zhn,Yobin Zhng,Zisheng Zho,Zhiqing Zho,∗
a School of Ocean Science and Technology,Dalian University of Technology,Panjin 124221,China
b School of Ecology and Environment,Zhengzhou University,Zhengzhou 450001,China
c Key Laboratory of Industrial Ecology and Environmental Engineering (Dalian University of Technology),Ministry of Education,School of Environmental Science and Technology,Dalian University of Technology,Dalian 116024,China
Keywords:Fe(0)-cathode CH4 production Anaerobic digestion Direct electron transfer Microbial electrochemical system
ABSTRACT Considering that cathode of microbial electrochemical system (MES) is a good electrons source for methane production via direct/indirect electron transfer to electroactive microorganisms,and that Fe(0)is also a confirmed electron donor for some electroactive microorganisms through metal-microbe direct electron transfer (DET),Fe(0)-cathode was equipped into an MES digester to enhance cathodic methane production.The results of this study indicated that the potential DET participator, Clostridium possibly obtained electrons directly from Fe(0)-cathode via metal-microbe electrons transfer,then transferred electrons directly to the definite DET participators, Methanosarcina/Methanothrix via microbemicrobe electrons transfer for CH4 production.In addition, Methanobacterium is another specially enriched methanogen on Fe(0)-cathode,which might obtain electrons directly from Fe(0)-cathode to produce CH4 via metal/electrode-microbe DET.The increment of conductivity of cathodic sludge in Fe(0)-cathode MES digester (R1) further confirmed the enrichment of electroactive microorganisms participating in DET process.As a consequence,a higher CH4 production (1205–1508 mL/d) and chemical oxygen demand (COD) removal (79.0%-93.8%) were achieved in R1 compared with graphite-cathode MES digester(R2,720–1090 mL/d and 63.6%-85.6%) and the conventional anaerobic digester (R3,384–428 mL/d and 35.2%-41.0%).In addition,energy efficiency calculated indicated that the output energy of CH4 production was 8.16 folds of electricity input in Fe(0)-cathode MES digester.
Microbial electrochemical systems (MES) are promising alternative processes for wastewater treatment and energy recovery using a biofilm on the electrode as the biocatalyst [1–4].Microbial bioanodes and biocathodes constitute the heart of these systems [5].Anode respiring bacteria metabolize biodegradable organic compounds then discharge electrons to anodic surface as an extracellular electron acceptor,for bacterial respiration,viaseveral electrontransfer pathways,such as direct electron transfer (DET) through membrane-bound c-type cytochrome [6],transfer using conductive pili [7],and self-mediated transferviaendogenous redox-active metabolites [8].Electrons flow to cathode and participate in the cathodic synthesis of chemical products,such as H2,CH4,acetate,with the aid of an external applied voltage and/or microorganism community [9–11].
Fe(0) is an alternative electrode material that has been applied in anaerobic wastewater treatment and electrochemical metal reduction,because of its unique reducibility [12–14].An anaerobic reactor equipped with a pair of Fe(0)-graphite plate electrodes increased azo dye wastewater treatment efficiency significantly,which resulted from the special enrichment of the functional microorganisms and the increment of extracellular polymeric substance on anodic surface [15].Fe(0)-electrode also achieved superior Cr(VI) removal efficienciesviareduction by Fe(0)-anode and adsorption of Cr(VI) to Fe(OH)3precipitates produced by Fe(0)oxidation [16].Moreover,Fe(0)-electrode can also help the cathodic synthesis of chemical products in MES.Methane production from the high-solid anaerobic digestion of waste sludge was enhanced by 22.4% in microbial electrolysis cells (0.3 V) with Fe(0)-graphite electrodes,due to the bioaugmentation effect on both anodic bacteria for enhancing VFA formation and cathodic archaea for methane production [17].Similarly,considerable formate (672 mg/L) was produced by cathodic CO2reduction from waste activated sludge digestion in an MES (−0.6 V) with Fe(0)plate and carbon pillar as the electrodes [18,19].However,most researchers equipped MES with Fe(0) as anode because of its active reducibility,which made Fe(0) sacrifice itself and result in the wastage of anode frequently [16,17].The anaerobic corrosion of Fe(0) structure is expensive to repair and can be environmental concern [12].
Actually,it has been known for over 100 years that the presence of anaerobic respiratory microorganisms can accelerate Fe(0) corrosion [12].Multiple studies have suggested that there are sulfate reducers,methanogens,and acetogens that can accept electrons from Fe(0) to support sulfate or CO2reduction [20–22].However,for a long time,all of the strains studied were believed to use H2as an electron donor for growth,because H2is known to be abiotically produced from Fe(0) [23,24].Recently,direct metal-microbe electron transfer was proved to occur between Fe(0) andGeobacter sulfurreducensstrain ACL,an autotrophic strain that was previously shown to grow with electrons derived from a graphite-cathode as the sole electron donor [12].Strain ACL still grew with Fe(0) as the sole electron donor and fumarate as the electron acceptor although the genes for the uptake of hydrogenase and formate dehydrogenases were deleted [12].It provides a possibility that strain ACL or some other species similar to strain ACL,may grow better with Fe(0)-cathode as direct electron donors.Considering that CO2reduction for methane or other organic products occurs possibly at cathode of MES [25,26],the microorganisms able to obtain electrons and live depending on Fe(0) or cathode would be further enriched with Fe(0) as cathode to produce more organic products.On one hand,the microorganisms,for example,methanogens,receive electrons directly or indirectly from cathode to participate in the cathodic reductive reactions.On the other hand,the electroactive microorganisms obtained electronsviadirect Fe(0)-microbe electron transfer from Fe(0)-cathode to form cathodic reductive products.Moreover,since the cathodic microorganisms capture electrons easily from cathode,Fe(0) will be protected from corrosion,consequently lowering the operating costs for replenishing Fe(0)materials.
