Tailoring biochar by PHP towards the oxygenated functional groups(OFGs)-rich surface to improve adsorption performance
Xinyue Xiong,Zhanglin Liu,Li Zhao,Mei Huang,Lihun Dai,Dong Tian,Jianmei Zou,Yongmei Zeng,Jinguang Hu,Fei Shen,∗
a Institute of Ecological and Environmental Sciences,Sichuan Agricultural University,Chengdu 611130,China
b Rural Environment Protection Engineering &Technology Center of Sichuan Province,Sichuan Agricultural University,Chengdu 611130,China
c Key Laboratory of Development and Application of Rural Renewable Energy,Biogas Institute of Ministry of Agriculture and Rural Affairs,Chengdu 610041,China
d Chemical and Petroleum Engineering,Schulich School of Engineering,the University of Calgary,Calgary T2N4H9,Canada
Keywords:Biochar Oxidative modification Phosphoric acid Hydrogen peroxide Oxygenated functional groups
ABSTRACT In this work,a modification method of H3PO4 plus H2O2 (PHP) was introduced to targetedly form abundant oxygenated functional groups (OFGs) on biochar,and methylene blue (MB) was employed as a model pollutant for adsorption to reflect the modification performance.Results indicated that parent biochars,especially derived from lower temperatures,substantially underwent oxidative modification by PHP,and OFGs were targetedly produced.Correspondingly,approximately 21.5-fold MB adsorption capacity was achieved by PHP-modified biochar comparing with its parent biochar.To evaluate the compatibility of PHP-modification,coefficient of variation (CV) based on MB adsorption capacity by the biochar from various precursors was calculated,in which the CV of PHP-modified biochars was 0.0038 comparing to 0.64 of the corresponding parent biochars.These results suggested that the PHP method displayed the excellent feedstock compatibility on biochar modification.The maximum MB adsorption capacity was 454.1 mg/g when the H3PO4 and H2O2 fraction in PHP were 65.2% and 7.0%;the modification was further intensified by promoting temperature and duration.Besides,average 94.5% H3PO4 was recovered after 10-batch modification,implying 1.0 kg H3PO4 (85%) in PHP can maximally modify 2.37 kg biochar.Overall,this work offered a novel method to tailor biochar towards OFGs-rich surface for efficient adsorption.
Biochar,a carbon-rich solid,is producedviabiomass pyrolysis in air-free environments,which provides an effective way for waste management [1].As an adsorbent,biochar has been widely investigated in water treatment to remove the organic and inorganic contaminants [2].Substantially,the adsorption performances of biochar greatly relate to its physicochemical characteristics,such as porous structure,surface functional groups (SFGs),ion exchange capacity [3].These characteristics mainly control the adsorption of various pollutants through the mechanisms of physical holding,hydrogen bonding,electrostatic interaction,complexation [4,5],etc.
However,it is undeniable that the prepared biochar displayed unstable properties from the precursors,the carbonization conditions,and the preparing processes [6].Consequently,various modification methods are widely employed to tailor biochar for the stable characteristics and the targeted performances [7–9].Chemical modification,mainly including acids,alkaline and oxidant,has been widely investigated,by which the surface characteristics were improved,especially,the SFGs were introduced abundantly,the adsorption performances to the pollutants thereby can be intensified[10–15].Besides,another modification method,physical modification contributed to the porous structure,the promoted adsorption performances are also closely associated with the improvement of the accessibility of SFGs [16–18].Hence,enriching SFGs on the biochar will be a powerful strategy to targetedly improve the pollutant removal in the environmental application.
Among various SFGs on biochar,the oxygenated functional groups (OFGs,e.g.,carboxyl,hydroxyl,carbonyl and ester groups)substantially more contribute to the adsorption behaviors of biochar [19,20].As reported,the hydroxyl group,carboxyl group and carbonyl group on biochar were formed after modification by KOH [21],phosphomolybdic acid [22],O3[23]and H2O2[15,24],and these modified biochars strengthened the adsorption of phenol,methylene blue (MB) and the heavy metals through electrostatic effect,hydrogen bonding,cation exchange and complexation,respectively.Thus,it can be hypothesized that oxidative modification in acid conditions will produce more carboxyl and carbonyl groups.In previous investigations,H3PO4plus H2O2(PHP) was employed to pretreat lignocellulosic biomass,in which the recovered by-product of lignin displayed abundant OFGs and efficient MB adsorption capacity [25,26].These results inspired us that PHP can offer the oxidative modification in acid conditions,by which biochar can be targetedly tailored for abundant OFGs,and improve the adsorption capacity of pollutants.
