Plasma-induced chemical etching generating Ni3S2 for formaldehyde detection
Jiaxin Zhou,Li Zhao,Qiang Wang,Lixin Zuo,Ana Zhao,Huimin Yu,Xue Jiang,Xiaoli Xiong
College of Chemistry and Materials Science,Sichuan Normal University,Chengdu 610068,China
Keywords:Chemical etching Microplasma Electrochemical detection Formaldehyde
ABSTRACT In this paper,Ni3S2 nanosheet (NS) was generated by chemical etching with sodium sulfide directly on the nickel foam (NF),which was induced by dielectric barrier discharge plasma in liquid.Compared with other chemical etching methods of nickel-based nanomaterials,this method was not only rapid (40 min)and mild (at room temperature and atmospheric pressure),but also showed consistent stability and good reproducibility.The Ni3S2 NS/NF electrode showed excellent performance in the electrochemical detection of formaldehyde under alkaline conditions.It had a good linear relationship with the concentration of formaldehyde in the range of 0.002-5.45 mmol/L (R2=0.9957) and the limit of detection (LOD) was 1.23 μmol/L (S/N=3).The sensitivity was 1286.9 μA L mmol–1 cm–2,and the response time was about 5 s.The plasma-induced chemical etching strategy provides a simple and stable electrode preparation method,which has great application prospects in nonenzymatic electrochemical sensors.
In 2006,the International Agency for Research on Cancer of the World Health Organization (WHO) placed formaldehyde in the list of carcinogens [1].On July 23,2019,formaldehyde was included in the list of toxic and harmful water pollutants (the first batch)by the National Health Commission of the Ministry of Ecology and Environment,China.However,many chemical productions currently require formaldehyde,for instance,as a chemical raw material used in chemical manufacturing,textile industry and anticorrosive materials.This means that a large amount of industrial waste water discharged may contain formaldehyde and contaminate urban sewers.Therefore,it is extremely necessary to develop highefficient and portable methods to detect formaldehyde.At present,the main detection methods of formaldehyde are spectrophotometry,fluorimetry and high-performance liquid chromatography [2–5].The biggest shortcomings of these methods are complex operation in labs,large instruments,and expensive costs.Contrast that with the above methods,electrochemical sensors have great advantages in the detection of formaldehyde because of their simple operation,high sensitivity and low price.Compared with noble metals,transition metals such as cobalt,nickel and copper have a great application prospect in electrochemical sensors,because they are cheap,readily available,and easy to lose electrons to have high redox activity.Some traditional synthesis methods for preparation of transition metals based nonenzymatic electrochemical sensors usually are time-consuming,laborious,and complicated in operation steps.For example,the NiMnO3(NMO) nanosheet was synthesizedviathe solvothermal synthesis method (180 °C,12 h),followed by the calcination (500 °C,3 h) [6].Nickel phosphate(NiPh)/carbon fibers (CF) were synthesized with the precursor of NiPh by reflux at 90 °C for 12 h [7].Therefore,there is an urgent need to develop a simple,mild and short time-consuming synthesis method to construct electrode materials of electrochemical sensors.
Chemical etching is a fast way to synthesize nanomaterials,and it is a redox reaction process with selective dissolution of metal in solution to produce the required material in a step of chemical etching.In recent years,chemical etching has made great achievements in the synthesis of modified silicon-based nanomaterials.Zhanget al.reported the synthesis of large-area uniform silicon nanowire arrays by multiple acid etching with metal assistance [8].Saverinaet al.synthesized porous nano-silicon materials by electrochemical etching in ionic liquid without HF acid solution [9].
In addition,microplasma is a kind of plasma with high electron density and high stability,which is limited to submillimeter size [10].The microplasma could be induced by dielectric barrier discharge (DBD),and the copper wire as one discharge electrode wound around the glass tube while the other discharge electrode in the coaxial of the glass tube.When the DBD tube is connected to a high-voltage power supply,it will generate a large number of high-energy electrons,ions and free radicals.These substances with high energy density provide sufficient energy for the synthesis of materials at atmospheric pressure in a very short time [11].Recently,we have successfully prepared complex metal-organic frameworks compound (MOFs) and covalent organic frameworks(COFs) by barrier discharge plasma in liquid [12,13].In our previous work,the Co(OH)F nanoflowers on carbon cloth synthesized by dielectric barrier discharge microplasma was used as a nonenzymatic electrochemical sensor for glucose.In order to further improve the stability and repeatability of the electrodes,we try to develop a microplasma induced chemical etching method,which is expected to be a widely used technique for the synthesis of nanomaterials and electrode preparation.
