Real-time effects of nicotine exposure and withdrawal on neurotransmitter metabolism of hippocampal neuronal cells by microfluidic chip-coupled LC-MS
Zhiyu Chen,Lei Fu,Xin-An Liu,Zhiyi Yng,Wenbo Li,Fng Li,∗,Qin Luo,c,∗
a Institute of Biomedical and Health Engineering,Shenzhen Institute of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,China
b University of Chinese Academy of Sciences,Beijing 100049,China
c Shenzhen Engineering Laboratory of Single-molecule Detection and Instrument Development,Shenzhen 518055,China
d Guangdong Provincial Key Laboratory of Brain Connectome and Behavior,CAS Key Laboratory of Brain Connectome and Manipulation,the Brain Cognition and Brain Disease Institute (BCBDI),Shenzhen Institute of Advanced Technology,Chinese Academy of Sciences,Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions,Shenzhen 518055,China
Keywords:Nicotine Microfluidic chip-coupled LC-MS Hippocampal neuronal cells Neurotransmitter metabolism Cognition
ABSTRACT Nicotine ingested from smoking exerts neuroprotection and developmental neurotoxicity in central nervous system.It can produce several changes of cognitive behaviors through regulating the release of different neurotransmitters in the brain.However,the effects of nicotine exposure or withdrawal on neurotransmitter metabolism of hippocampus are still unclear.In this study,we real-time evaluated the dynamic alterations in neurotransmitter metabolism of hippocampal neuronal (HT22) cells induced by nicotine exposure and withdrawal at relevant exposure levels of smoking and secondhand smoke by using a microfluidic chip-coupled with liquid chromatography-mass spectrometry (MC-LC-MS) system.We found HT22 cells mainly released related neurotransmitters of tryptophan and choline metabolism,both nicotine exposure and withdraw altered its neurotransmitters and their metabolites release.Exposure to nicotine mainly altered the secretion of serotonin,kynurenic acid,choline and acetylcholine of HT22 cells to improve hippocampal dependent cognition,and the change are closely related to the dose and duration of exposure.Moreover,the altered metabolites could rapidly recover after nicotine withdrawal,but picolinic acid was elevated.MC-LC-MS system used in present study showed a greater advantage to detect unstable metabolites than conventional method by using in vitro model,and the results of dynamic alterations of neurotransmitter metabolism induced by nicotine might provide a potential targets for drug development of neuroprotection or cognitive improvement.
Nicotine,a natural psychoactive ingredient in tobacco smoke,can easily cross the blood brain barrier and enter into the brain within 20 s after inhalation [1].The impacts of nicotine in central nervous system remains a paradox,it is not only a main disruptor of the functional development of fetal brain [2],but also can improve learning,memory and attention as a cognitive enhancer [3].As reported,nicotine can active nicotinic acetylcholine receptors,which mediated by changes in the release of neurotransmitters [4,5].Hippocampus serves as a fundamental and important structure of cognitive behaviors,and plays a role in nicotinemediated cognitive enhancement basing on the high expression of nicotinic acetylcholine receptors in this brain region [6].Therefore,the changes of hippocampal neurotransmitters release might be one of the important factors that nicotine affects cognitive behaviors of humans and animals.Characterizing hippocampal neurotransmitter metabolism might be an important strategy to elucidate the mechanisms of nicotine effects on cognition.Bothin vitroandin vivostudies have found that nicotine exposure and withdrawal caused the changes of acetylcholine,dopamine,serotonin,andγ-aminobutyric acid in hippocampus [7,8].However,the dynamic effects of nicotine on hippocampal neurotransmitters have been not extensively investigated,and were mainly limited to realtime and simultaneous detection of neurotransmitters.
Many analytical methodologies,such as electrochemical and optical biosensors,have been developed to continuously monitor the levels of neurotransmitters,but it is difficult to simultaneously measurement all neurotransmitters [9,10].Liquid chromatography coupled with mass spectrometry (LC-MS) is the most common analytical method for simultaneous identification and quantitation of different neurotransmitters due to its high sensitivity and selectivity [11].However,conventional LC-MS method cannot perform the real-time analysis of neurotransmitters.Recently,microfluidic chipcoupled with LC-MS (MC-LC-MS),integrating online cell dynamic culture and real-time LC-MS analysis,has great advantages in exploring cell-cell communications and interactions [12–15].Thus it has great potential in real-time monitoring the alterations of neurotransmitter metabolism in neuronal cells during different pharmacological or physiological processes.In this study,we investigated the acute and chronic effects of nicotine with human exposure levels on hippocampal neurotransmitter metabolism by using MC-LC-MS system.The results were expected to better understand the possible mechanism of nicotine ingested from smoking on cognitive.