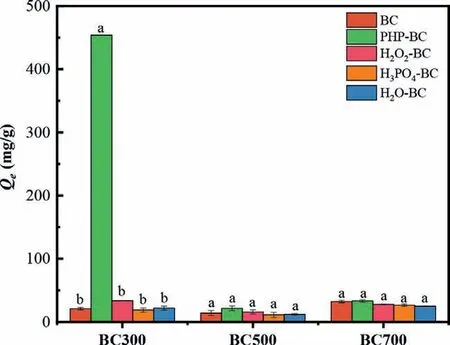
Fig.1.MB adsorption using PHP-modified biochars that were prepared at different pyrolysis temperatures.The modified conditions were 3.0 h and 60 °C,and the final fraction of H3PO4 and H2O2 in the employed PHP solution was 65.2% and 7.0%,respectively.
In this context,current work aimed to check the possibility of enriching OFGs on biochar by PHP modification.MB,an organic cationic pollutant,can be typically combined with OFGs on biochar by electrostatic interaction and hydrogen bonding [22].Thereby,MB was selected for adsorption test to reflect the potentially enriched OFGs by PHP modification.The physico-chemical characteristics of biochar before/after modification were investigated to check the aforementioned hypothesis.Biochars derived from various precursors were also modified by PHP to check the technical compatibility.Besides,the main modification conditions and the recovery of H3PO4were also discussed to show the potential in application.To achieve aims,the biochars were prepared,modified,characterized and analyzed,and their detailed information was displayed in Supporting information.
As mentioned above,PHP modification was hypothesized to oxidatively tailor biochar for more OFGs,by which the properties of biochar will be potentially stabilized,and targetedly improve the adsorption capability.To clarify this hypothesis,the harvested biochar at different pyrolysis temperatures using oak sawdust (300–700 °C) was attempted for modification.
According to Fig.1,MB adsorption (21.1–32.4 mg/g) of the parent biochars derived from different temperatures was not in good performances,suggesting the MB adsorption was controlled by multiple mechanisms.For example,the decreased adsorption(BC500
Besides,Fourier transform infrared spectroscopy (FT-IR) spectra of PHP-modified biochar before/after adsorption (Fig.S2 in Supporting information) indicated the typical peaks of MB at 885 cm−1and 1332 cm−1obviously appeared on the modified biochar after adsorption;the vibration of –C=O–O at 1704 cm−1was weakened after adsorption,suggesting the MB adsorption by the OFGs on the biochar [24].It was reported that H3PO5or H4P2O8will exist in PHP solution,which will be the strong oxidants for the modification [30].In this part,the PHP-modification was performed at 60 °C,and the thermal activation of the formed peroxy acids into free radicals,such as•OH and1O2,at this temperature [31].Besides,the persistent free radicals on the biochar will be a potential activator for the homolysis of peroxy acids to form the free radicals [32,33].These derived free radicals will trigger the oxidative modification for the biochar to generate more OFGs on the biochar[34].