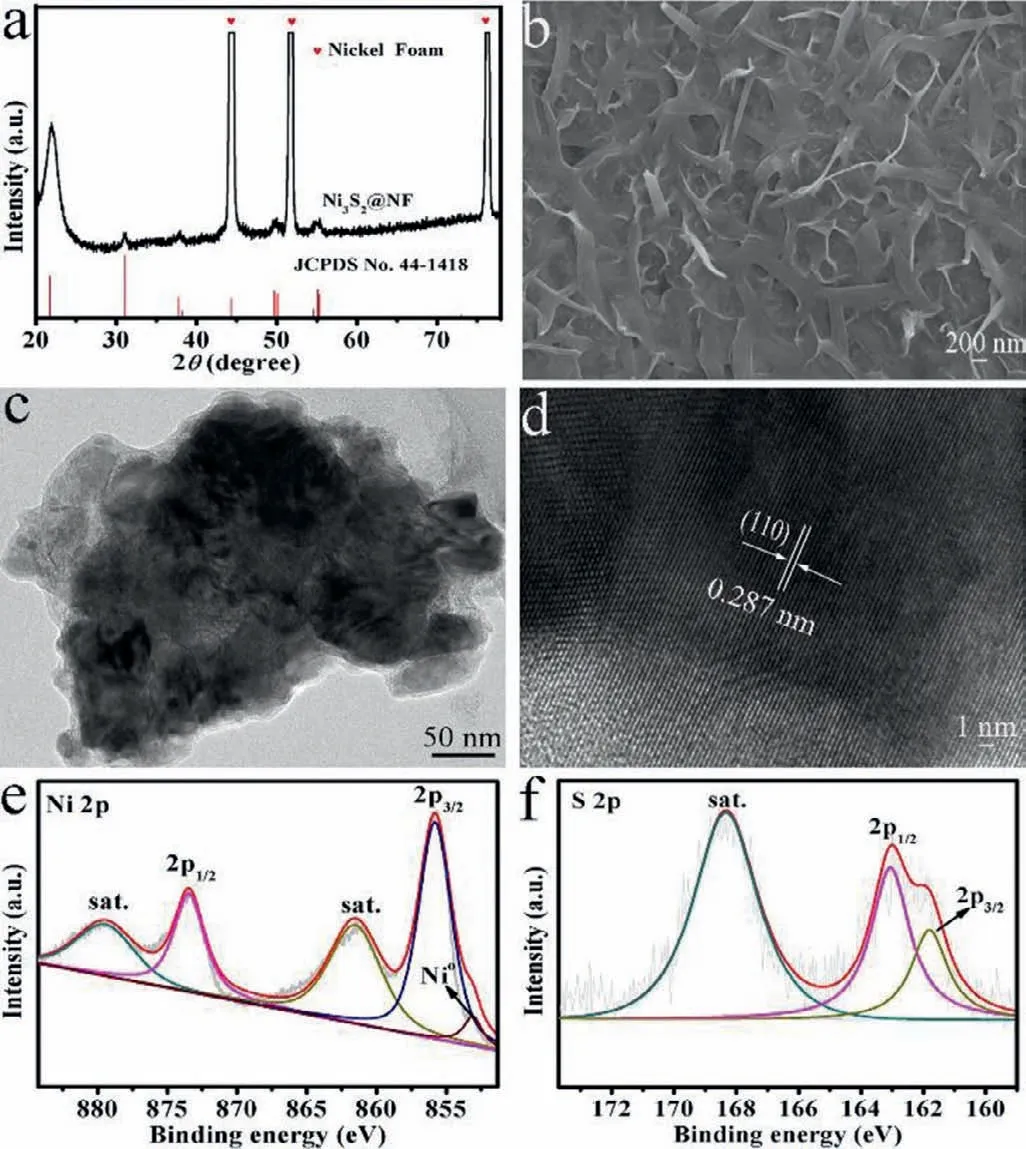
Fig.1.(a) XRD patterns for Ni3S2 NS/NF and blank NF.(b) SEM image for Ni3S2 NS/NF.(c) TEM image of Ni3S2 NS/NF.(d) HRTEM image taken from Ni3S2 NS/NF.(e) and (f) XPS spectra of Ni and S,respectively.