Therefore,a pair of graphite-Fe(0) (graphite as anode and Fe(0)as cathode) electrodes imposed with an applied voltage of 0.6–1.2 V was equipped into an anaerobic digester to construct a Fe(0)-cathode MES digester.The artificial wastewater containing cellulose was used as the target pollutant,since it represented the complicated organic wastewater possibly produced both in industry and agriculture [27,28].The cellulose was aimed to be degraded at graphite-anode,and the electrons produced flowed to Fe(0)-cathode to participate in the reductive reactions for organic products.Scanning electron microscope (SEM),high-throughput sequencing and fluorescencein situhybridization (FISH) were used to analyze the microbial community structure on the anodic and cathodic surface and the abundance of functional microorganisms.In addition,the conductivity of sludge on cathodic surface was detected with the aim of further evaluating the presumption of possible direct electron transfer on Fe(0)-cathode.
In this experiment,a Fe(0) plate electrode(100 mm × 30 mm × 3 mm,cathode) and graphite plate electrode (100 mm × 30 mm × 3 mm,anode) with a distance of 30 mm between two electrodes were equipped in an up-flow anaerobic digester (Φ60 mm × 354 mm,working volume of 1 L) to form a graphite-Fe(0) MES digester (hereafter referred to as R1,named Fe[0]-cathode MES digester).The control experiments were conducted in the following digesters: a conventional electrochemical anaerobic digester that was the same structure and size as R1 but with graphite plates as both the cathode and anode (hereafter referred to as R2,named graphite-cathode MES digester);and a conventional anaerobic digester that was the same as R1 but without electrodes (hereafter referred to as R3,named conventional anaerobic digester).The information about the pretreatment for Fe(0) and graphite plates was descripted in Supporting information.The electrodes of R1 and R2 were supplied by a regulated DC power source of 0.6–1.2 V.These three digesters were operated under a hydraulic retention time (HRT)of 24 h,and the temperature was controlled at 35 ± 1 °C using a heating jacket system.
The anaerobic sludge collected from an anaerobic reactor in our laboratory (glucose as substrate with a concentration of 1000 mg/L) was used as seed sludge,which has never been explored to cellulose.The ratio of volatile suspended solids to total suspended solids (VSS/TSS) of the sludge was 0.68,and the initial TSS was about 15 g/L.Artificial cellulose wastewater composed of sodium hydroxyethylcellulose was employed in this study.NH4Cl and KHPO4were added as the nitrogen and phosphorus sources,along with trace elements (Zn,Cu,Ni,Co,H3BO3and EDTA) [13].
After being seeded with sludge,three digesters were fed with the artificial cellulose wastewater with a constant chemical oxygen demand (COD) of 5000 mg/L.The digesters were operated for about 180 days including three stages (each for 60 days): (1) applied voltage of 0.6 V,(2) applied voltage of 0.9 V,(3) applied voltage of 1.2 V.Each digester was sampled one time a day to monitor the effluent,and for each time,the triplicate samples were prepared to be determined to get the standard deviation in statistics.
COD,VSS and TSS were determined according to standard methods for the examination of water and wastewater.The concentration of Fe2+was determined using ortho phenanthroline spectrophotometry at 510 nm (Techcomp,UV-2301,Shanghai,China).But there was almost no Fe2+detected in this study.The pH was recorded using a pH analyzer (Sartorius PB-20,Germany).Concentration of methane,hydrogen and carbon dioxide produced were analyzed with a gas chromatograph (Shimadzu,GC-14C) equipped with a thermal conductivity detector and a 1.5 m stainless-steel column (Molecular Sieve,80/100 mesh).Temperatures of injector,detector and column were kept at 100,105 and 60 °C with argon as carrier gas at a flow rate of 30 mL/min [29].Volatile fatty acids (VFAs) were measured with another gas chromatograph (Shimadzu,GC2010) with a GC-flame ionization detector,FID (Shimadzu,Model 14B) and a 30 m × 0.32 mm × 0.5 μm fused silica capillary column (DB-FFAP).The operating temperatures for the injection port and the FID were 170 °C.The temperatures for the oven were gradually increased from 100 °C to 130°C at a rate of 5 °C/min.N2was used as the carrier gas with a flow rate of 30 mL/min.The conductivity of sludge in different digesters was measured according to Zhaoet al.[18].Reducing sugar was measured with a phenol-sulfuric acid method using glucose as a standard solution [17].
