Rigid chelating dicarbene ligands based on naphthyridine-fused bisimidazolium salts
Yan Liu,Zhijie She,Qinze Zheng,Xuesong Zheng,Tianbao Wang,Ge Gao
Key Laboratory of Green Chemistry and Technology of Ministry of Education,College of Chemistry,Sichuan University,Chengdu,610064,China
Keywords:NHC Dicarbene ligand Naphthyridine Phenanthroline
ABSTRACT Naphthyridine-fused bisimidazolium salts were designed and synthesized for the first time.The study of the Cu(II) and Pd(II) complexes demonstrated that the deprotonated dicarbene ligands are rigid chelating C,C-ligands with strong electron-donating ability in analogy with the classic phenanthroline N,N-ligands.
1,10-Phenanthroline (phen,A,Fig.1) is a tricyclic and planar nitrogen-containing heteroarene [1].It has relatively weakσdonating ability due to its electron-poor characteristic.However,this drawback is largely compensated by its hydrophobicity,two rigid pre-organized nitrogen atoms with a perfect cone angle,andπ-acceptor nature.Therefore,it still exerts strong and entropically favored metal binding.Phen has become a classic chelating bidentate N,N-ligand,and together with its various derivatives been widely serving in coordination chemistry,supramolecular chemistry,transition metal catalysis,and photoactive materials [2,3].On the other hand,N-heterocyclic carbenes (NHCs) are strongσ-donors and weakπ-acceptors [4–7].Since the synthesis and isolation of the first stable NHC,1,3-di(adamantyl)imidazol-2-ylidene,by Arguengoet al.in 1991 [8],a plethora of different NHCs have been continuously created and extensively studied,resulting in enormous attractive applications [9–14].Imidazolium salts are the most commonly used precursors to construct multidentate NHC ligands due to their easy access and stability.In 1995,Herrmannet al.first showcased the catalytic activity of a Pd(II) complex formed by a methylene-bridged dicarbene ligand (B,Fig.1) in the Heck coupling of aryl halides.Unfortunately,complex decomposition was witnessed in solution above 70 °C,probably related to the flexibility of the ligand [15].
It was not until 15 years later that a rigid tricyclic bidentrate NHC ligand named vegi [16]was synthesized based on pyridazine by the group of Kunz to be a C,C-analogue of phen (C,Fig.1).Due to the contracted five-membered imidazolium moieties,the two carbene sites is fixed in a distance of 2.97 ˚A with a wider cone angle in comparison with the two nitrogen atoms in phen,and the molecule is planar.The vegi ligand acted as a chelating C,C-ligand to form mononuclear complexes with alkali metals [17]as well as common transition metals such as Rh(I) and Ir(I) [16,18,19].However,it acted as a bridging ligand with coinage metals Ag(I),Cu(I)and Au(I) to form the corresponding binuclear complexes [16,20],implying it is still relatively flexible [21].To attain a phen analogue with a smaller cone angle,Monkowiuset al.later introduced a mixed C,N-ligand (D,Fig.1) in 2012 [22].It formed chelating Pd(II)and Rh(I) complexes [23,24],but acted as a monodentate C-ligand for Ag(I),Au(I),Au(III) and Cu(I) [22,25].Interestingly,nonorbitalbased dispersion-type interactions were found between the nitrogen and Au atoms in the Au(III) complexes [22].
Due to our continuous interests in novel azolium compounds as functional materials and ligands [26–32],our design of a C,Canalogue of phen ligand (1,Fig.1) is based on the following considerations: 1) two rigid and pre-organized NHC sites to exert strongerσ-donating ability than phen;2) a tetracyclic over tricylic system with a smaller cone angle than C and D to reinforce chelating metal coordination;3) different R groups as wingtips to fine tune the metal coordinating environment.Herein,we present the synthesis of naphthyridine-fused bisimidazolium salts 1·2HCl.The deprotonated dicarbene coordinates Cu(I) to afford a mononuclear Cu(II) complex in tetrahedron geometry,suggesting its strongσ-donating and suitable chelating ability.It coordinates Pd(II) to form a distorted square planar complex,which surpasses its counterparts,the rigid N,N-ligand and flexible C,C-ligand in the Suzuki cross-coupling reactions.
The precursors 1·2HCl could be easily obtained following a three-step procedure starting from 2,7-dimethyl-1,8-naphthyridine(Scheme 1).The two methyl groups were first transformed into the formyl groups by oxidation using selenium dioxide.The dicarbaldehyde then reacted with different primary amines to form diimines.Finally,cyclization of diimines with paraformaldehyde followed by treatment with a dioxane solution of hydrochloride(4 mol/L) afforded the designed precursors 1·2HCl in nice yields,which were isolated and purified by column chromatography on silica gel.Their structures were characterized by NMR and high resolution mass spectroscopy (HRMS).The1H NMR spectra showed that their protons on the precarbene sites are significantly acidic as they appear in low field at 11.05 (DMSO–d6),12.14 (DMSO–d6),and 10.90 (CD3OH) ppm for 1a-c,respectively.Meanwhile,the protons on the pre-abnormal carbene sites are in high field at 8.98,8.85,and 8.56 ppm,respectively.The large differences (up to 3.29 ppm)implied that the formation of carbenes by deprotonation of 1·2HCl in the presence of a base should be less interfered by the formation of abnormal carbenes.