Herein,Ni3S2nanosheet (NS) wasin-situgenerated on the nickel foam (NF) by microplasma-induced chemical etching,and it was used as a catalytic electrode for formaldehyde oxidation under alkaline conditions.Importantly,the chemical etching method was fast (40 min) and low energy consumption (<90 W),and the details of the experimental section were shown in the Supporting information.Furthermore,the formaldehyde electrochemical sensor based on Ni3S2NS/NF,showed good electrocatalytic performance with excellent stability and repeatability.
To determine the crystal phase information of the prepared material,the X-Ray Diffraction (XRD) image of blank NF and the obtained Ni3S2NS/NF was displayed in Fig.1a.It was exactly consistent with the standard (JCPDS No.44-1418),and the peaks located at 21.8°,31.1°,37.8°,49.7°,54.6° could be indexed to (101),(110),(003),(113) and (104) planes,respectively [14].The scanning electron microscope (SEM) was adopted to investigate the morphology of Ni3S2NS/NF,which showed abundant nanosheet materials grown on NF in Fig.1b.Importantly,the transmission electron microscope (TEM) image of Ni3S2NS/NF (Fig.1c) further confirmed that the synthesized material was nanosheet structure and the measured well-resolved lattice fringe space of 0.287 nm matched the (110) crystal plane of Ni3S2NS/NF (Fig.1d).Energy dispersive system (EDS) spectroscopy (Fig.S2 in Supporting information) confirmed the presence of Ni and S in synthetic materials.The corresponding EDS element mapping images also indicated that S and Ni elements were uniformly distributed on the surface of Ni3S2nanomaterials (Fig.S3 in Supporting information).
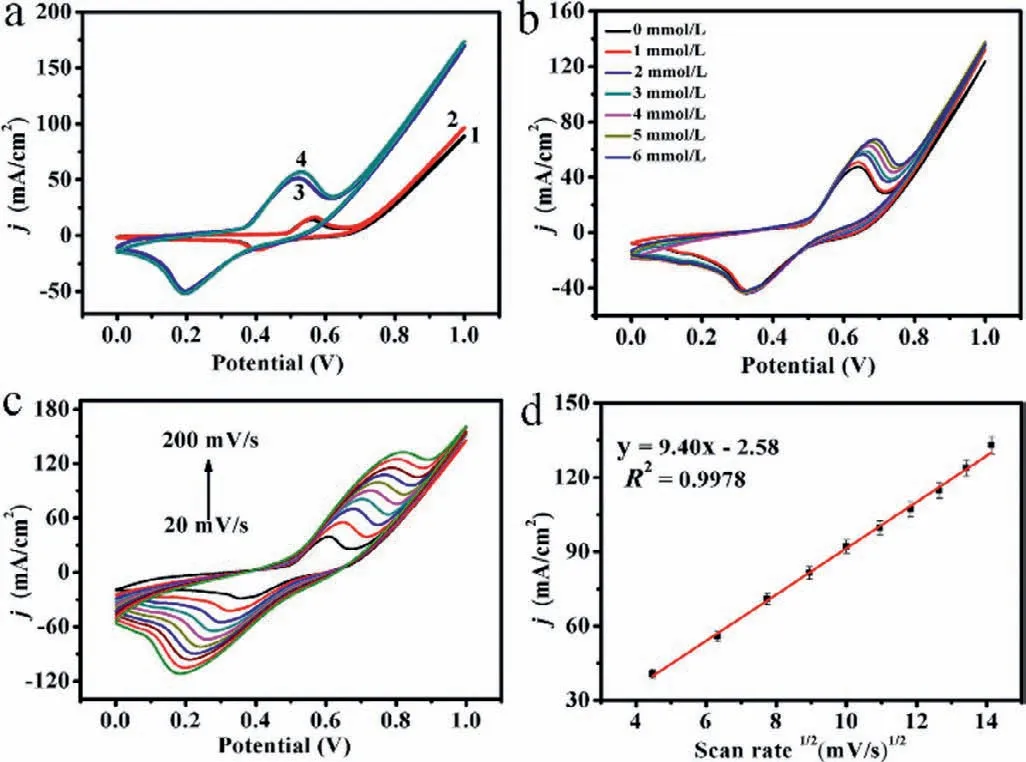
Fig.2.(a) CVs (curves 1 and 2) in the absence and presence of 1 mmol/L formaldehyde on the blank NF;CVs for Ni3S2 NS/NF without and with 1 mmol/L formaldehyde (curves 3 and 4) in 0.1 mol/L NaOH respectively.Scan rate: 50 mV/s.(b) CVs of variational formaldehyde concentrations (0-6 mmol/L) in 0.1 mol/L NaOH at Ni3S2 NS/NF electrode.(c) Cyclic voltammetry of the change of scanning rate of Ni3S2 NS/NF from 20 mV/s to 200 mV/s in 1 mmol/L formaldehyde solution.(d) The linear curve of the peak anode current and the square root of the scan rate.
