A chemo-mechanical switchable valve on microfluidic chip based on a thermally responsive block copolymer
Sifeng Mo,Xiohong Hu,Yumi Tnk,Lin Zhou,Chenhn Peng,Nhoko Ksi,Hizuru Nkjim,Shungo Kto,Ktsumi Uchiym
a Department of Applied Chemistry,Graduate School of Urban Environmental Sciences,Tokyo Metropolitan University,Hachioji-shi,Tokyo 192-0397,Japan
b University Education Center,Tokyo Metropolitan University,Hachioji-shi,Tokyo 192-0397,Japan
Keywords:Microchip Valve Flow control NIPPAm Chemo-mechanical switch Thermo-responsive polymer
ABSTRACT Microfluidic devices have become a powerful tool for chemical and biologic applications.To control different functional parts on the microchip,valve plays a key role in the device.In conventional methods,physio-mechanical valves are usually used on microfluidic chip.Herein,we reported a chemo-mechanical switchable valve on microfluidic chip by using a thermally responsive block copolymer.The wettability changes of capillary with copolymer modification on inner surface were investigated to verify the function as a valve.Capillaries with modification of poly-(N-isopropylacrylamide-co-hexafluoroisopropyl acrylate) (P(NIPAAm-co-HFIPA)) with a 20% HFIPA was demonstrated capable of control aqueous solution stop or go through.Then short capillaries with copolymer modification were integrated in microchannels as valves.With the temperature changing around lower critical solution temperature (LCST),the integrated chemo-mechanical switchable valve exhibited excellent “OPEN–CLOSE’’behavior for microflow control.After optimization of the block copolymer sequences and molar ratio,a switching time as low as 20 s was achieved.The developed micro valve was demonstrated effective for flow control on microchip.
Microfluidic chip developed from 1990s has been widely used for sample pretreatment [1,2],immunoassay [3-5],chemical synthesis [6,7],cell analysis [8-10].Valve,as one of the key function parts,helps to control microflow in microfluidic device in desirable sequence [11,12].Various types of valves have been integrated in microchannels,including shut-off valve [13],normally closed valve [14,15],monolithic elastomer valves [16],and surface tension plug [17].Limited to the large size,most of the valves were difficult to integrate into channels on microscale [13–15].Surface tension plug was easy to integrate in microchannel,but it is not reversible.Recent years,Smart valves have attracted considerable attentions for dynamically controlling microflow transport [18,19].Micro/nano structured channels modified with stimuli-responsive polymers are frequently be designed as switchable smart valves[20].
Poly(N-isopropylacrylamide) (PNIPAAm) has been reported as an excellent thermal responsive polymer that owns a lower critical solution temperature (LCST) [21].The LCST could be adjust as wish when designing different copolymer with PNIPAAm [22,23].Surface grafted with the polymer could change its hydrophilicity as the environment temperature changes[24].Most studies have focused on surface property change and drug release [25,26].However,the design of a practical switchable valve based on PNIPAAm on microchip for temperature controlling the transportation of microflow remains challenging.
In this work,we report on a switchable valve integrated on microfluidic chip by using a thermally responsive block copolymer for temperature controlling the transportation of microflow.As illustrated in Fig.1a,a T-shaped microfluidic chip was designed with two functional switchable valve (Valve 1 and Valve 2) in the two branch channels.The two branch channels with two individual valve were positioned on two individual thermoelectric cooler for temperature control.By adjusting the temperatures of the two thermoelectric cooler,the injected flow could be controlled to flow left or flow right only.The results suggested the valve could switch in short time (20 s).The switchable valve was fabricated by grafting a thermally responsive block copolymer brush poly-(Nisopropylacrylamide-co-hexafluoroisopropyl acrylate) (P(NIPAAmco-HFIPA)) on the inner side of glass capillaries which were then integrated in the micro channels (Fig.1b).As demonstrated in other reports,PNIPAAm was never hydrophobic whenever the temperature was above or below the LCST [27].Therefore,HFIPA was added to adjust hydrophilicity and hydrophobicity while PNIPAAm governed the thermal properties.In design,high molecule ratio of HFIPA would cause a higher contact angel (CA) that mean higher hydrophobicity.On the modified surface,the C=O and N−H groups of the PNIPPAm and (P(NIPAAm-co-HFIPA) parts generated intermolecular hydrogen bonding with water molecules,which would enhance the hydrophilicity (Fig.1b).In contrast,the C−F groups of the (P(NIPAAm-co-HFIPA) part would result in hydrophobic (Fig.1b).When the temperature (T) was below the LCST (Fig.1c),the polymer brush had a stretched state where the inter-molecule hydrogen bonding between C=O/N−H and water molecules contributed to the hydrophilic property.As a result,the aqueous solution could pass the channel easily,where the valve state was defined as “OPEN”.In contrast,at the temperature above the LCST,the inner surface of the valve became hydrophobic because of the intra-molecule hydrogen bonding between C=O and N-H while C=O and N−H group difficult to interact with water molecules.The valve with hydrophobic inner surface would stop aqueous solution to go through the valve,where the valve state was defined as “CLOSE” (Fig.1d).Therefore,the valve state could be controlled by temperature to control the flow stream in the microchannels.
