Achieving a stable COF with the combination of “flat” and “twist”large-size rigid synthons for selective gas adsorption and separation
Jingyng Li,Ying He,Yongcun Zou,Yn Yn,Zhigung Song,∗,Xiodong Shi,∗
a State key Laboratory of Supramolecular Structure and Materials,College of Chemistry,Jilin University,Changchun 130012,China
b University of South Florida,Tampa,FL 33620,United States
Keywords:N-2-Aryl triazole ETTA Covalent organic framework Gas adsorption and separation
ABSTRACT A new type of covalent organic framework (COF) was achieved using combination of structrally rigid and conformationally othorganal building blocks.The N-2-aryl-substituted triazole derivative (NAT-CHO)was prepared with co-planar conformation among the three aromatic rings as the “flat” building block.The 4,4′,4′′,4′′′-(ethene-1,1,2,2-tetrayl)tetraaniline) (ETTA) was applied as the “twist” building block.A 2D sheet of network was obtained through imine formation.The resulting NAT-COF gave excellent thermal and chemical stability,survived aqueous solutions from pH 5 to 13.With large-size building blocks,the porous framework NAT-COF gave efficient gas adsorption with excellent selectivity of C3 propane over C1 me-thane,suggesting its potential application for selective gas capture and separation.
As a new class of extended porous crystalline materials,covalent organic frameworks (COFs) have received tremendous attention in the past decades [1,2].The covalent linkage along with high surface area,uniform pore size,thermal and chemical stabilities,and tunable structural properties make COF a promising material for applications in catalysis,drug delivery,gas storage,and separation [3–5].The structure and function relationship of the resulting frameworks depends on versatile synthetic strategy to develop the linker between building blocks [6,7].On the other hand,the geometry of building blocks determines both structural topology and functionality [8,9].In general,structurally rigid building blocks are required to produce polymeric crystalline frameworks [10–12].In addition,to reach controllable pore size,large building blocks are needed,which often requires long synthesis [13–15].Therefore,new COF systems constructed from readily available building blocks with good porosity and adsorption selectivity are of great interest.
Among the literature reported polymerization strategies,aldehyde-amine condensation (formation of imine) is of the most popular approaches due to mild reaction condition (dehydration)and conformation control [16–19].As shown in Scheme 1A,with aromatic aldehyde and aniline,the resulting Schiff base bearing extended conjugation of C=N with two arenes give good product stability.Moreover,although imine could give two different double isomers,the equilibrium nature of the reaction could lead to formation of more stable polymeric structures through building block conformation control [20–22].Based on this strategy,various new COFs have been successfully prepared with interesting chemical and physical properties.
Clearly,the size and conformation of organic linker is crucial for the capability to form good COF materials [23–25].To reach a desirable size of pore,rigid long linker is required.Linked aromatic hydrocarbon is one general strategy to achieve this goal.However,as shown in Scheme 1B,the arene could not form co-planar conformation due to the A-1,3 repulsion [26].The resulted twisted conformation often caused repulsion between 2D network,making it less ideal linkage for COF synthesis.Full conjugated aromatic system,such as pyrene,has been used to overcome this problem by keeping the flat conformation with large distance between binding units [27–30].However,the synthesis and derivatization of those compounds could be high cost and made it less practical for large scale COF production.Our group recently developed theN-2-aryltriazole (NAT) moiety as an alternative to reach co-planar conformation through readily available synthesis [31].With this new conformationally rigid large linker,we proposed the combination of“flat” and “twisted” building blocks for construction of COF through simple Schiff-base formation process.Herein,we report the synthesis of a new COF from a flat building block NAT-CHO and a twisted building block ETTA amine.With these two readily available building blocks,NAT-COF could be easily prepared in large scale.This new organic framework showed excellent stability toward various solvents,acidic and basic aqueous solution (pH 5 to 13).The large pore size makes this new COF good material for gas adsorption,with 190:1 selectivity in adsorbing larger size C3H8over CH4at 298 K,along with good adsorption selectivity toward CO2over N2.
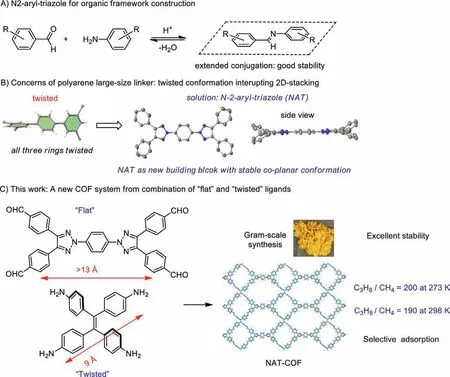
Scheme 1.NAT as the unique building block for material construction.