The X-ray photoelectron spectroscopy (XPS) was used for identifying the surface chemical states of Ni3S2NS/NF.Fig.1e showed peaks of Ni 2p3/2and Ni 2p1/2at 855.8 eV and 873.5 eV as well as their satellite peaks at 861.5 eV and 879.6 eV,respectively,suggesting that Ni3S2existed with the form of Ni2+[15–17].Moreover,there was another peak here located at 853.0 eV related to Ni0in Ni3S2[18].Fig.1f exhibited the representative S 2p spectra of the Ni3S2that the S 2p3/2and S 2p1/2peaks were observed at 161.8 eV and 163.1 eV together with the satellite peak at 168.3 eV,which could be marked as S2−in Ni3S2matching to S 2p3/2and S 2p1/2,respectively [19].
The formaldehyde electrochemical tests were performed in a traditional three-electrode electrochemical work station in 0.1 mol/L NaOH solution at a scan rate of 50 mV/s.To determine the effect of pH on the electrooxidation of formaldehyde,the voltammograms of the prepared Ni3S2NS/NF under different pH conditions were studied,and the pH value of 13 was finally determined as the optimal pH value for the maximum electrooxidation of formaldehyde under Ni3S2NS/NF (Fig.S4 in Supporting information).Therefore,all subsequent measurements were made in a solution with a pH of 13.In cyclic voltammetry (CV) testing,the applied potential range was 0-1.0 V.As is shown in Fig.2a,the electrochemical behavior of the prepared Ni3S2NS/NF electrode and bare NF electrode towards formaldehyde was studied by CV measurement in 0.1 mol/L NaOH.In the presence of formaldehyde,the current response value of the NF electrode (curve 2) was not significantly different from that in the absence of formaldehyde (curve 1),which was almost negligible.This indicated that empty NF could not be directly used as a material for formaldehyde detection.In contrast,the synthesized Ni3S2NS/NF produced a visible current change in 1 mmol/L formaldehyde (curve 4),compared to the case where no formaldehyde was added (curve 3).The above results indicated that Ni3S2NS/NF electrode showed excellent electrocatalytic activity.This makes it very clear that the Ni3S2NS/NF electrode has a pair of redox pairs belonging to Ni2+/Ni3+at the potential voltage.On this basis,combining with XPS analysis data,we have reasons to believe that this redox mechanism might look like this: oxidation of formaldehyde in NaOH solution with Ni3S2NS/NF was similar to nickel base electrode that reported antecedently as follows (Eqs.1 and 2) [7,20]:
Fig.2b displayed CVs of Ni3S2NS/NF in 0.1 mol/L NaOH under different formaldehyde concentrations.When the concentration of formaldehyde changed as 0–6 mmol/L,the current value of the corresponding oxidation peak also increased uniformly.This also indicated that our Ni3S2NS/NF material was effective in catalyzing the oxidation of formaldehyde.In order to study the kinetics of formaldehyde oxidation in an alkaline solution,Fig.2c demonstrated the CV curves of Ni3S2NS/NF in 1 mmol/L formaldehyde at varied scan rates from 20 mV/s to 200 mV/s.The peak oxidation current of the positive electrode was proportional to the square root of the scan rate (R2=0.9978),which indicated that the catalytic oxidation mechanism of formaldehyde on Ni3S2NS/NF was a process of diffusion control (Fig.2d) [21,22].