After the whole experiment,the sludge on all the electrodes was collected to analyze the microbial community composition.The electrodes in R1 and R2 were soaked in phosphate-buffered saline (PBS,0.13 mol/L NaCl and 10 mmol/L Na2HPO4at pH 7.2) for 2 h and washed three times to collect the sludge attached to them.In R3,the sludge was washed three times directly.Then the sludge samples were harvested by centrifugation (110 × 100gfor 15 min at 4 °C).For high-throughput sequencing analysis,the sludge on the anodic surface was used to analyze the bacterial community and the sludge on the cathodic surface was used to analyze the archaea community.For FISH,the sludge samples on both anodic and cathodic surfaces were analyzed to determine the abundance ofArchaea(red),Bacteria(green) andClostridiumspecies (blue).The FISH images obtained were imported to Image-Pro-Plus 6.0 for analysis of the relative abundance of microorganisms.The detailed methods of high-throughput sequencing and FISH were described in Supporting information.Cyclic voltammetry (CV) was used to evaluate the electrochemical response of cathodic sludge at the end of the experiment.The electrochemical measurements were performed on an electrochemical workstation (Zhenhua,CHI1030C,China).The detailed method of CV was described in Supporting information.Cellulase activities including endoglucanase,exoglucanase andβ-glucosidase were measured according to the operating manual of kit (mlbio,Shanghai,China),respectively.
Current density was calculated according to the following Eq.1:

whereIdis the current density (A/m2),Iis the measured current (A) andSis the effective area of the anodic electrode (here,3 × 10−3m2).
Anodic coulombic efficiency (CE) was calculated as the following Eq.2:

whereIis the measured current (A),tis the HRT (24 × 3600 s),nis the amount of the electrons (4 for 1 mol COD),Fis faradays constant (96,485 C/mol),Mis the molecular weight(32 × 103mg/mol),Vis the working volume of the digesters (1 L),andC0andC1are the COD concentrations in the influent and the effluent respectively (mg/L).
Energy efficiency (η[%]) relative to the electric energy supply(WE[J]) and energy output (WCH4[J]) was calculated by the following Eqs.3-5:
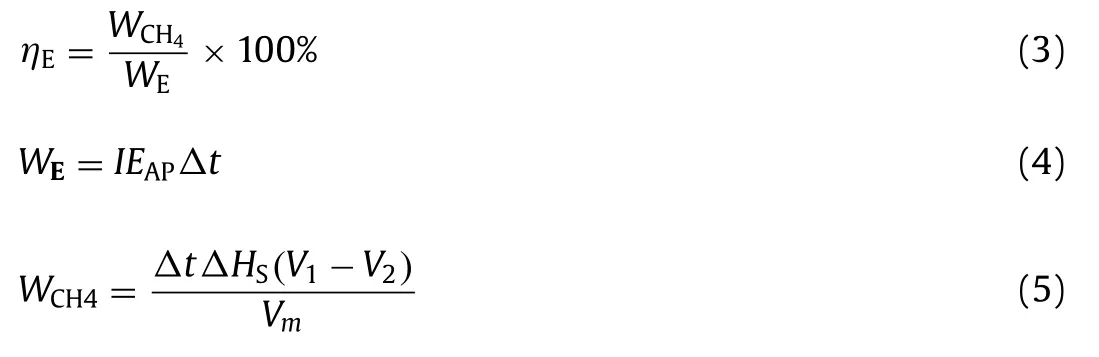
whereIis the average current per day (A),EAPis the applied voltage (1.0 V),Δtis the time of experiment (24 × 3600 s per HRT),ΔHsis the energy content of CH4based on the heat of combustion(upper heating value) (890.3 × 103J/mol),V1is the accumulative CH4production in the MES digesters (mL/h,R1 or R2),V2is the accumulative CH4production in the conventional anaerobic digester(mL/h,R3) andVmis molar volume of the gas under room temperature and atmospheric pressure (24.8 L/mol).
Cellulose is a chemically homogeneous linear polymer with up to 10,000 D-glucose molecules connected byβ-1,4 glucosidic bonds,which is considered as the most prominent single organic compound [30,31].The structural subunit of cellulose is cellobiose composed of glucose tilted 180° towards its neighbor[30].Therefore,hydrolysis of cellulose needs the participation of hydrolytic enzyme to produce soluble reducing sugar [32–34].The hydrolytic enzyme commonly includes endoglucanase,exoglucanase andβ-glucosidase: (1) Endoglucanase hydrolyzes the accessible intramolecularβ-1,4-glucosidic bonds [35,36];(2) Exoglucanase cleaves cellulose chains at the ends to release soluble cellobiose or glucose [35,36];(3) Thenβ-glucosidase hydrolyzes cellobiose to glucose [35,36].After hydrolysis,the reducing sugar was fermented and finally produced CH4[37].Therefore,COD,soluble reducing sugar,CH4and the hydrolytic enzymic activities were all detected in the experiment.
The effluent COD concentration was detected each day during the entire experiment as shown in Fig.1A.It showed clearly that the effluent COD in Fe(0)-cathode MES digester (R1) was lower than those in graphite-cathode MES digester (R2) and the conventional anaerobic digester (R3) throughout the experiment.In detail,with the applied voltage of 0.6 V,the effluent COD concentration gradually stabilized at about 530 mg/L,1820 mg/L,and 3240 mg/L in R1,R2,and R3 at the end of stage I.With the applied voltage increasing to 0.9 V,the effluent COD concentration decreased in R1 and R2 (310 mg/L and 720 mg/L),while it almost kept stable at 3100 mg/L in R3 similar to stage I.However,when the applied voltage increased to 1.2 V,the effluent COD concentration in R1 and R2 did not decrease further but increased in stage III compared with stage I and stage II.It meant that an applied voltage of 0.9 V was better for COD removal of cellulose wastewater,compared with a higher voltage (1.2 V) or a lower one (0.6 V).It may be because that a higher applied voltage could accelerate the anodic oxidative reaction and cathodic reductive reaction to some extent (0.9 V in this study for cellulose wastewater),but 1.2 V of applied voltage inhibited the reactions due to the occurrence of water electrolysis.