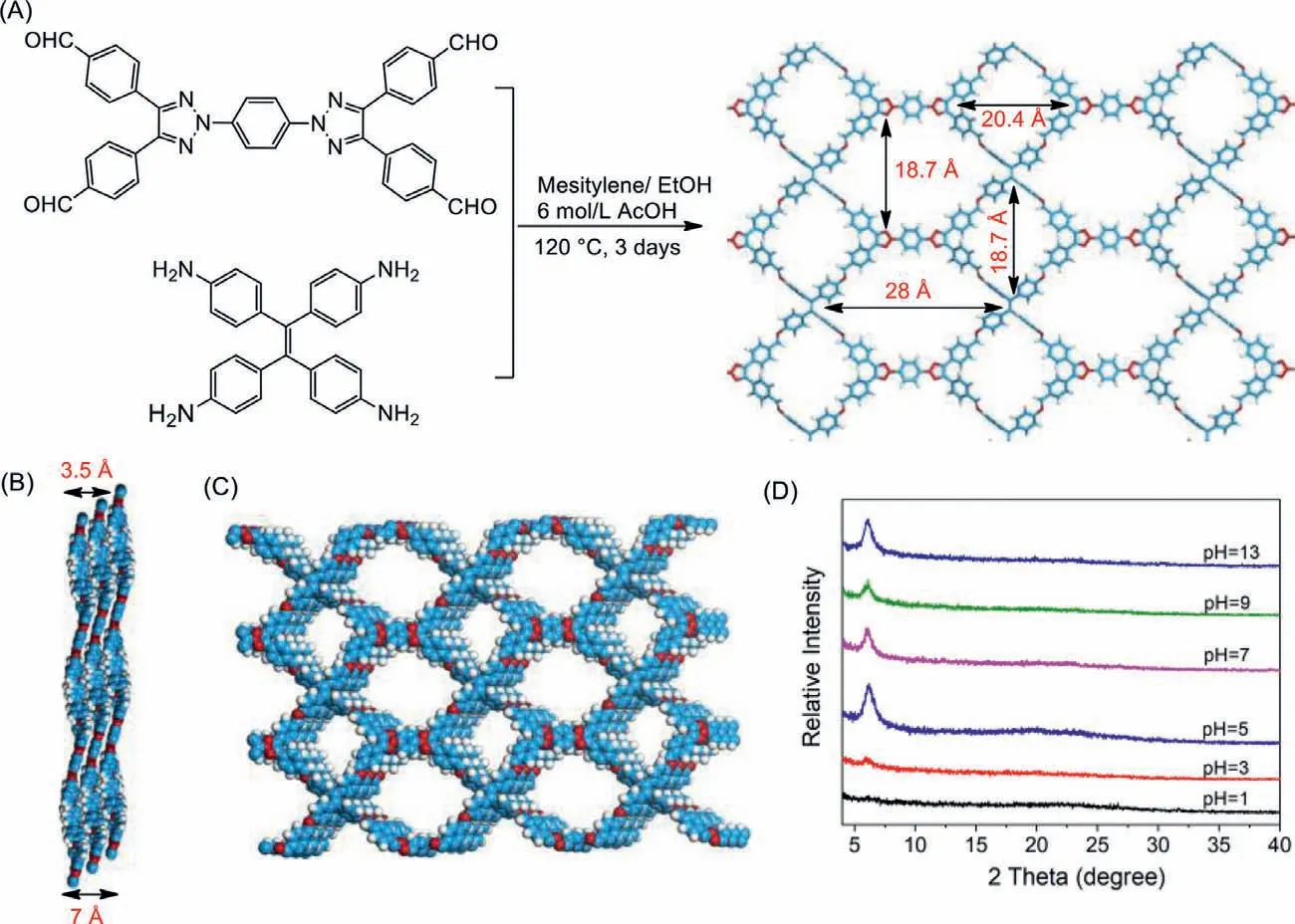
Fig.1.(A) Synthesis of NAT-COF;(B) Side view of stacking of 2D NAT-COF;(C) Top view of stacking of 2D sheets;(D) Stability of NAT-COF in aqueous solution from pH 1 to pH 13 by PXRD.