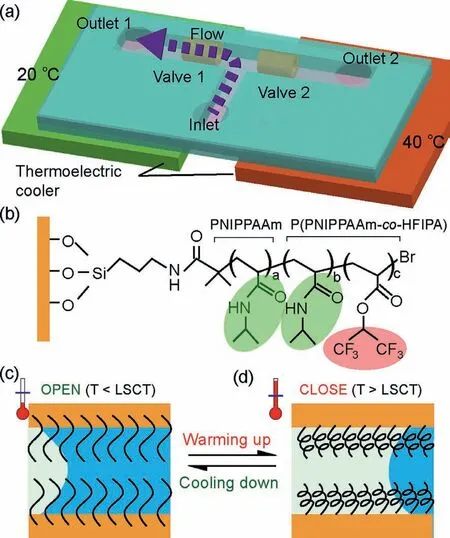
Fig.1.Chemo-mechanical switchable valve on microfluidic chip for flow control.(a) Illustration of the integrated device with switchable valves.(b) Structure of the copolymer brush.(c) Valve at the “OPEN” state when temperature was below the LCST.(d) Valve at the “CLOSE” state when temperature was below the LCST.
To graft the P(NIPAAm-co-HFIPA to the substrate,the substrate(capillary or glass plate) was first cleaned and modified to generate –OH groups on the surface.Then,the substrate was aminated by treating with 3-aminopropyltrimethoxysilane (APTMS) and amidated by treatment with 2-bromoisobutyryl bromide (BBiB).In polymerization process,the substrate was reacted with NIPAAm solution for 1 h at 60 °C.The reaction would allow to proceed from another 1 h at 60 °C after addition of HFIPA.The entire polymerization details are described in Supporting Information and Fig.S1 (Supporting information).Energy-dispersive X-ray spectroscopy(EDX) was used to investigated the surface chemical composites.Compare to the Energy-dispersive X-ray spectroscopy (EDX) analysis of bare slide glass (Fig.2a),the element contents of carbon and nitrogen (Fig.2b) increased significantly after amine functionalization.The peak relative to bromine (Fig.2c) from BBiB was observed after amidation.After polymerization,the peak relative to fluorine (Fig.2d) from HFIPA was observed.The results indicated that the polymerization of P(NIPAAm-co-HFIPA) on the substrate was achieved.
To confirm the function of the valve,the surface tension of polymerization substrate should have sufficient change between hydrophilicity and hydrophobicity.For achievement of the “OPEN”state,the water contact angle need to be below 90°.Meanwhile,the water contact angle need to be above 90° after switch to achieve the state of “CLOSE”.To improve the switch function of the valve,HFIPA was added in the polymerization process to ensure the water CA to be above 90° when the temperature was higher than LCST.Before applying to glass capillary,the optimization of HFIPA ratio was carried out by polymerize the copolymer on slide glass substrate.The water CA on the prepared substrate was measured by a self-assembled system under conditions of saturated humidity (Fig.S2 in Supporting information).A thermoelectric cooler (ECE-F15P-D12,OHM Electric Co.,Ltd.,Japan) was used to control the temperature during measurements.After 5 μL of deionized water was dropped onto the substrate surface and became stable,Image of the water droplet on substrate were recorded by a Dino-Lite digital microscope.The water CA were measured according to half angle formula (Fig.S3 in Supporting information).For one substrate,the water CAs at different positon were measured,and the water CA angle of the substrate was determined to be the average.
