In-situ growth of PbI2 on ligand-free FAPbBr3 nanocrystals to significantly ameliorate the stability of CO2 photoreduction
Ning-N Guo,Zho-Lei Liu,Yn-Fei Mu,Meng-Rn Zhng,Yun Yo,Min Zhng,∗,Tong-Bu Lu
a MOE International Joint Laboratory of Materials Microstructure,Institute for New Energy Materials and Low Carbon Technologies,School of Materials Science and Engineering,Tianjin University of Technology,Tianjin 300384,China
b MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage,School of Chemistry and Chemical Engineering,Harbin Institute of Technology,Harbin 150001,China
Keywords:CO2 reduction Halide perovskite Photocatalysis Stability Charge transfer
ABSTRACT Excellent optical properties involving strong visible light response and superior carrier transport endow metal halide perovskites (MHP) with a fascinating prospect in the field of photocatalysis.Nevertheless,the poor stability of MHP nanocrystals (NCs) in water-contained system,especially without the protection of long alkyl chain ligands,severely restricts their photocatalytic performance.In this context,we report an effortless strategy for the generation of ligand-free MHP NCs based photocatalyst with high water tolerance,by coating PbI2 on the surface of ligand-free formamidinium lead bromide (FAPbBr3) NCs via the facile procedure of in-situ conversion with the aid of ZnI2.Under the protection of PbI2 layer,the resultant FAPbBr3/PbI2 composite exhibits significantly ameliorated stability in an artificial photosynthesis system with CO2 and H2O vapor as feedstocks.Moreover,the formation of compact PbI2 layer can accelerate the separation of photogenerated carriers in FAPbBr3 NCs,bringing forth a remarkable improvement of CO2 photoreduction efficiency with an impressive electron consumption yield of 2053 μmol/g in the absence of organic sacrificial agents,which is 7-fold over that of pristine FAPbBr3 NCs.
Using clean solar energy to directly transform CO2into valueadded fuels and chemicals through artificial photosynthesis,known as photocatalytic CO2reduction reaction [1],is one of the most promising strategies to settle the global ever-growing energy crisis and environmental problems [2].To promote the economic efficiency of this technology,a vast variety of semiconductor photocatalysts [3–8]have been explored during the last decade.In this regard,increasing the light-harvesting capacity and improving the separation efficiency of photogenerated carriers are two essential motivations for most of the studies [9],which play critical roles in the final photocatalytic performance.In this context,low-cost metal halide perovskite (MHP) nanocrystals (NCs) have been considered as fascinating candidates for photocatalytic CO2reduction recently [10–14],on primary account of their strong visible light response and superior carrier transport.The photocatalytic CO2reduction activity of MHP NCs has been greatly improved through surface modification [15–18],morphology control [19–22]and heterojunction engineering [23–34]strategies in the past few years.
Unfortunately,when the photocatalytic reaction is carried out in a water-contained system,the stability of MHP NCs is poor owing to their highly ionic nature [35],especially in the absence of the protection of long alkyl chain ligands,which seriously limits their photocatalytic performance for a long time.Moreover,the long alkyl chain ligand capping on the surface of the perovskites will hinder the separation of surface photogenerated carriers as confirmed in our previously reported work [36,37].Therefore,the development of ligand-free MHP NCs is beneficial for improving the photocatalytic activity by increasing the probability of contact between the reaction substrate and the surface of MHP NCs.Nevertheless,the problem of how to improve the stability of MHP NCs without long alkyl chain ligands has not been well solved.In order to conquer this dilemma,herein we prepared a novel ligand-free formamidinium lead bromide (FAPbBr3) based composite byin-situforming PbI2on the surface of FAPbBr3NCs (FAPbBr3/PbI2)viathe strategy of ZnI2isopropanol (IPA) solution assistance.Owing to the high moisture resistivity of PbI2,the resultant FAPbBr3/PbI2composite exhibits significantly improved stability and activity in comparison with pristine FAPbBr3NCs for photocatalytic CO2reduction.