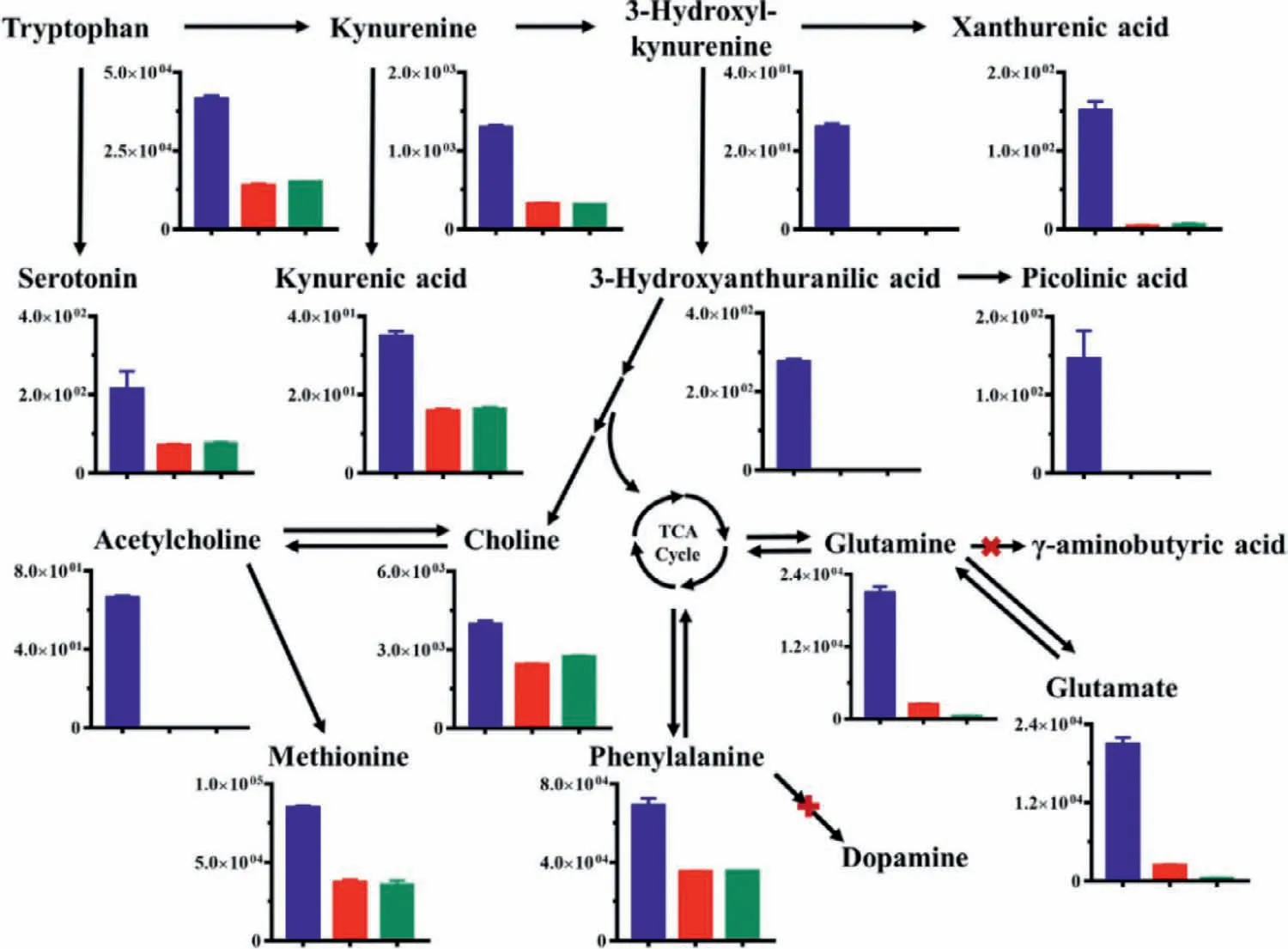
Fig.1.Secretion of neurotransmitters and their metabolites from HT22 cells culture on microfluidic chip and Petri dish.Data were presented in mean ± SD (n=4,the repetitive data was from the four repeated experiments).The y-axis is the concentration (ng/mL) of neurotransmitters and their metabolites in different groups.Blue,red and green columns represented the group of cells on microfluidic chip,cells on Petri dish and blank medium without cells,respectively.