As shown in Fig.3a,the water CA of the substrate raised with the increasing ratio of HFIPA.When the HFIPA ratio reached 20%,the water CA was below 90° (69.2° ± 1.2°) at 20 °C and above 90°(96.0° ± 1.1°) at 40 °C.The ratio of HFIPA was optimized as 20%.Moreover,the response time was also investigated.The substrate placed on the temperature control plate with a temperature of 20°C,the water CA was measured.Then,the substrate with droplet was moved carefully to another temperature plate with a temperature of 40 °C,and the water CA was recording with different time.The water CA increased quickly with the passage of time increases,and reach a maximum after 20 s (Fig.3b).The results suggested that the copolymer with 20% HFIPA was suitable for valve manufacture.Satisfactorily,the response time was as short as 20 s,which was capable of flow control in microchannels.
After optimization of HFIPA ratio,the polymerization was applied to Square-Miniature Hollow Glass Tubing.The glass capillary owned a square inner diameter of 500 μm and a square outer diameter of 700 μm (Fig.S4 in Supporting information).The glass capillaries were modified using the same polymerization as before a HFIPA ratio of 20%.In the experiment,the modified capillaries were inserted in water with different temperature.Image was obtained using a digital camera when the height (H) of water in the capillary became stable.
As shown in Fig.4a,when the temperature of water was kept at 20 °C that was lower than the LCST (T
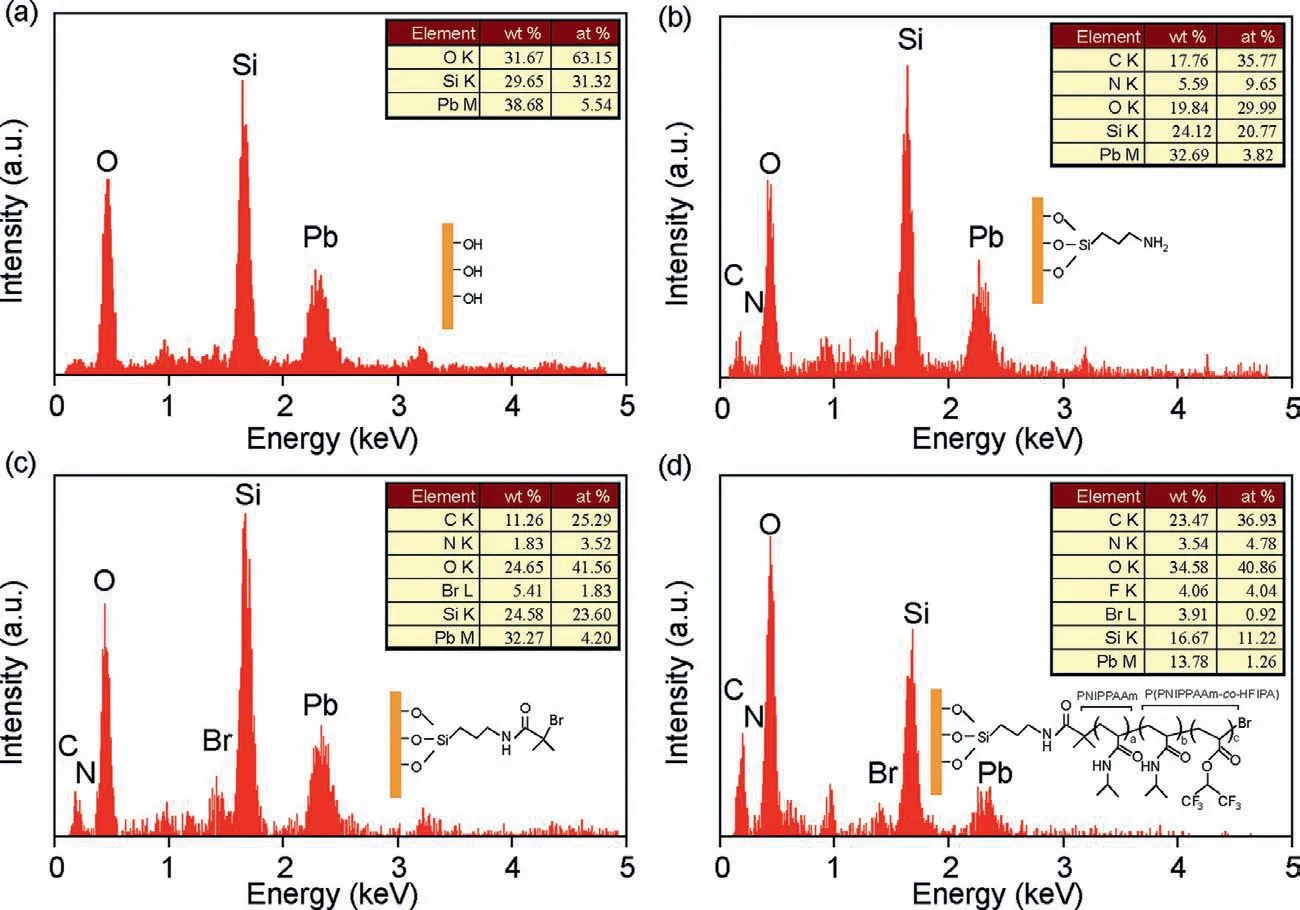
Fig.2.Characterization of the substrate with each polymerization step by EDX.(a) Bare slide glass.(b) Substrate after amine functionalization.(c) Substrate after Amidation.(d) Substrate after polymerization.