As shown in Fig.1A,NAT-COF was synthesized with stoichiometric amount of NAT-CHO and ETTA in mixed solvents (mesitylene:EtOH:AcOH (6 mol/L)=5:5:2,v/v/v) at 120 °C (Fig.1A).After 3 days,yellow powder was obtained and washed by anhydrous THF,anhydrous trichloromethane and anhydrous methanol to obtain the pure NAT-COF powder in 90% yield.FT-IR was used to confirm imine formation (Fig.S3 in Supporting information).It can be found that characteristic C=N stretching at 1623 cm−1formed while typical bands of C=O at 1703 cm−1from NAT-CHO and NH at 3357 cm−1from ETTA almost disappeared after new material formation.
Having successfully obtained the NAT-COF,its crystal structure was simulated through Materials Studio (Fig.S2 in Supporting information).The simulated structure showed a 2D AA stacking structure which was linked by imine functional groups.In each layer,two pores were identified,suggesting this extended backbone could provide large porosity for promising gas sorption ability(Fig.1A).Simulated NAT-COF from Materials Studio represents the stacking of layers with ordered pore channels of NAT-COF and the distance between neighboring layers was calculated to be around 3.5 ˚A (Figs.1B and C).
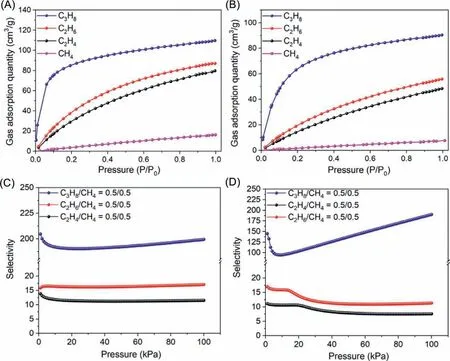
Fig.2.Gas adsorption of C3H8,C2H6,C2H4 and CH4 for NAT-COF at 273 K (A) and 298 K (B).Selectivity of C3 hydrocarbon over C1 for NAT-COF at 273 K (C) and 298 K (D).
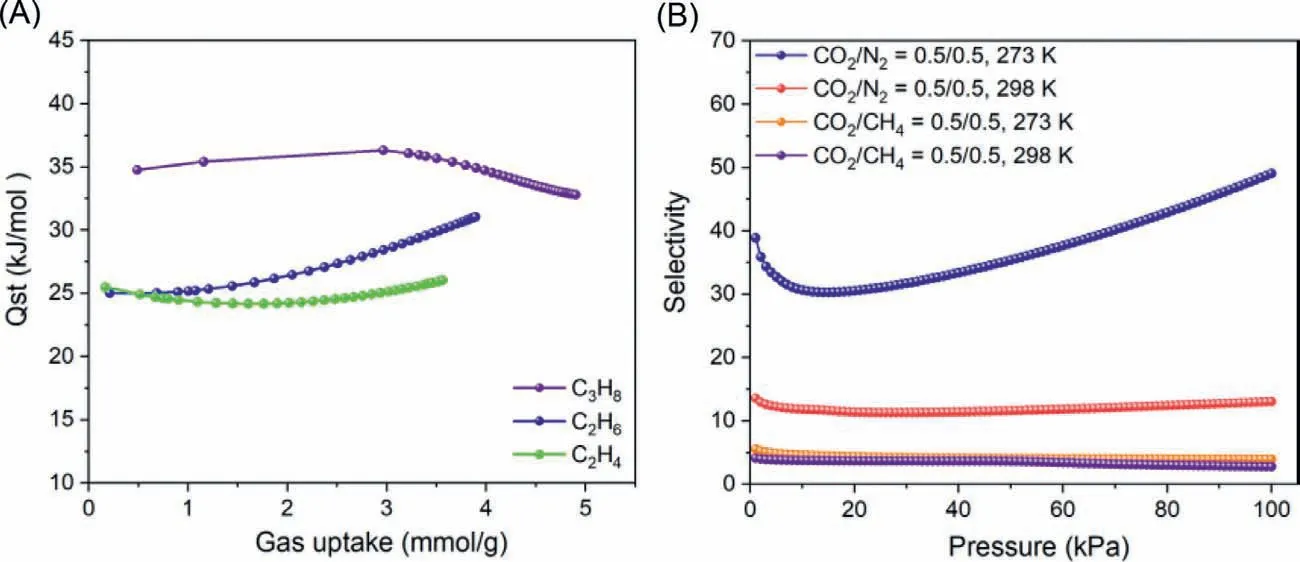
Fig.3.(A) Qst values of C3H8,C2H6 and C2H4;(B) Gas selectivity of CO2 over CH4 and CO2 over N2 at 273 K and 298 K.