It is well known that the applied potential will affect the electrocatalytic oxidation reaction of formaldehyde in electrochemical sensing.Hence,it is extremely important to optimize the test voltage.Fig.3a depicted the current response of the Ni3S2NS/NF electrode under varying potential (0.40–0.60 V) when 1 mmol/L formaldehyde was continuously added to 0.1 mol/L NaOH,in which the selected test voltages were all in the range around the oxidation peak.The time required for this current response value to reach the stable platform was about 5 s,indicating that oxidation of formaldehyde by the Ni3S2NS/NF electrode had a fast current response.Fig.3a showed the optimized spectrum of the test potential.It was not difficult to see that the noise value of 0.55 V and 0.60 V was relatively large,which would affect the accurate measurement of formaldehyde,while the test current response value of 0.40 V and 0.45 V was lower than 0.50 V.Therefore,0.50 V was selected as the optimal test potential in the following electrochemical tests.Electrodes were usually evaluated by measuring the current response at a fixed potential when the analyte was added.Fig.3b indicated the current value response on the Ni3S2NS/NF electrode towards formaldehyde concentration changed over time at the potential of+0.50 V.As shown in Fig.3c,the current response value showed a linear relationship with the formaldehyde concentration in the range of 0.002-5.45 mmol/L,and the correlation coefficient was 0.9957 at 0.50 V in 0.1 mol/L NaOH with a sensitivity of 1286.9 μA L mmol–1cm–2.The detection limit was 1.23 μmol/L (S/N=3).Table 1 listed the performance comparison with other nickel−based formaldehyde sensors.Compared with the other nickel−based materials that had been reported,the Ni3S2NS/NF electrode was with outstanding detection limit,sensitivity and wide linear range.This proved that the Ni3S2NS/NF electrode had excellent performance in formaldehyde detection.
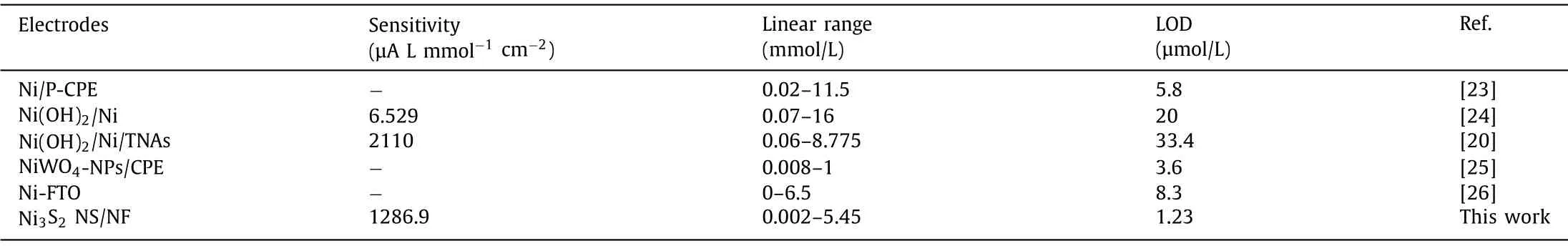
Table 1 Performance comparison of our catalyst with other reported nickel−based formaldehyde sensors.