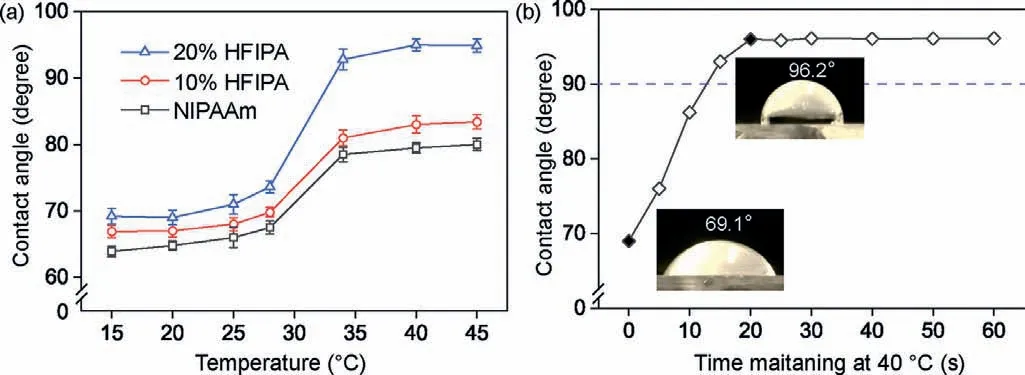
Fig.3.Optimization of the copolymer on slide glass.(a) Water CA on slide glass polymerized with different ratios of HFIPA (0,10%,20%).(b) The water CA at different heating time on a temperature control plate of 40 °C.
The microfluidic chip was designed with “T” shape.All channels were with a width of 700 μm and a height of 700 μm.The Microfluidic chip was fabricated using polydimethylsiloxane (PDMS)by standard soft lithography and replica molding techniques as previous report [28].Before the PDMS layer with channel was irreversibly sealed with another PDMS as substrate layer by oxygen plasma treatment (Electro-technic products,Inc.,Japan),two individual capillaries with polymerization function as valves were placed in the two downstream microchannels (Fig.S5 in Supporting information).The two downstream microchannel parts were placed on two individual thermoelectric coolers for temperature control.In all the application experiments,temperature of 20 °C was used to turn the valve to “OPEN” state,while temperature of 40 °C was used to turn the valve to “CLOSE” state.When the left thermoelectric cooler was set at 20 °C and the right one was set at 40 °C,an aqueous solution containing 200 μmol/L Rhodamine 6G at a speed of 1000 μL/h was injected into the microchannel.The results showed that the injected solution flowed into the left downstream microchannel which no solution flowed into the right downstream microchannel (Fig.4d).Instead,the injection solution flowed right (Fig.4e) when thermoelectric cooler was set at 40 °C.All those results demonstrated that the valve state could turn to“OPEN” and “CLOSE” conveniently by changing the temperature.In further applications,the total system will include cell culture part,valve part,and assay part.Each part can be controlled with desirable temperature.Therefore,the temperature in the valve part will not limit the temperature requirements in assay part.The reported valve made from polymer brush,thus it could work for limited times in real sample detection because of the adsorptions of metabolites from cells.The property of the valve depended on brush density and molecule ratio of HFIPA.By changing the percent of HFIPA,the LCST could be adjusted to meet the requirements of different applications.