As illustrated in Table S1 (Supporting information),C content in PHP-BC300 significantly decreased to 38.06%,which meant the carbon fraction in the BC300 chemically participated in the PHP modification.Besides,the O content of PHP-BC300 was 56.46%,which was significantly higher than the other biochars.Moreover,the relatively higher O/C and H/C of PHP-BC300 suggested the polarity was promoted,while the aromaticity was reduced by PHP modification,resulting in more hydrophilic surfaces and fewer aromatic structures [11].The FT-IR spectra (Fig.S3 in Supporting information) further indicated that PHP-BC300 exhibited significant differences with the BC300 at 1704 cm−1,and the increase of peak intensity corresponded to an increase in –C=O–O stretching vibration of carboxyl and ester,which was greatly related to the produced OFGs by PHP [24].The peaks of 1603 cm−1and 1514 cm−1on BC300 were mainly attributed to aromatic and aliphatic carbon–C=C stretching vibration [35,36],but the disappearance of 1514 cm−1and weakened vibration at 1603 cm−1happened on PHPBC300,which also supported the oxidation modification by PHP.The broadened adsorption peak at 3408 cm−1of PHP-BC300,corresponding to the stretching vibration of –OH in hydroxyl groups,also partially reflected more OFGs (–OH) formation after PHP modification.According to the X-ray photoelectron spectroscopy (XPS)spectra (Fig.S4a in Supporting information),the PHP-BC300 surface displayed the lowest C content and the highest O content comparing with other biochars.These results substantially proved the formation of OFGs by PHP modification.In addition,when the C 1s spectra were further de-convoluted (Fig.S4b in Supporting information),the aromatic and aliphatic –C–C/–C=C sharply decreased from 56.43% (BC300) to 38.28% after PHP modification,while the –C–O,–C=O,and –C=O–O in C 1s were significantly increased to 44.65%,8.79%,and 8.28%,respectively,comparing with their corresponding intensity in BC300 (37.73%,3.94%,and 1.90%,respectively) [24].These results well responded to the elemental content and the FT-IR spectra (Table S1 and Fig.S3),and again directly proved the largely generated OFGs after PHP modification.Additionally,in contrast with the BC300,a very lower ash content (0.58%) of PHP-modified biochar suggested the ash function on MB adsorption can be neglected (Table S1).Besides,no significant differences on X-ray diffractometry (XRD) spectra implied the oxidative modification did not alter the chemical skeleton (the graphitized structure) (Fig.S5 in Supporting information).Moreover,specific surface area (SSA),total pore volume,pore size,and scanning electron microscopy (SEM) images (Table S1 and Fig.S6 in Supporting information) reflected that the porous structure was not developed well regardless of the modification or not.These results suggested that the physical adsorption will not dominate the MB removal by PHP-modified biochar [37].Based on these discussions,it was concluded that the oxidative modification substantially happened in PHP modification to targetedly introduce OFGs on biochar,and facilitated the OFGs-dominated adsorption.
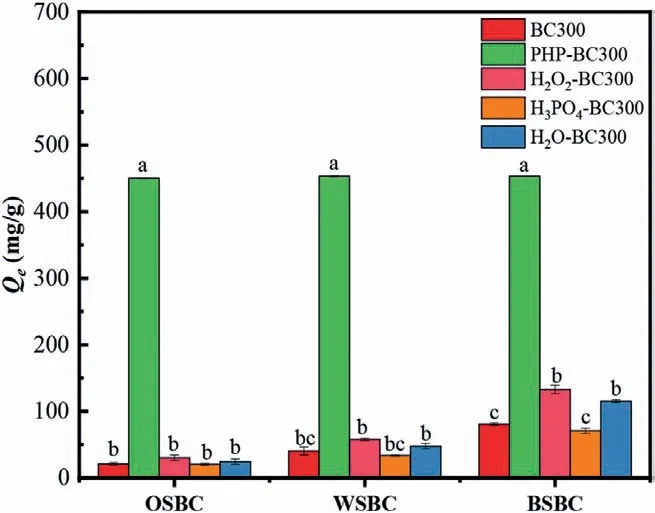
Fig.2.MB adsorption by the PHP-modified biochars derived from various lignocellulosic precursors.Carbonization temperature for parent biochars was 300 °C;the modified conditions were 3.0 h and 50 °C;the employed PHP solution with the final fraction of H3PO4 and H2O2 was 65.2% and 7.0%,respectively.
To investigate PHP-modification compatibility on the biochar from various precursors,oak sawdust (OS),wheat straw (WS) and birch sawdust (BS) were employed to prepare the corresponding biochars at 300 °C.In contrast with the BC300,the significant promotion on MB absorption did not happen on the separately“modified” biochars by the H3PO4and H2O regardless of the variety of parent biochars for the modification (Fig.2),suggesting the modification function by the separated H3PO4was very weak.Although the H3PO4was reported to be a modifier to improve the biochar adsorption capacity,the different modification processes and parameters may cause the inconsistent results [38,39].The H2O2-BC300 can promote the MB adsorption by 42.9%−64.3% using these three parent biochars,this may be attributed to the oxidative modification by the H2O2to introduce OFGs [15].By contrast,the PHP modified biochars significantly promoted the MB adsorption to 450.1–453.1 mg/g,which was 5.6–21.3 folds and 3.4–14.9 folds higher than the corresponding parent biochars and the corresponding H2O2-BC300,respectively.It was obviously proved that PHP displayed a positive modification on biochar by the synergism of H3PO4and H2O2,and achieved an excellent adsorption capacity.