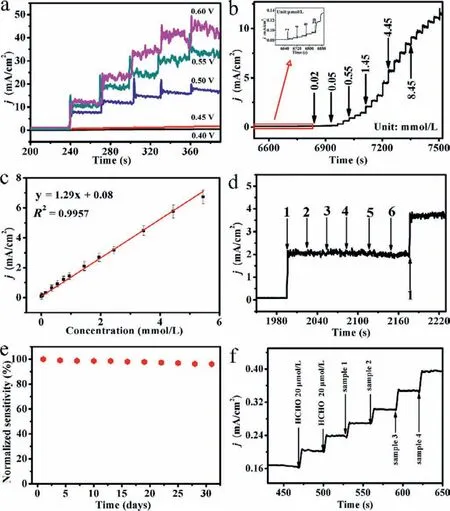
Fig.3.(a) Time-range amperometric diagrams of Ni3S2 NS/NF electrode at different potentials (0.40-0.60 V) with sustaining addition of 1 mmol/L formaldehyde in 0.1 mol/L NaOH.(b) The current response of Ni3S2 NS/NF in 0.1 mol/L NaOH with continuous addition of formaldehyde at 0.50 V (inset: the current response of electrode towards the addition of formaldehyde from 2 μmol/L to 20 μmol/L).(c) Calibration curve of Ni3S2 NS/NF electrode to successive additions of formaldehyde in 0.1 mol/L NaOH at 0.50 V test voltage.(d) Amperometric response of Ni3S2 NS/NF electrode in the presence of 1 mmol/L formaldehyde,1 mmol/L formic acid,1 mmol/L ethanol,1 mmol/L acetic acid,1 mmol/L propanol and 1 mmol/L acetone in 0.1 mol/L NaOH at the applied potential of 0.50 V.(e)Normalized sensitivity of Ni3S2 NS/NF as the working electrode to formaldehyde tested every 3 days within a month.(f) Amperometric response of Ni3S2 NS/NF on the addition of two 20 μmol/L standard formaldehyde solution,two 20 μmol/L real samples,and two 30 μmol/L real samples at 0.50 V in 0.1 mol/L NaOH.
The selectivity of the fabricated sensor is one of the very important studies.Therefore,the selectivity of Ni3S2NS/NF electrode was presented in Fig.3d.The interferences from some electroactive substances were recorded when the same concentration was injected respectively at a steady potential of 0.50 V in 0.1 mol/L NaOH (pH 13).In Fig.3d,1-6 represented formaldehyde,formic acid,ethanol,acetic acid,propanol and acetone,respectively.It was found that interfering substances could hardly cause current value response,while the addition of 1 mmol/L formaldehyde caused a large current change,demonstrating that the Ni3S2NS/NF electrode was with good selectivity and could be used to detect formaldehyde.Besides,we also tested the stability of the Ni3S2NS/NF electrode for 31 days using the current-time method.The specific process was to test the response of the modified electrode to 1 mmol/L formaldehyde in 0.1 mol/L NaOH every 3 days.After each measurement,the material was rinsed with deionized water 3 times and kept dry.The normalized sensitivity of multiple tests was as high as more than 95% (Fig.3e),indicating that the material showed good repeatability and long-term stability.In addition,we also tested the XRD of the Ni3S2NS/NF after detecting formaldehyde.As shown in Fig.S5 (Supporting information),the test result was still very consistent with the standard card,which further proved that the material was with good stability.
In order to test the effectiveness of the prepared materials in the detection of real samples,Ni3S2NS/NF was used to detect formaldehyde in water samples in different environments by the standard addition method.According to previous literature reports,the National Standard stipulates that the formaldehyde content in drinking water does not exceed 30 μmol/L [27].Hence,it is meaningful that the sensor can detect real water samples containing less than 30 μmol/L formaldehyde.To verify the electrochemical sensor we proposed was suitable for the detection of formaldehyde in real water samples,20 μmol/L and 30 μmol/L formaldehyde were added to real water samples for recovery experiments.Fig.3f was amperometric response of the Ni3S2NS/NF on the addition of two 20 μmol/L standard formaldehyde solution,two 20 μmol/L real samples,and two 30 μmol/L real samples at an applied potential of 0.50 V in 0.1 mol/L NaOH solution.The recovery value of the real samples in Table S1 (Supporting information) was fairly satisfactory,showing that the proposed sensor could be used for the detection of formaldehyde.
In summary,Ni3S2NS/NF was rapidly andin-situsynthesized by microplasma-induced chemical etching.This method provided a new and promising way for preparation of other nickel-based and metal nanomaterials for the electrochemical sensor.Ni3S2NS/NF was used as an electrode for electrochemical detection of formaldehyde in alkaline solution,which showed an extremely wide detection range (0.002–5.45 mmol/L),good sensitivity (1286.9 μA L mmol–1cm–2) and limit of detection (LOD) (1.23 μmol/L).Moreover,the prepared electrode was excellent in stability and sensing performance,which demonstrated the potential application in the determination of trace formaldehyde.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Foundation of Sichuan Normal University (No.XJ20210047) and Foundation of Sichuan Department of Science and Technology (No.2017FZ0079) for financial support.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.09.024.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