HT22 cells were seeded into the cell chamber on microfluidic chip (Fig.S1 in Supporting information) and incubated in MC-LCMS system under 5% CO2air atmosphere at 37 °C.The morphology of cell on microfluidic chip was directly obtained and showed the cells grew well on the microfluidic chip (Fig.S2 in Supporting information),and the extracellular neurotransmitters and their metabolites were real-time analyzed by using LC-MS.The mass precursor ions,mass product ions,retention time,collision energy,Q1 prebias,Q3 prebias,limits of detection,limits of quantitation of these compounds were listed in Table S1 (Supporting information).As shown in Fig.1,total of 14 neurotransmitters and their metabolites of HT22 cells,consisting of tryptophan,serotonin,kynurenine,3-hydroxykynurenine,kynurenic acid,3-hydroxyanthranilic acid,xanthurenic acid,picolinic acid,choline,acetylcholine,glutamate,glutamine,phenylalanine,and methionine,were detected after dynamic culture on microfluidic chip.
Among them,10 compounds were also found in blank medium,but their extracellular levels in HT22 cells were significantly higher than blank medium (t-tests,P <0.05).It was suggested that HT22 cells mainly synthesized and released neurotransmitters involved in tryptophan and choline metabolism.The results are consisted with neuronal properties of HT22 cells,which exhibits functional cholinergic and serotoninergic properties,and mainly release serotonin,choline and acetylcholine [16].5-Hydroxyindoleacetic acid,γ-aminobutyric acid,dopamine,levodopa,epinephrine,norepinephrine,tyramine and 3,4-dihydroxyphenylethanol,were not detected in secretion of HT22 cells,might be attributed to HT22 cell-line that lack of the abilities to mediate dopamine metabolism andγ-aminobutyric acid metabolism [17].Compared with the secretions of HT22 cells cultured on Petri dish,our used online method showed greater potential to several metabolites,such as 3-hydroxykynurenine,3-hydroxyanthranilic acid,picolinic acid and acetylcholine (Table S2 in Supporting information).It was reported that both 3-hydroxykynurenine and 3-hydroxyanthranilic acid were rapidly oxidized under physiological conditions [18,19],and it is difficult to extract picolinic acid from biological matrix due to it was easily chelate with some metal ions [20,21].
Then HT22 cells were dynamic cultured and exposed to nicotine at concentrations of 12 ng/mL (low dose) or 50 ng/mL (high dose) under the flow rate of 10 μL/h.We evaluated the effects of nicotine acute (8 h) and chronic (48 h) exposure on neurotransmitter metabolism of HT22 cells by using partial least squaresdiscriminant analysis (PLS-DA) with unit variance scaling transformation.The established PLS-DA models had high goodness of fit and predictability (acute exposure:R2=0.879 andQ2=0.650;chronic exposure:R2=0.731 andQ2=0.439).The score plots of PLS-DA showed profound separations between different doses of nicotine exposure along the component 1 direction at acute exposure (Fig.2A),and only the high-dose group was significantly different from control group at chronic exposure (Fig.2B).It was revealed that nicotine exposure significantly altered the neurotransmitter release of HT22 cells,and its acute and chronic effects were closely related to the exposure doses.As shown in Figs.2C and D,after acute exposure to nicotine at low dose,the level of serotonin was significantly decreased and kynurenic acid was increased,and the levels of tryptophan,kynurenic acid,choline and acetylcholine were increased at high dose.Similar to acute exposure,the level of choline were also significantly increased at chronic exposure to high dose of nicotine.Meanwhile,kynurenic acid and kynurenine were significant down-regulated at chronic exposure to nicotine at high-dose group,and it was different from the effects of acute exposure.