Furthermore,based on the MB adsorption capacity of the biochars from these three precursors,the coefficients of variation(CV) of BC300,H2O2-BC300,H3PO4-BC300,and H2O-BC300 were calculated as 0.64,0.72,0.63 and 0.76,respectively,which suggested the distinguishable adsorption performances because of the different characteristics of precursors [40].By contrast,the CV of PHP-BC300 was only 0.0038,suggesting a relatively compatibility of the PHP-modification to the biochars derived from different lignocellulosic precursors regardless of the existing differences in biochar characteristics.Besides,according to literature comparison (Table S2 in Supporting information),MB adsorption by PHPmodified biochars from these 3 precursors were all superior to the mostly reported biochars (41.0–433.1 mg/g),although some of these reported biochars had higher SSA [22,24,41–44];this also proved that the superior MB adsorption efficiency by PHP-modified biochar was mainly attributed to the chemisorption of OFGs,rather than physical adsorption.
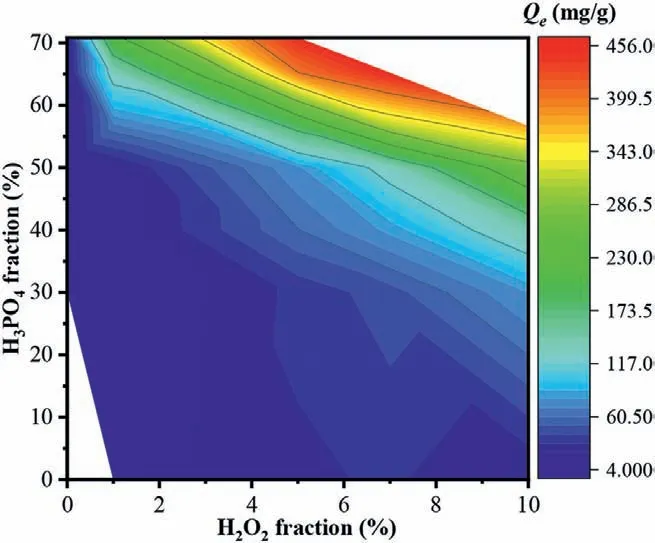
Fig.3.Effects of H3PO4 and H2O2 on the adsorption performances of the PHPmodified biochars.Carbonization temperature for parent biochars was 300 °C;the modified conditions were 3.0 h and 60 °C.
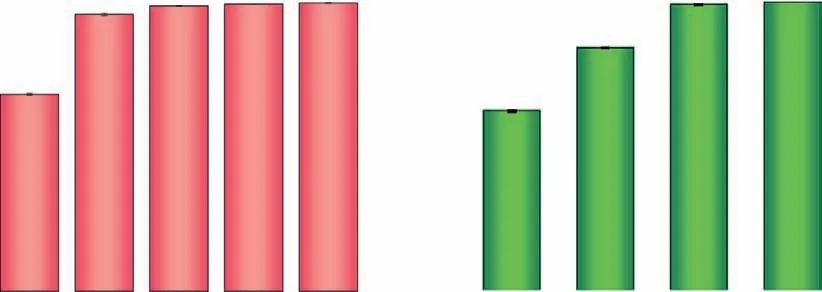
Fig.4.Effects of (a) modification time and (b) modification temperature on adsorption performances.Carbonization temperature for parent biochars was 300 °C;the employed PHP solution with the final fraction of H3PO4 and H2O2 was 65.2% and 7.0%,respectively.