The COD removal supported the trends observed in the cellulose degradation results.The activities of hydrolytic enzyme and the concentration of the hydrolytic products,reducing sugar,were analyzed to compare the effects of Fe(0)-cathode MES digester on cellulose hydrolysis in three digesters.The activities of endoglucanase,exoglucanase andβ-glucosidase detected at the end of the experiment are shown as Fig.1C.Although all three types of cellulose hydrolytic enzyme activities increased in R1,compared with R2 and R3,theβ-glucosidase hydrolytic cellobiose activity increased the most.The activity ofβ-glucosidase was 0.44 U/mg in R3,while they were 0.63 U/mg and 0.58 U/mg in R1 and R2,respectively.It implied that microbial electrochemical process was beneficial for the hydrolysis of cellulose,especially in the process that Fe(0)-cathode participated in.The improvement of this process might be related to the quick consumption of reducing sugar in chemical reaction dynamics on the anodic surface of R1.This deduction was confirmed by the results of reducing sugar (Fig.1B).It showed that the concentration of reducing sugar in R1 was lower evidently compared with R2 and R3.For example,when the applied voltage was 0.9 V (stage II),the concentration of reducing sugar in R1 was as low as 7.5 mg/L,while they were about 24 mg/L and 79 mg/L in R2 and R3,respectively.It indicated that the hydrolytic product was metabolized further as intermediates and Fe(0)-cathode participating in microbial electrochemical process could also help accelerate the degradation of reducing sugar.
Since the concentration of reducing sugar in R1 was the lowest,it implied the occurrence of the reducing sugar fermentation to VFAs.Therefore,the concentration of VFAs was detected at the end of each stage to compare the effects of Fe(0)-cathode MES digester on fermentation of reducing sugar to VFAs.As shown in Fig.S1 (Supporting information),the concentration of VFAs including acetate,propionate and butyrate in R1 was also the lowest in all three digesters,similar to the results of reducing sugar.It showed that propionate and acetate constituted the majority of VFAs,and both of them in R1 were lower evidently than those in R2 and R3.Moreover,when the applied voltage was 1.2 V,the concentration of propionate in R2 (168 mg/L) was even higher than that in R3(128 mg/L),while it was only 51 mg/L in R1 that was lower evidently compared with R2 and R3.It implied the acceleration of all types of VFAs degradation by Fe(0)-cathode MES digester throughout the whole experiment.
During anaerobic digestion,VFAs were finally transferred into CH4,H2and CO2that were detected at the end of each stage in three digesters (Fig.S2 in Supporting information) [38].The results showed that at all three stages,the yield of CH4was the highest in R1,followed by R2 and that in R3 was the lowest.For example,at stage II (applied voltage of 0.9 V),the CH4yield was 1508 mL/d,1090 mL/d,and 384 mL/d in R1,R2,and R3.The result implied the acceleration of methanogenesis process in Fe(0)-cathode MES digester.However,H2production showed a different trend.H2was almost not detected in R1,while they were produced in R2 and R3,especially R2.It may be because that H2produced in R1 was consumed for methane production more quickly than that in R2 and R3.Although Fe(0) could react with water or H+to produce H2,Fe(0) as cathode would protect Fe(0) from corrosion.Therefore,the difference in H2production from Fe(0) corrosion could be negligible in this study,which was also confirmed by no detection of Fe2+concentration.
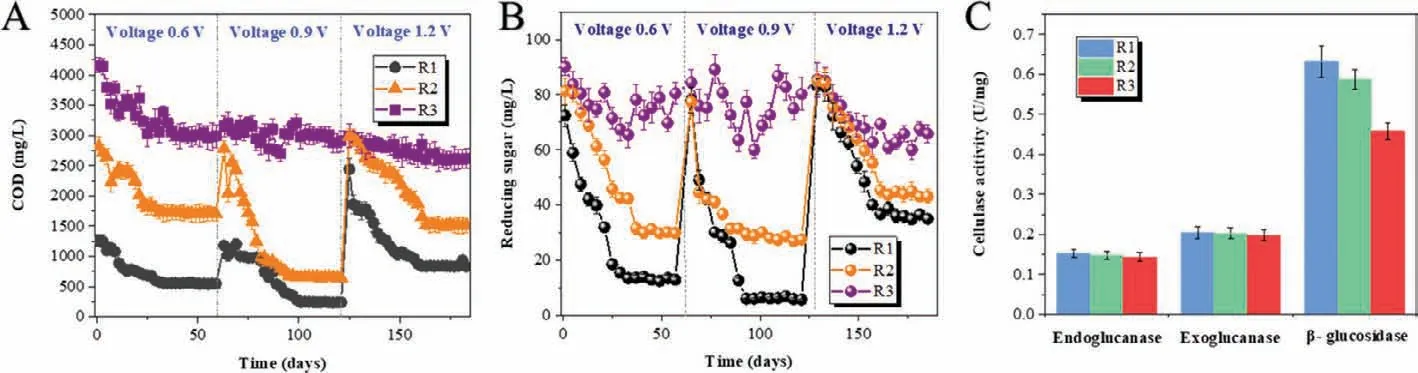
Fig.1.(A) Effluent COD concentration in three digesters during the 180-day experiments.(B) Effluent reducing sugar concentration in three digesters during the 180-day experiments.(C) The activities of cellulolytic enzyme in three digesters at the end of the experiment.Error bars represent the standard deviations of triplicate samples in each group.