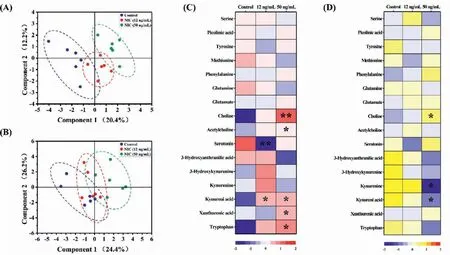
Fig.2.PLS-DA score plots and heat map obtained neurotransmitters profile of HT22 cells from the acute (A,C) and chronic (B,D) exposure group at different dose and control group.∗P < 0.05 and ∗∗P < 0.01 represented significantly differences between control and exposed group (n=6,the repetitive data was from the six repeated experiments.).
Choline is associated with brain health because it could be converted into acetylcholine,which plays a role in cognitive behaviors.Promotion of cholinergic transmission improved cognitive performanceviamodulating oxidative and neurochemical status in brain [22].The high dose of nicotine inhibited HT22 cell intake choline from medium,but enhanced release of acetylcholine only during acute exposure,indicating the choline acetyltransferase was activated.The release of hippocampal acetylcholine is important in memory processes,and nicotine as a cholinergic agonist can regulate its release in hippocampusin vitro[23].Moreover,nicotine can induced the high expression of hippocampal vesicular acetylcholine transporter and choline acetyltransferase [24].Kynurenic acid,a metabolite in the kynurenine pathway of tryptophan metabolism,is a neuroprotectant,antioxidant,and anα7 nicotinic acetylcholine receptor antagonist [25],and acute exposure to nicotine increased the release of kynurenic acid from HT22 cells.Notably,the alterations in tryptophan metabolism of HT22 cells at different nicotine exposure doses were not consistent,and the balance between the kynurenine and serotonin pathways was broken.It was reported that tryptophan depletion impaired cognition mediated by serotonin system [26].Low dose of nicotine competitively inhibited the serotonin biosynthesis,and the kynurenine pathway was activated at high exposure dose.It was consistent with the enhancement in kynurenine aminotransferase (KAT) activities of mice brain by nicotine administration [25,27].Chronic exposure to nicotine,the release of kynurenic acid was no changed at low dose,but a decrease was found at high dose,which might further enhance neuronal vulnerability [28].Our study extends earlier reports using hippocampal synaptosomes [29],and the depletion of serotonin in hippocampus facilitates spatial learning and memory by increasing hippocampal theta activity [30].Therefore,we inferred that the effects of nicotine on kynurenic acid metabolism might be multiphasic and be related to the exposure dose and duration of nicotine exposure,and nicotine exerted the neuroprotective effect on hippocampal neuronal cells and the positive effects in cognitive impairment.Meanwhile,the result of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay also showed an obvious improvement of cell viability after exposure to nicotine for 48 h at high dose (Fig.S3A in Supporting information).
Time-dependent response of neurotransmitter metabolism to nicotine exposure is of great important to better understanding the mechanisms of nicotine exposure on neuronal cells.The dynamic alterations of neurotransmitters and their metabolites induced by acute and chronic exposure to nicotine is showing in Fig.3.The fold-change of these compounds approximately ranged from 0.83 to 1.38 after exposure to nicotine at different dose,except for serotonin.Exposure to low dose of nicotine,serotonin was significantly decreased from 0.5 h to 16 h,a significant increase in kynurenic acid was found from 4 h to 8 h,and the decrease of 3-hydroxyanthranilic acid was only found at 16 h.Exposure to nicotine at high dose,the extracellular tryptophan,kynurenic acid,choline and acetylcholine were significantly increased from 0.5 h to 8 h.In the meantime,the increase of xanthurenic acid and 3-hydroxyl-kynurenine was only found at 0.5 h and 4 h,respectively,and the decrease of serotonin was only at 4 h.It is worth noting that the decreased kynurenine,kynurenic acid,and 3-hydroxyanthranilic acid,and the elevated choline were both found after 24 h when exposed to nicotine at high dose (Fig.S4 in Supporting information).These results demonstrated that serotonin pathway was more susceptible to lower dose of nicotine,but high dose of nicotine was more likely to disturb kynurenine and choline metabolism pathways.