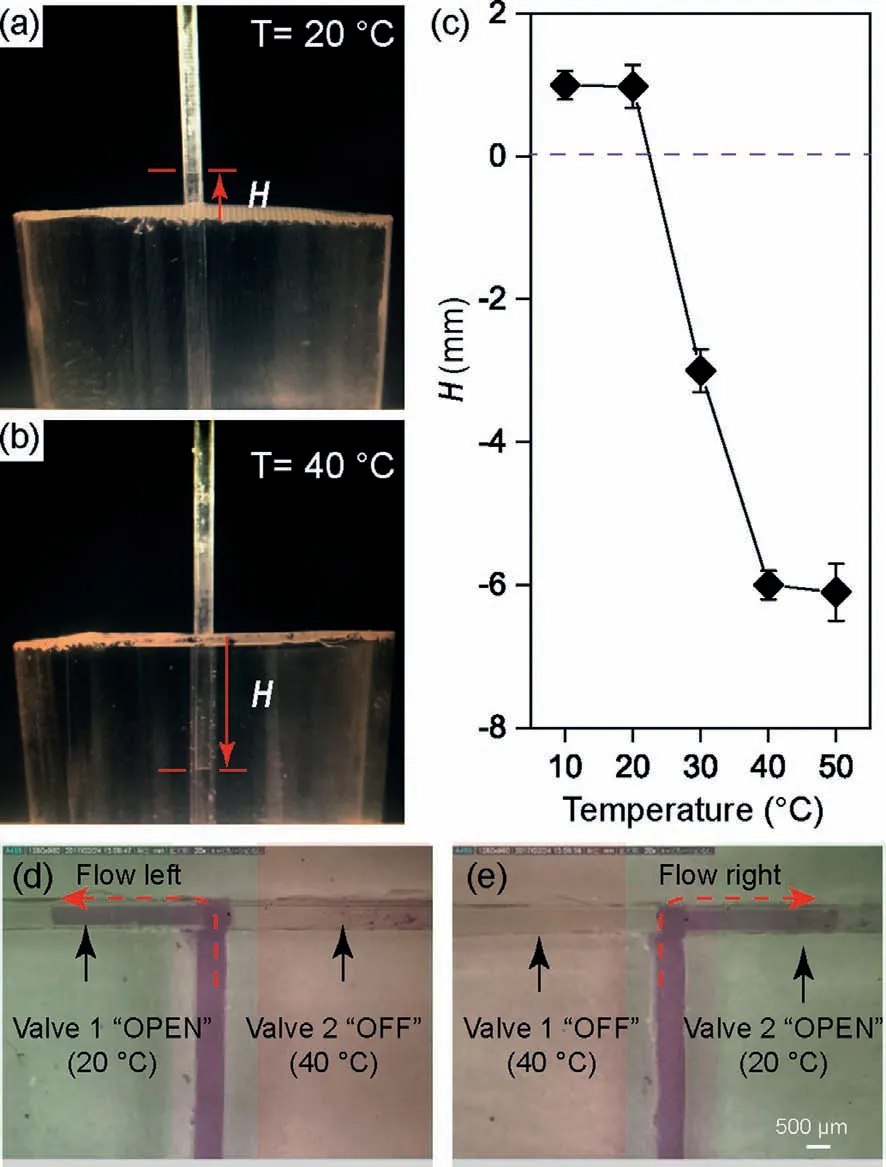
Fig.4.Conformation of the hydrophobicity and hydrophilicity switch in polymerized glass and application as a valve in microchannel.(a) The height of water (H)inside the capillary at 20 °C.(b) The height of water (H) inside the capillary at 40°C.(c) The H at different temperatures.(d) Flow direction when Valve 1 was “OPEN”and Valve 2 was “CLOSE”.(e) Flow direction when Valve 1 was “CLOSE” and Valve 2 was “OPEN”.
In summary,we have developed a switchable valve that is capable of integrating in microchannel for control flow stream using a thermally responsive block copolymer.The ratio of HFIPA at 20% in the P(NIPAAm-co-HFIPA) was demonstrated to be optimal.The water contact angle changed from 69oto 96owhen then temperature changed from 20 °C to 40 °C.The integrated valve in microchannel showed excellent performance on flow control.The method provides a potential approach for valve manufacture on microfluidic chip,which will be much benefit for integration of various function parts.In further applications,every channel with valve could connect with a functional assay channel where different metabolites from cells could been analyzed.The valve channel could also connect with mass spectrometer.By controlling the valve,metabolites at different time could be collected and detected.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgment
We acknowledge the financial support from JSPS KAKENHI Grants (Nos.JP21K14653,JP20K22555 and JP20K05557).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.065.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