The bacteria on anodic surface and archaea on cathodic surface in MES digesters (R1 and R2) were analyzed by high throughput sequencing as shown in Fig.S3 (Supporting information),which clearly showed that the abundance of bacteria and archaea were different in all three digesters.Petrimonas,Levillinea,Sphingomonas,Sedimentibacter,Saccharofermentans,SyntrophomonasandClostridiumwere the dominant bacteria in three digesters (abundance>1%),among whichLevillineandSyntrophomonashave the absolute outstanding abundance,especially in R3 (the suspended sludge).Instead,the abundance ofPetrimonasandClostridiumincreased at anodic surface in R1 (2.3% and 8.1%) compared with R2 (1.6% and 3.2%) and R3 (the suspended sludge,1.7% and 3.2%).Especially,Saccharofermentans,PetrimonasandLevilineaare typical sugar-fermenting bacteria commonly detected in the digestate of hemicellulose and cellulose (Liet al.,2018).SyntrophomonasandClostridiumare well-known H2and VFAs producers,coupled with H2-consumers,for example,methanogens [39].In addition,Clostridiumis well-known for producing a multienzyme cellulosedegrading complex called cellulosome [40].Recently,Clostridiumwas characterized as an electroactive bacteria because of its possible type IV pili gene,which could participate in the direct electron transfer between microorganisms and/or metal materials [41,42].Moreover,Geobacter,the most common electroactive bacteria,was also detected on the anodic surface in MES digesters (0.9% in R1 and 0.5% in R2),especially in Fe(0)-cathode MES digester,although its abundance was lower than 1%.In the conventional anaerobic digester (R3),there was noGeobacterdetected.Therefore,in Fe(0)-cathode MES digester (R1),after the oxidation of organics (reducing sugar included) at the anodic surface,electrons released by organics were transferred to anode accomplished by anodic electroactive microorganisms,ClostridiumandGeobacterdetected by high throughput sequencing (Fig.S3A) in this study.Then the electrons flew through external circuit to cathode to participate in the reductive reactions.
The archaea community structure on the cathodic surface (Fig.S3B) in MES digesters (R1 and R2) showed that the abundance ofMethanothrixandMethanosarcinain R1 (43.3% and 4.3%) were higher than those in R2 (37.8% and 0.9%) and R3 (the suspended sludge,40.7% and 1.8%).Instead,the total abundance of other methanogens includingMethanosphaerula,Methanospirillum,Methanobacterium,etc.were higher in R2 (54.5%) and R3 (52.7%)than those in R1 (50.1%).MethanosarcinaandMethanothrixboth have the ability to receive electrons directly from other electroactive microorganisms [43–45].MethanosphaerulaandMethanospirillumare both defined as hydrogenotrophic methanogens using H2as electron carriers for CH4production.In addition,Methanobac-teriumwas proved could obtain electrons directly from the surface of metal,like Fe(0) [46],and also was described mostly enriched on the cathodic surface of MES [26,47].Methanospirillumwas also observed on the surface of C-cathode in previous studies [48],which was consistent with the result in graphite-cathode MES digester in this study.
FISH was conducted to further compare the abundance of functional bacteria and archaeain situamong three digesters.The images (Fig.2) showed that the abundance of both bacteria on the anodic surface and archaea on the cathodic surface in R1 were higher than those in R2 (bacteria on the anodic surface and archaea on the cathodic surface) and R3 (the suspended sludge).Moreover,bacteria were also detected on the cathodic surface,especially in R1,which was higher than those in R2 (bacteria on the cathodic surface) and R3 (the suspended sludge).The special enrichment bacteria in R1 analyzed by high throughput sequencing,Clostridium,was also targeted by fluorescent dye (Cy5,blue) in FISH images.Clostridiumwas not only detected on the anodic surface in R1 and R2,but also detected abundantly on the cathodic surface in R1 with an abundance of 4.5%,while it was only 1.8%on the cathodic surface of R2 and 3.0% in the suspended sludge of R3.It indicated thatClostridiumwas also specially enriched on the cathodic surface of Fe(0)-cathode MES digester.