Nicotine withdrawal cause irritability,anxiety,increased eating,dysphoria,and hedonic dysregulation.After withdrawal to nicotine for 24 h,all neurotransmitters and their metabolites had no significant difference,except for picolinic acid at high-dose group(Fig.4).It was suggested that the alterations of neurotransmitter metabolism induced by nicotine exposure have been recovered to normal,especially for secondhand smokers.Picolinic acid as an important antioxidant molecular and endogenous chelator,exerts a various bioactivities in central nervous system including neuroprotective abilityviaaltering metal-binding and intact glutamatergic afferent [31,32].MTT assay also showed the cell viability of HT22 cells was improved by withdrawal from nicotine for 48 h (Fig.S3B in Supporting information).Notably,picolinic acid was significantly increased from 0.5 h during withdrawal from nicotine.After withdrawal from nicotine for 0.5 h,the extracellular levels of kynurenine,kynurenic acid and choline of HT22 cells were not significantly different from controls,and the level of acetylcholine have no alteration after 4 h.It was indicated that the alterations of kynurenine and choline metabolism induced by nicotine exposure had returned to normal after nicotine withdrawal.
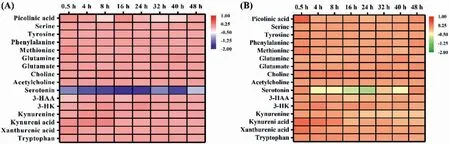
Fig.3.Real-time alteration of neurotransmitters levels of HT22 cells induced by exposure to nicotine at 12 ng/mL (A) and 50 ng/mL (B) based on log2 (fold-change).
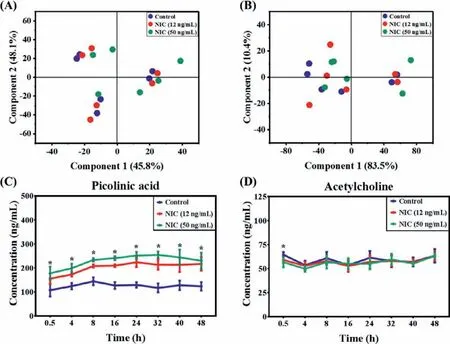
Fig.4.PLS-DA score plots obtained neurotransmitters profile of HT22 cells from the nicotine withdrawal for 24 h (A) and 48 h (B).Line graph showed dynamic alterations in extracellular levels of picolinic acid (C) and acetylcholine (D) of HT22 cells during nicotine withdrawal.Data are presented in means ± SD (n=6,the repetitive data was from the six repeated experiments.).∗P < 0.05 represented significant differences between control and nicotine-withdrawal group.
In summary,the effects of nicotine exposure and withdrawal on neurotransmitter metabolism of HT22 cells were investigated by using an integrated MC-LC-MS system for the first time,and this study highlighted possible protective mechanisms of nicotine on hippocampal dependent cognition.Exposure to nicotine at relevant levels of smoking and secondhand smoke significantly altered the tryptophan and choline metabolism related neurotransmitters release of HT22 cells,and their alterations was closely associated with the exposure dose and duration (Fig.S5 in Supporting information) [33].The acute exposure to nicotine is beneficial to protect the neurons,especially cognitive enhancement,and the elevated picolinic acid continually protected neuronal cognitive function after nicotine withdrawal.Therefore,nicotine could be considered to use for preventive action from cognitive deficits and memory impairment in neurodegenerative disorders.But it is worth noting that nicotine also has systemic adverse effects,such as highly addiction,organic damage,and carcinogenicity.Moreover,the ingestion of nicotine by cigarette smoking also needs to be considered carefully,because smoking is a well-known risk factor for psychiatric disorders and other diseases.In addition,our results need to further be verified by animal experiments and the effects of nicotine metabolites need to be evaluated.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.22076197),the Scientific Instrument Developing Project of the Chinese Academy of Sciences (No.YJKYYQ20200034),Shenzhen Engineering Laboratory of Single-molecule Detection and Instrument Development (No.XMHT20190204002),Shenzhen Science and Technology Innovation Commission (No.JCYJ20200109115405930),Basic and Applied Basic Research Foundation of Guangdong Province (No.2020B1515120080).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.060.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