The ligand-free FAPbBr3NCs were preparedviaone-step spin coating process on the filter paper substrate (Fig.S1 in Supporting information).The powder X-ray diffraction (XRD) pattern of as-prepared FAPbBr3(Fig.S2 in Supporting information) verifies the successful synthesis of cubic phase FAPbBr3with high purity,displaying distinct characteristic diffraction peaks corresponding to cubic phase FAPbBr3(Pm¯3m,No.221).In addition,high-resolution transmission electron microscopy (HRTEM) image of FAPbBr3NCs(Fig.S3a in Supporting information) displays the well-defined interplanar distance of 0.42 nm and 0.59 nm,corresponding to the(011) and (001) crystal planes of cubic phase FAPbBr3[38],respectively,further confirming the successful generation of cubic phase FAPbBr3.Moreover,a sequence of ordered bright spots can be clearly observed in the corresponding fast Fourier transform(FFT) pattern of FAPbBr3(Fig.S3b in Supporting information),proving once again the well-crystallinity of FAPbBr3NCs.The composite of ligand-free FAPbBr3/PbI2was prepared byin-situconstructing PbI2on the surface of FAPbBr3NCsviathe strategy of ZnI2IPA solution assisted method as illustrated in Fig.S1,and the details are provided in Supporting information.Briefly,the dried filter paper coated with FAPbBr3was immersed in ZnI2IPA solution and annealed at 80 °C for 10 min to remove the residual IPA.It is well known that IPA can be used as a weak extractant to extract formamidinium halide from formamidinium lead halide perovskite [39,40],and its extraction ability can be enhanced by introducing ZnI2through the reaction of ZnI2with halide ions to form ZnX3-and ZnX42-[41],where the X denotes the halide ions.Meanwhile,the residual PbI2could crystallize and retain on the surface of FAPbBr3owing to its insolubility in IPA.The mass ratio of PbI2in composite can be determined based on X-ray photoelectron spectroscopy (XPS) analysis,being 2.6%.
To further explore the structure of the FAPbBr3/PbI2composite,we first carried out the XRD measurement to analyze the composition of the as-prepared composite.Compared to pristine FAPbBr3NCs,the main diffraction peaks of cubic phase FAPbBr3were well maintained on the XRD pattern of FAPbBr3/PbI2(Fig.S2),while no XRD characteristic peaks of PbI2can be observed in FAPbBr3/PbI2,which may be due to the low content and small size of PbI2on the surface of FAPbBr3.While the transmission electron microscopy(TEM) (Fig.1a) and HRTEM (Fig.1b) images of FAPbBr3/PbI2composite display the lattice spacings of 0.42 nm and 0.24 nm for (011)lattice plane of FAPbBr3and (109) lattice plane of PbI2(JCPDSPbI2: 01–073–9472),respectively,implying the existence of PbI2on the surface of FAPbBr3.High-resolution scanning electron microscopy (HRSEM) measurements revealed that the as-prepared FAPbBr3sample is irregular nanoparticle with the average size of∼20 nm (Fig.1c),and there are obvious agglomerations due to the lack of surface ligands.ZnI2IPA solution treatment leads to further increase in reunion (Fig.1d).
The influence of ZnI2IPA solution treatment on the interfacial electron structure of halide perovskite was further investigated by recording the XPS spectra of FAPbBr3NCs and FAPbBr3/PbI2composite.As shown in Fig.2a,C,N,Pb and Br co-exist in both as-prepared FAPbBr3NCs and FAPbBr3/PbI2composite.Two new peaks at 630.60 and 619.10 eV corresponding to I 3d3/2and I 3d5/2can be clearly observed in the FAPbBr3/PbI2composite as presented in Fig.2b,which could be attributed to the formation of PbI2on the surface of FAPbBr3NCs.It is noted that the characteristic peaks of Br 3d for Br 3d5/2and Br 3d3/2in FAPbBr3/PbI2composite are located 68.25 and 69.25 eV,respectively,which are perceivably lower than those of Br 3d (68.40 and 69.40 eV) in pristine FAPbBr3NCs as shown in Fig.2c,indicating the presence of effective electron coupling between FAPbBr3and PbI2owing to the close contact between them.This inference can be further confirmed by scrutinizing the changes of Pb 4f signals in FAPbBr3NCs and FAPbBr3/PbI2composite (Fig.2d),which displays the same trend as Br 3d.Moreover,the Fourier transform infrared (FTIR)spectra of as-prepared samples were further measured to scrutinize the interaction between FAPbBr3and PbI2.With respect to pristine FAPbBr3,there is a new peak occurring near 3550 cm-1for FAPbBr3/PbI2(Fig.S4 in Supporting information),which can vest in the Pb-I stretching vibration mode [42],indicating the formation of PbI2on FAPbBr3nanocrystals.In addition,compared with pristine PbI2,a perceptible shift is occurred for the Pb-I vibration of FAPbBr3/PbI2,suggesting a strong interaction between FAPbBr3and PbI2.This effective electron coupling should be beneficial to the interfacial electron transfer between FAPbBr3and PbI2.