To clarify the effects of H3PO4and H2O2on the MB adsorption performances,the wide scale modulation on the concentration of H3PO4(0%−70.8%) and H2O2(0%−10%) was investigated on oak biochar modification.Fig.3 showed that increasing H2O2concentration from 0% to 10% did not improve MB adsorption significantly (P >0.05),when no H3PO4was involved in the modification;the increase of H3PO4also did not significantly promote the MB adsorption (P >0.05) without H2O2involvement.These results suggested the desired modification performances could not be successful by the sole H3PO4or H2O2.Overall,MB adsorption was obviously promoted by PHP mixture.The increase of the H3PO4fraction can significantly promote MB adsorption,especially at final H3PO4concentration ≥30%;increasing H2O2fraction in PHP solution also improved the MB adsorption.These results indicated the synergistic effect for oxidative modification was intensified by the involved H3PO4or H2O2.It can be explained that the intensity of formed peroxy acid in PHP solution will be intensified by the input H3PO4and H2O2.The highest 10% H2O2fraction was involved in PHP modification achieved 6.3 mg/g increase on MB adsorption by 1.0% promotion on H3PO4fraction.By contrast,1.0% promotion on H2O2fraction increased MB adsorption by 7.6–86.2 mg/g on average.Obviously,the effect of H2O2fraction in PHP on biochar modification was stronger than that of H3PO4.The maximum MB adsorption of 454.1 mg/g was harvested with the PHP-modified biochar at the final concentration of H3PO4and H2O2was 65.2%and 7.0% in PHP solution,respectively.
Besides,oak biochar was PHP-modified for 1.0–5.0 h at 50 °C to clarify the effects on MB adsorption.Fig.4a indicated that adsorption capacity of PHP-BC300 arrived at 310.8 mg/g at modification time of 1.0 h;By contrast,adsorption capacity of H3PO4-BC300 and H2O2-BC300 (the modification time was 3.0 h) was only 18.7 and 33.8 mg/g,respectively (Fig.1).This result once again proved that the PHP-modification was more beneficial to the biochar adsorption of MB than that of H3PO4and H2O2alone.The adsorption capacity significantly promoted to 450.1 mg/g (P <0.05)with the removal efficiency (Re) of 90.1%,however,no significant variations were identified as longer modification were employed(>3.0 h) (P >0.05).It could be basically deduced that the chemical reaction existed in the PHP-modification,and the reaction will be equilibrated with longer reaction time.As displayed in Fig.4b,the PHP-modification temperature was promoted from 30 °C to 60 °C,MB adsorption of the modified biochar was increased significantly (P <0.05),and reached the maximum adsorption capacity of 454.1 mg/g withReof 90.8% after 60 °C modification.The improved modification effects by promoting temperature also suggested the chemical modification happened substantially,such as thermal activation of the formed peroxy acids into free radicals,which was greatly temperature-dependent in 20–60 °C [31].Besides,the very gentle modification temperature by PHP involvement exhibited the potential advantages on simplifying the modification process and reducing the energy input comparing with the reported H3PO4involved methods for biochar modification,such as H3PO4impregnation (47.5%−85%) plus pyrolysis (450 °C) [11,39].
Although the PHP-modification targetedly generated more OFGs on the modified biochar,the large amounts of employed H3PO4triggered us to recycle the input H3PO4for the modification.As showed in Fig.S7 (Supporting information),MB adsorption of modified biochar by the PHP using the recycled H3PO4still reached 437.3–448.0 mg/g in a successive 10 batches modification (P >0.05),which was closed to the first batch (454.1 mg/g).Besides,the average 94.5% H3PO4was recovered and recycled for the next batch modification,which meant PHP solution prepared by 1.0 kg H3PO4(85%) can modify 2.37 kg biochar in total;this will greatly decrease the input cost of H3PO4.Of course,average 5.5% H3PO4will be consumed in the water washing process for the modified biochar of each batch,thereby the P valorization in wastewater should be considered.According to current experience,the calcium hydroxide and calcium oxide were suggested to introduce the wastewater for the neutralization and the recovery P by precipitation.Besides,struvite [45]and lanthanum-based nanomaterial[46]were considered as advantageous methods to recover phosphorus from wastewater.Integrating this process will potentially make the whole process cleaner and more cost-efficient.
In summary,PHP was successfully employed to tailor biochar prepared at a low temperature (300 °C)viathe oxidative modification in acid conditions;OFGs were targetedly introduced on biochar,by which extremely high MB adsorption performances were achieved comparing with its parent biochar.PHP modification displayed wide adaptability to the biochars from various typical lignocellulosic precursors,suggesting the feedstock compatibility.The modification was significantly intensified by increasing temperature and time,and the H3PO4and H2O2fraction in PHP positively related to the modification,especially H2O2proportion.Besides,approximately 94.5% H3PO4was recovered for the next batch modification,suggesting 1.0 kg H3PO4(85%) in PHP can modify 2.37 kg biochar in total.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No.21978183).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.099.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