Commonly,on cathode of anaerobic MES,the electrons are captured by H+/CO2to form H2and/or CH4viachemical/biochemical process [25,26].In this study,CH4was produced in R1 with a higher yield compared with R2 and R3 during the whole experiment.Instead,H2was not detected in R1,but its yield in R2 was the highest.The results implied that CH4production was specially enhanced in Fe(0)-cathode MES digester.The FISH analysis showed that the abundance of bacteria (fluorescence intensity of green)was not only enhanced on the anodic surface,but also on the cathodic surface in R1,which were both higher than that in R2 (the anodic surface and the cathodic surface,respectively) and R3 (the suspended sludge).Interestingly,Clostridiumwas not only detected on the anodic surface,but also enriched on the cathodic surface of R1 with a higher abundance (4.5%) than R2 (1.8%,the cathodic sludge) and R3 (3.0%,the suspended sludge).The special enrichment ofClostridiumon Fe(0)-cathode in MES digester implied its relationship with Fe(0) materials.Actually,Fe(0) has been used to enhance anaerobic organics degradation for a long time,because of its unique reducibility [12,13].Fe(0) was also equipped into MES as anodes to donate more electrons for cathodic reductive reactions [16,17].As a consequence,the acceleration of cathodic reductive reactions was achieved at the sacrifice of Fe(0)-anode,which would result in the operating cost of replenishing Fe(0)-anode [16].Recently,Fe(0) was proved as the sole electron donor for fumarate reductionviathe direct metal-microbe electron transfer process[12].It indicated that some electroactive microorganisms,for example,Geobacter,could obtain electrons directly from Fe(0) [12].Clostridiumis well-known for its ability to metabolize complex organics into small pieces in anaerobic digestion [28].More than that,Clostridiumis also a potential DET participator likeGeobacter,since it has type IV pili [42].Type IV pili are thin filaments that are extended out from the cell,attach to a surface and then are retracted back into the cell,thereby pulling the bacterium in the direction of the site of attachment.The electrically conductive pili (e-pili) is one of type IV pili,and the bacteria owning e-pili is a typical symbol for having the ability of DET [41].Therefore,the special enrichment ofClostridiumon Fe(0)-cathode (R1,abundance of 4.5%),but not on graphite-cathode (R2,abundance of only 1.8%),was possibly because thatClostridiumobtained electrons directly from Fe(0)viametal-microbe electrons transfer.What is more,in this study,the electrons donated by Fe(0) did not result from the oxidation of Fe(0),but from cathodes,which consequently avoided the loss of Fe(0) materials.In addition,Clostridiumenriched on Fe(0)-cathode might relate closely with the improved CH4yield in Fe(0)-cathode MES digester.
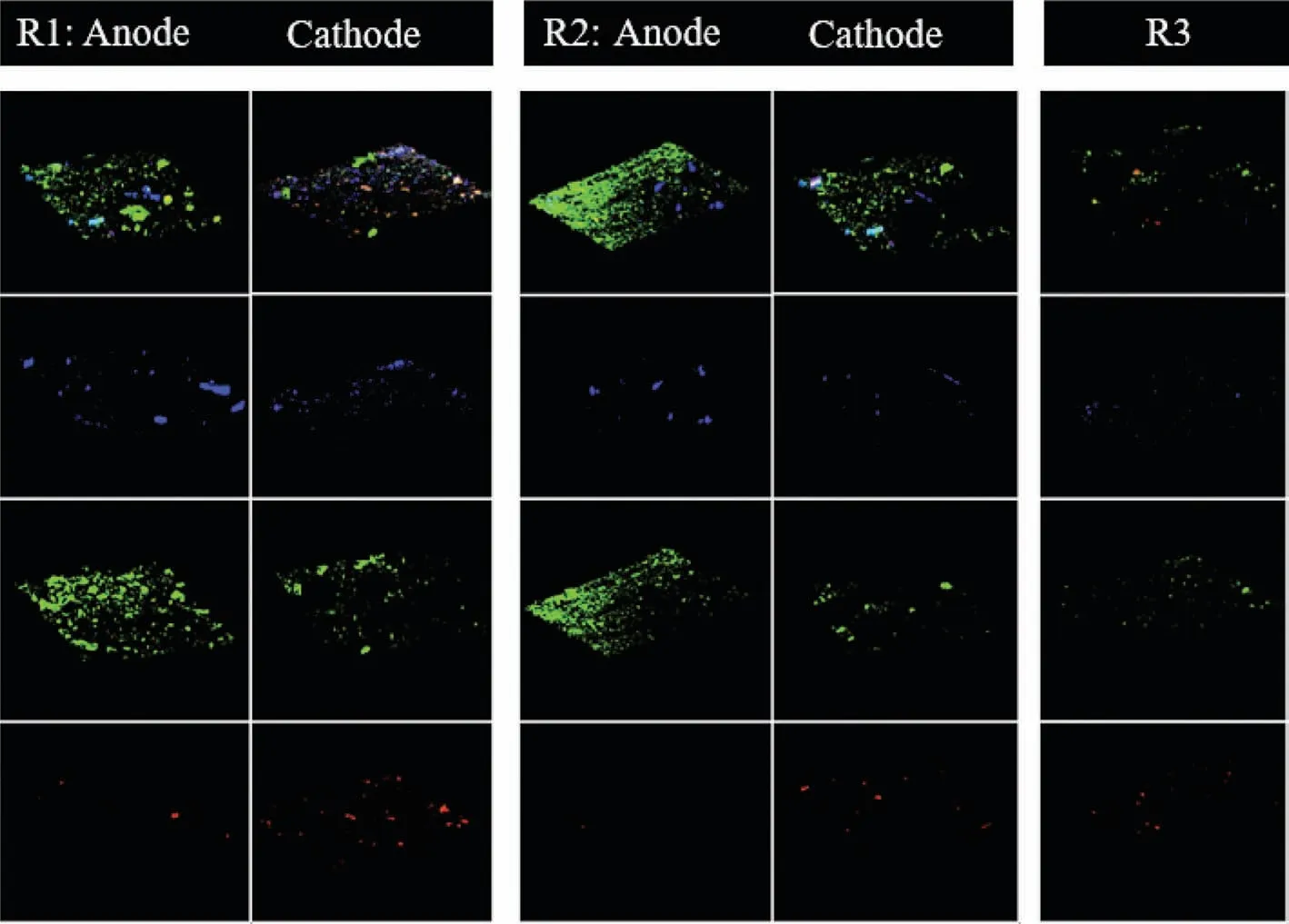
Fig.2.FISH images of Archaea (red), Bacteria (green) and Clostridium (blue) in the sludge of three digesters at the end of the experiment.