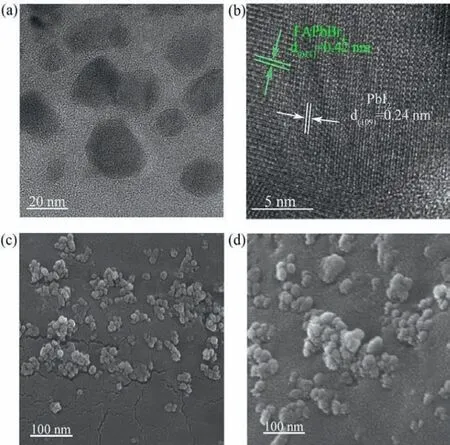
Fig.1.(a) TEM and (b) HRTEM images of FAPbBr3/PbI2.HRSEM images of (c)FAPbBr3 and (d) FAPbBr3/PbI2.
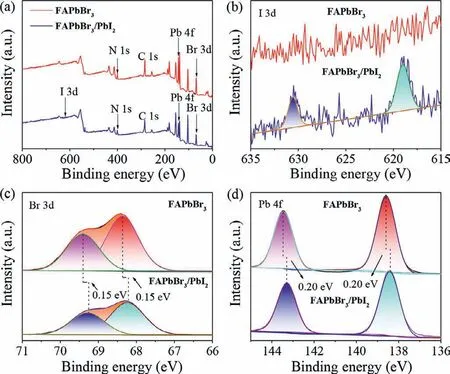
Fig.2.XPS spectra for FAPbBr3 and FAPbBr3/PbI2: (a) full spectra,(b) I 3d,(c) Br 3d and (d) Pb 4f.
In order to inspect the possible electron transfer orientation between the interface of FAPbBr3/PbI2composite,we first performed the ultraviolet-visible diffuse reflectance spectroscopy (UV–vis DRS) and electrochemical measurements to obtain the thermodynamic information of components.As presented in Fig.S5(Supporting information),the absorption spectrum of FAPbBr3/PbI2shows a perceivable red-shift compared with pristine FAPbBr3,indicating the existence of strong interaction between FAPbBr3and PbI2[43],which is consistent with the results of XPS measurements.The corresponding Tauc plots of FAPbBr3and FAPbBr3/PbI2are presented in Fig.S6 (Supporting information),where the band gaps (Eg) of FAPbBr3NCs and FAPbBr3/PbI2composite can be determined to be 2.25 and 2.22 eV,respectively.The values of conduction band edge potentials (ECB) can be derived from the Mott-Schottky curves (Fig.S7 in Supporting information),being −1.10 and −0.98 Vvs.the normal hydrogen electrode (NHE) for FAPbBr3and FAPbBr3/PbI2,respectively.In combination with the values ofEg,the values of valance band edge potentials (EVB) for FAPbBr3and FAPbBr3/PbI2can be obtained as 1.15 and 1.24 Vvs.NHE,respectively.In addition,according to the previously reported values [41],the values ofECBandEVBfor PbI2are −1.00 and 1.17 Vvs.NHE,respectively.Therefore,a type-Ⅱband alignment could be formed between the interface of FAPbBr3and PbI2,as illuminated in Fig.S8 (Supporting information),indicating that the photogenerated electron transfer from FAPbBr3to PbI2is thermodynamically feasible.In addition,ultraviolet photoelectron spectra (UPS)of FAPbBr3and FAPbBr3/PbI2were further measured to obtain the information of Fermi levels (EF),which also play an important role in the interfacial free electron transfer process.As depicted in Fig.S9 (Supporting information),the calculated value ofEFfor FAPbBr3(−4.14 eVvs.vacuum) is lower than that of FAPbBr3/PbI2composite (−3.93 eVvs.vacuum),indicating that theEFof PbI2on the FAPbBr3surface is higher than that of FAPbBr3.When FAPbBr3and PbI2are in contact,the free electrons in PbI2will transfer into FAPbBr3to realize theEFequilibrium of composite.This is in line with the XPS results in Fig.2,in which the binding energies of Pb 4f and Br 3d in composite shift towards lower values compared to FAPbBr3,which can be attributed to Pb and Br in composite exposing to a more negative chemical environment.A built-in electric field pointing from PbI2to FAPbBr3will be constructed by free electron movement at the interface between FAPbBr3and PbI2,which also facilitates the photogenerated electron transfer from FAPbBr3to PbI2.