To explore the property and functionality of this new network,we first evaluated its thermal and chemical stability.Thermogravimetric analyses (TGA) revealed good thermal stability of NAT-COF(Fig.S4 in Supporting information).Then the stability of COF in solution was investigated.NAT-COF was immersed in aqueous media with wide range of pH for 15 days at room temperature.The PXRD patterns showed that NAT-COF maintained the structural integrity from pH 5 to 13 (Fig.1D).For organic solvents,NAT-COF maintained good stability in various organic solvents (acetone,MeOH,EtOH,DMF,DCM,MeCN and THF) after being immersed over 15 days at room temperature (Fig.S5 in Supporting information).
The porosity of NAT-COF was first examined by N2adsorption at 77 K using activated samples.The Brunauer-Emmett-Teller (BET)and Langmuir surface area were calculated to be 785.3 m2/g and 945.6 m2/g (Fig.S6 in Supporting information).Pore width distribution of NAT-COF suggests that NAT-COF contained both micropores and mesopores which facilitate gas adsorption (Fig.S7 in Supporting information).
Impurities from natural gas usually contain ethane and propane,which limited CH4utilization in natural-gas processing industry[32,33].Considering good stability and moderate BET surface area,gas adsorption of NAT-COF for hydrocarbons including C3H8,C2H6,C2H4and CH4at 273 K and 298 K were recorded with the trend of C3H8>C2H6>C2H4>CH4,highlighting the highest adsorption value of 109.8 cm3/g (4.9 mmol/g) at 273 K and 90.4 cm3/g(4.0 mmol/g) at 298 K for C3H8among all tested gas,and detailed value for other gas are deposited in Figs.2A and B).
The selectivity of gas molecules was calculated by Ideal solution adsorbed theory (IAST) with a good correlation factor (R2 >0.999).The selectivity curve of C3 hydrocarbon over CH4is shown in Figs.2C and D.The results suggested a similar trend of C3H8>C2H6>C2H4for the selectivity of C3 hydrocarbon over C1.The selectivity of C3H8/CH4is 200 at 273 K and 190 at 298 K.Both high sorption capability and selectivity towards C3 hydrocarbon over C1 could be attributed to size matching between C3H8and large amount of micropores in the framework.Weak interactions between host and gas molecules (van der Walls forces) also have influenced on selectivity.Relatively more hydrophobic C3 hydrocarbon has stronger interaction with lipophilic ETTA.Isosteric heat of adsorption (Qst) could also provide some insight for addressing this result.Qstvalues C3H8,C2H6and C2H4were calculated and summarized in Fig.3A.Among all tested gas,C3H8gave the highest absoluteQstvalue (34.7 kJ/mol) in all cases which could account for higher selectivity of C3 hydrocarbon over C1.
Carbon capture and sequestration (CCS) technology plays a crucial role in tackling environmental concerns related to growing atmospheric CO2emissions caused by fossil fuels [34–37].Beside,CO2and CH4molecules have close kinetic diameters of 3.3 ˚A and 3.8 ˚A,which makes their separationviamolecular sieving diffi-cult.The nitrogen atoms in NAT-CHO could increase CO2adsorption capacity.Moderate selectivity between CO2and CH4(4 at 273 K and 2.8 at 298 K) was obtained (Fig.3B),which can be contributed to higherQstvalue (Fig.S8 in Supporting information) of CO2(26.7 kJ/mol) compared with that of CH4(21.5 kJ/mol).Similarly,the selectivity of CO2/N2is 49.1 at 273 K and 13.1 at 298 K,which is consistent with CO2-philicity of NAT-COF owing to more dipole-dipole interactions.
In summary,combination of “flat” and “twisted” building blocks for the construction of COF was successfully achieved by tethering polar NAT-CHO and lipophilic ETTA through imine formation.Taking advantage of extended scaffold of co-planar NAT-CHO which was supported by “twisted” backbone of ETTA,NAT-COF exhibited excellent gas selectivity of hydrocarbons and CO2.Notably,NATCOF demonstrated efficient gas adsorption and selectivity in C3 propane over C1 methane,at the same time good with CO2over N2,making it a promising candidate for both natural gas purification and CCS.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.12.011.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