In theory,methanogens on the cathodic surface were reported to gain electrons from cathode directly or indirectly for CH4production [25,47].For example,the microbial electrosynthesis of CH4was successfully achieved usingMethanobacteriumattached to the graphite-cathode of anaerobic MES digester [26,47].Most researchers believed thatMethanobacteriumobtained electrons directly from cathode to produce CH4with CO2and H+[26,47].Similarly,cathodic methanogenesis process was also noted in some other methanogenic species,such asMethanospirillum,Methanococcus,MethanobrevibacterandMethanothermobacterin some previous studies [25,47,49],but if they could produce CH4viaDET from cathode was still unknown.However,sinceMethanobacterium,Methanospirillum,Methanococcus,MethanobrevibacterandMethanothermobacterwere all typical hydrogenophilic methanogens,they definitely could obtain electrons from H2produced by cathodes to reduce CO2for CH4production [1,51].In this study,Methanospirillumwas specially enriched on graphite-cathode of MES digester(R2) compared with other digesters.And H2yield in R2 was the highest among three digesters,which indicated that H2was possibly the electrons donor forMethanospirillumto produce CH4.However,in Fe(0)-cathode MES digester (R1),althoughMethanospirillumwas also more abundant compared with R3,it was less enriched than that in R2.Instead,Methanothrix,MethanosarcinaandMethanobacteriumwere specially enriched on the cathodic surface of R1.MethanosarcinaandMethanothrixwere recently proposed to participate in DET process during methane production in MES,but they were notably to interact with co-culture species(e.g.,Geobacter metallireducens)viamicrobe-microbe DET process to obtain electrons that were transferred byG.metallireducensfrom cathode first [44,45].The special enrichment ofMethanosarcinaandMethanothriximplied the possible existence of microbe-microbe DET on Fe(0)-cathodic surface of R1.
However,the best microbe-microbe DET partner ofMethanosarcina/Methanothrix,Geobacterwas not detected on the cathodic surface of R1 (data not shown) in this study,while the potential DET participator,Clostridiumwas enriched obviously.Therefore,it was reasonable to deduce that microbe-microbe DET occurred betweenClostridiumandMethanosarcina/Methanothrixon Fe(0)-cathodic surface of R1 to produce CH4.Detailly,Clostridiumobtained electrons directly from Fe(0)-cathodeviametal-microbe DET,then transferred electrons toMethanosarcina/Methanothrix viamicrobe-microbe DET for CH4production.However,in graphite-cathode MES digester (R2),the abundance ofMethanosarcina/Methanothrixdid not increase but decreased compared with R3,which indicated the special enrichment ofMethanosarcina/Methanothrixby Fe(0)-cathode in R1.In addition,another enriched methanogens,Methanobacteriumon Fe(0)-cathodic surface,was also surprised to be proved to have the ability to obtain electrons directly from Fe(0) to produce CH4viadirect metal-microbe electron transfer,which might imply the other DET pathway on Fe(0)-cathode in MES digester.
To clarify the role of Fe(0)-cathode MES on the anaerobic digestion of cellulose,current density and coulombic efficiency of R1 and R2 had been measured and recorded in Fig.3A.From Fig.3A the current density increased from 12.0 A/m2to 34.8 A/m2in R1 during stage I (applied voltage of 0.6 V),while it only increased from 11.2 A/m2to 22.0 A/m2in R2,indicating that anodic oxidation of organic matters was accelerated by Fe(0)-cathode in MES digester.Similar to stage I,the current density in R1 was always higher than that in R2 throughout stage II and stage III.But with the increment of applied voltage,the current density did not increase with the applied voltage when it increased to 1.2 V.The highest current density in both R1 and R2 were achieved in stage II with the applied voltage of 0.9 V,coincidently with the COD removal efficiency.Energy efficiency was calculated to compare the ratio of output chemical energy of methane production and the electrical energy input.As shown in Fig.3A,the energy efficiency decreased with the increment of applied voltage in both R1 and R2.However,it was always higher in R1 than that in R2 throughout the experiment.For example,in stage I the energy output in CH4vsenergy input as electricity were 8.16 and 5.09 folds in R1 and R2,respectively,while they were only 3.56 and 3.36 folds (R1 and R2) in stage II.