The detailed charge transfer dynamics between FAPbBr3and PbI2were further evaluated by monitoring the steady state photoluminescence (PL) and time-resolved PL (TRPL) decay curves of FAPbBr3NCs and FAPbBr3/PbI2composite.As depicted in Fig.3a,pristine FAPbBr3NCs displayed a strong emission peak at 525 nm,while the PL emission intensity was sharply quenched to 12% afterin-situconversion of PbI2on the surface of FAPbBr3.This observation was in accordance with the photographs of FAPbBr3NCs and FAPbBr3/PbI2composite under UV illumination (Fig.S10 in Supporting information),revealing the occurrence of swift charge transfer between FAPbBr3and PbI2.Fig.3b displays the TRPL decay curves of FAPbBr3NCs and FAPbBr3/PbI2composite,where an obviously accelerated PL decay for FAPbBr3/PbI2can be identified compared with pristine FAPbBr3NCs.The corresponding fitting parameters based on a triple-exponential function were summarized in Table S1 (Supporting information).The PL average lifetimes for FAPbBr3NCs and FAPbBr3/PbI2composite are calculated as 39.2 and 18.0 ns,respectively,further demonstrating the swift charge transfer between FAPbBr3and PbI2.In addition,the photocurrent response (I-t) and electrochemical impedance spectroscopy(EIS) measurements further confirmed thatin-situformation of PbI2on the surface of FAPbBr3is beneficial to the charge separation.FAPbBr3/PbI2composite exhibits larger photocurrent intensity(Fig.3c) and smaller semicircular radius in Nyquist plots (Fig.3d)with respect to individual FAPbBr3NCs.
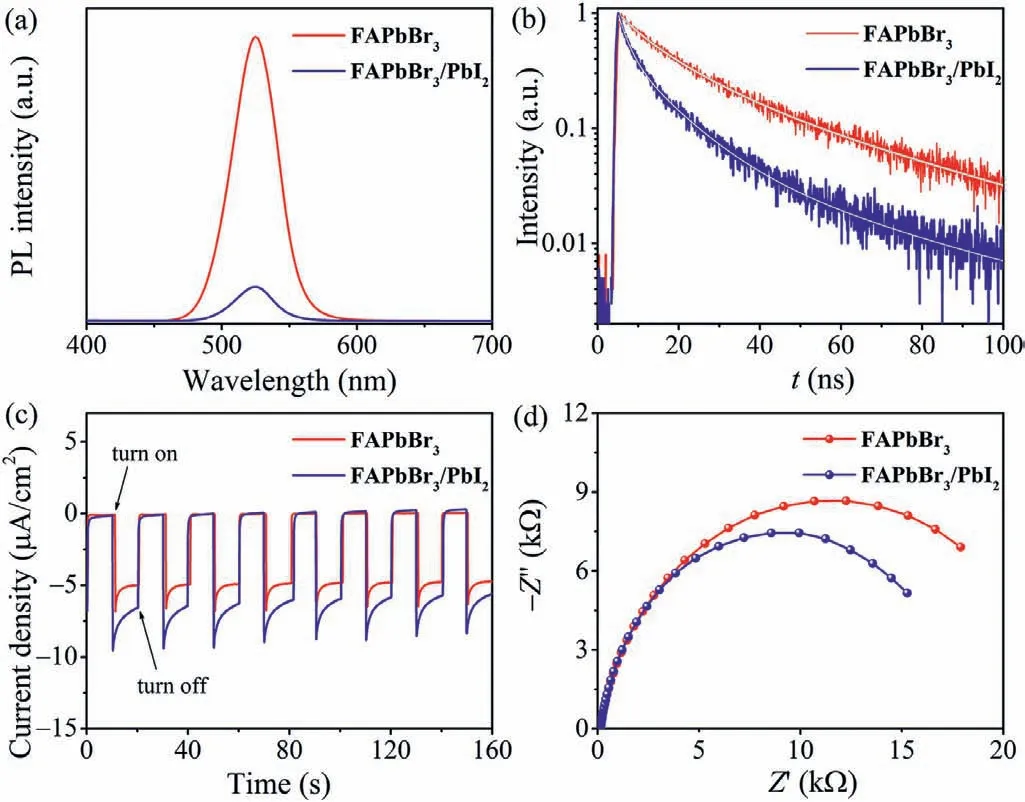
Fig.3.(a) Steady state PL spectra,(b) time-resolved PL decay curves,(c) photocurrent response,and (d) photoelectrochemical impedance spectroscopy (EIS) plots of FAPbBr3 and FAPbBr3/PbI2.
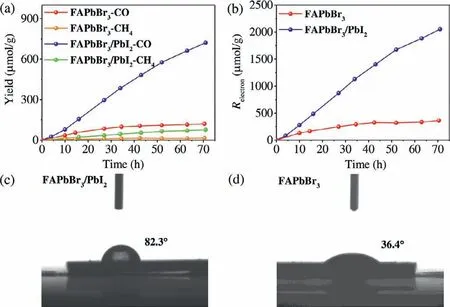
Fig.4.(a) The evolution of CO and CH4 yield and (b) corresponding Relectron with FAPbBr3,FAPbBr3/PbI2 as photocatalysts.Images of water droplets between water and (c) FAPbBr3/PbI2 or (d) FAPbBr3 on the silicon substrate.
The photocatalytic performance for CO2reduction was evaluated in a gas-solid system (see details in Supporting information).For FAPbBr3NCs and FAPbBr3/PbI2composite,the major products of CO2reduction are CO and CH4.As presented in Fig.4a,thein-situformation of PbI2on the surface of FAPbBr3can significantly enhance the photocatalytic activity and stability of FAPbBr3.The yields of CO and CH4for FAPbBr3/PbI2composite achieve impressive values of 720 and 76.5 μmol/g,respectively,after 70 h of irradiation.The corresponding electron consumption(Relectron=2nproduct(CO)+8nproduct(CH4),nproductdenotes the yield of product) yield is 2053 μmol/g for FAPbBr3/PbI2composite (Fig.4b),which is about 7-fold over that of pristine FAPbBr3NCs.What is more,there is no obvious decrease in CO evolution for FAPbBr3/PbI2composite after 70 h of illumination,while the pristine FAPbBr3loses its photocatalytic activity after 20 h,owing to the destroyed structure during photoreaction as identified by the XRD measurements (Fig.S11a in Supporting information).In contrast,FAPbBr3/PbI2composite can maintain its crystal structure well after photocatalytic reaction (Fig.S11b in Supporting information).Moreover,after the photocatalytic reaction,there is no change in XPS characteristic peaks (Fig.S12 in Supporting information) of FAPbBr3/PbI2composite,further suggesting the good stability of FAPbBr3/PbI2in this gas-solid reaction,which can be further confirmed by photocatalytic cycling experiment (Fig.S13 in Supporting information).The good stability of FAPbBr3/PbI2should be attributed to the high moisture resistivity of PbI2,which endows FAPbBr3/PbI2with a larger water contact angle of 82.3°(Fig.4c) than that of 36.4° (Fig.4d) for pristine FAPbBr3NCs,protecting FAPbBr3NCs from water corrosion.In addition,a sequence of control experiments was conducted with FAPbBr3/PbI2as catalyst.As shown in Fig.S14 (Supporting information),without catalyst,CO2or H2O vapor,there are negligible CO and CH4can be detected,indicating that the products of CO and CH4come from the photocatalytic reduction of CO2over catalyst.According to the test result of13CO2labeling experiment (Fig.S15 in Supporting information),the major reaction products13CO withm/zvalue of 29 and13CH4withm/zvalue of 17 can be obviously observed,further confirming that the C in CO and CH4products originate from the reduction of CO2by photogenerated electrons in FAPbBr3/PbI2.In addition,H218O labeling experiment was also performed (Fig.S16 in Supporting information),and18O2withm/zvalue of 36 can be also obviously observed,indicating that water is the reductant.
To summarize,we have demonstrated a facile strategy to improve the water tolerance of ligand-free MHP NCs in a watercontained photocatalytic reaction system,byin-situforming PbI2on the surface of ligand-free FAPbBr3NCs with the assistance of ZnI2IPA solution.The formation of PbI2layer not only significantly ameliorates the stability of MHP NCs for photocatalytic CO2reduction with H2O vapor as electron source,but also brings forth an accelerated interfacial charge separation as demonstrated by the photophysical and electrochemical measurements.The improved stability and charge separation endow the FAPbBr3/PbI2composite with a remarkable improvement of photocatalytic performance for CO2reduction,achieving an inspiring electron consumption yield of 2053 μmol/g without any organic sacrificial agents,over 7-fold higher than that of individual FAPbBr3NCs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Natural Science Foundation of Tianjin City (No.17JCJQJC43800).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.033.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