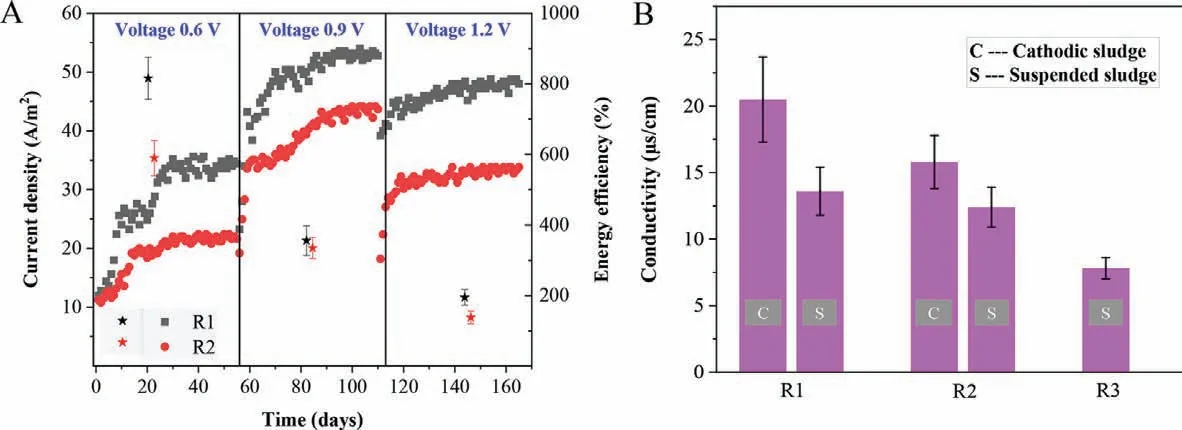
Fig.3.(A) Current density (square) and coulombic efficiency (star-shaped) in three digesters.Error bars represent the standard deviations of triplicate samples in each group.(B) Average energy efficiencies in MES digesters (R1 and R2) at each stage.Error bars represent standard deviations of data in each day of each stage.
T he conductivity of cathodic sludge was measured to prove the possible enrichment of electroactive microorganisms for DET(Fig.3B).It showed that the conductivity of the cathodic sludge in R1 was about 20.5 μS/cm,while it was 15.8 μS/cm of the cathodic sludge in R2.Although the conductivity of the suspended sludge in R1 (13.6 μS/cm) was also higher than that in R2 (12.4 μS/cm),the increment was not as much as that of the cathodic sludge.In addition,the conductivity of both the cathodic sludge and the suspended sludge in either R1 or R2 was higher than that of the suspended sludge in R3 (7.6 μS/cm).Since the electrically conductive pili (e-pili) is a typical symbol for the bacteria having the ability of DET [41],the increased conductivity of sludge in R1 possibly resulted from the enrichment of electroactive microorganisms as DET participators.Actually,MethanosarcinaandMethanothrixwere important DET participators,which,however,have no e-pili [50].Instead,Methanospirillumwas proved to have e-pili,which has not yet to be proved to participate in DET.Methanospirillumeven cannot accept electrons directly fromGeobacter sulfurreducensin coculture.It meant thatMethanospirillumcould contribute to a portion of conductivity of the cathodic sludge in R1 and R2,but obviously,it was not the participator of DET in this study.Interestingly,the abundance ofMethanospirillumon graphite-cathode in R2 was higher than that on Fe(0)-cathode in R1,but inversely the conductivity of the cathodic sludge in R2 was lower than that in R1.It implied thatClostridiummust contribute to another portion of the conductivity of the cathodic sludge in R1 and R2,especially in R1.The results further confirmed the possibility thatClostridiumcould participate in DET processes on Fe(0)-cathode of MES digester
In summary,Fe(0) was used as the cathode material of MES anaerobic digester to enhance the cathodic CH4production in this study.The possible pathways for CH4production in Fe(0)-cathode MES digester were shown in Fig.S4 (Supporting information).First,the possible combination of metal-microbe DET and microbemicrobe DET processes might occur on Fe(0)-cathodic surface.The potential DET participator,Clostridiumobtained electrons directly from Fe(0)-cathodeviametal-microbe DET.ThenClostridiumtransferred the electrons toMethanosarcina/Methanothrix viamicrobemicrobe DET to produce CH4(pathway 1 and reaction (i)).Second,Methanobacteriummight also obtain electrons directly from Fe(0)-cathodeviametal-microbe or electrode-microbe DET to produce CH4(pathway 2 and reaction (i)).Third,Methanobacterium,MethanosphaeralaandMethanospirillumproduced CH4viathe hydrogenophilic methanogenesis process with H2as electron donors generated by the cathodic reduction of H+(pathway 3 and reaction (ii)).Moreover,as shown in Fig.2,all the bacteria,especiallyClostridium,interwoved with methanogens (the colorful images formed by overlapping of blue,green and red) on Fe(0)-cathode in R1,which made the microorganisms transfer electrons easily between each other.Therefore,the CH4yield was strongly enhanced in Fe(0)-cathode MES digester.Instead,in graphite-cathode MES digester,the specially enrichedMethanospirillummainly produced CH4through hydrogenophilic methanogenesis process as the pathway 3 and reaction (ii).Of course,it cannot be ruled out the possibility of pathway 1 and pathway 2 occur in R2,sinceClostridium,Methanosarcina,MethanothrixandMethanobacteriumwere also detected,although their abundance was lower than those in R1.Finally,in Fe(0)-cathode MES digester,the highest current density and energy efficiency were achieved as 34.5−53.0 A/m2and 1.95–8.16 folds,accompanied with the highest COD removal (79.0%−93.8%) and CH4production (1205−1508 mL/d).However,the current density and energy efficiency were only 22.0−42.5 A/m2and 1.38−5.90 folds in graphite-cathode MES digester,and the COD removal was 63.8%−85.6% and CH4production was 720−1090 mL/d.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the financial support from the National Natural Scientific Foundation of China (No.52000020),and the National Natural Scientific Foundation of China (No.21876022).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.097.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