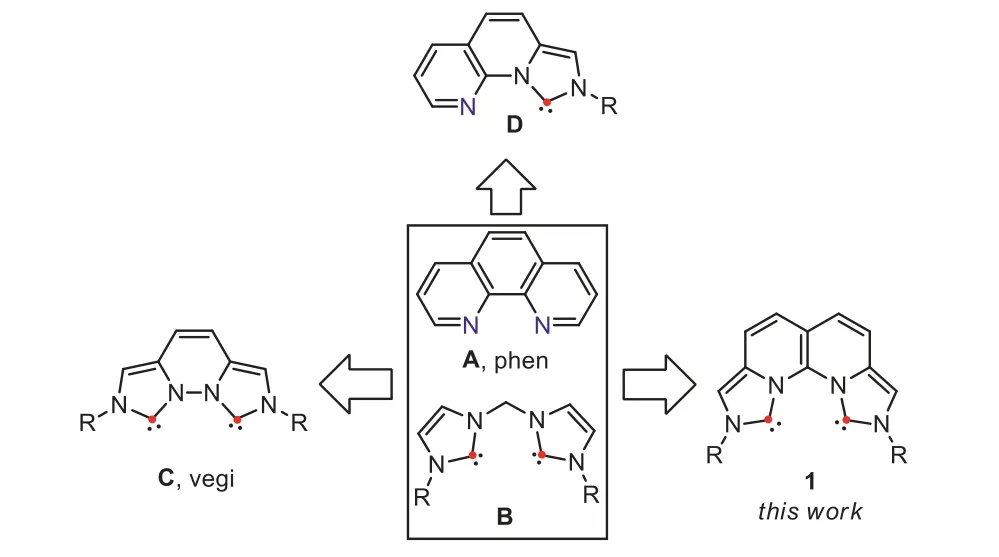
Fig.1.Structures of phen (A) and bidentate NHC ligands: flexible C,C-ligand (B);vegi (C);mixed C,N-ligand (D) and rigid C,C-ligand (1).
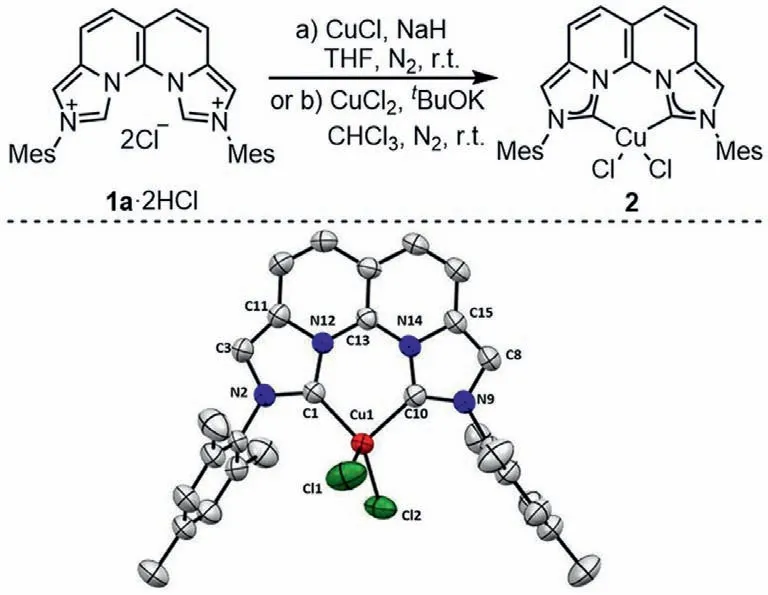
Scheme 2.Top: Synthesis of the Cu(II) complex 2.Bottom: ORTEP plot (50% probability thermal ellipsoids) of 2.Hydrogen atoms are omitted for clarity.
The single crystals of 1a·2HCl were cultivated in mixed dichloromethane (DCM)/methanol at room temperature and the structure was resolved by the X-ray diffraction analysis as shown in Fig.2.The aza[4]helicene structure renders 1a·2HCl nonplanar and the two imidazolium rings twist about 18.4°.The distance between the two precarbene carbons (C1 and C10) is 3.093 ˚A.This long distance is caused by repulsion between the two hydrogens on C1 and C10,and is expected to be significantly reduced after deprotonation.Moreover,the two mesityl groups sit almost orthogonally to the imidazolium planes with dihedral angles of 73.7° and 71.2°,respectively,providing a very nice shielding for the carbene sites.It is worth noting that the same protection by the aryl groups is not presented in the vegi ligand [21].
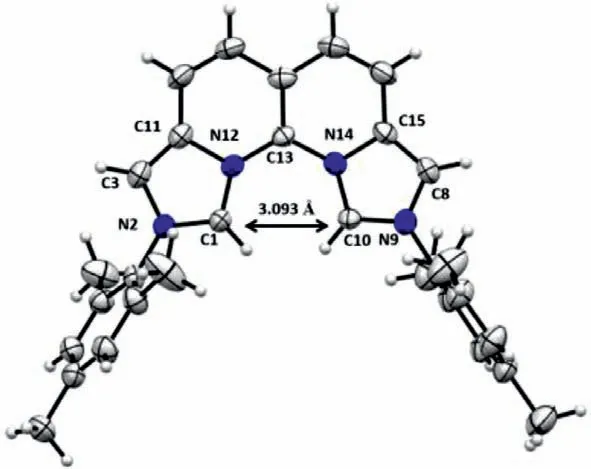
Fig.2.ORTEP plot (50% probability thermal ellipsoids) of 1a·2HCl.
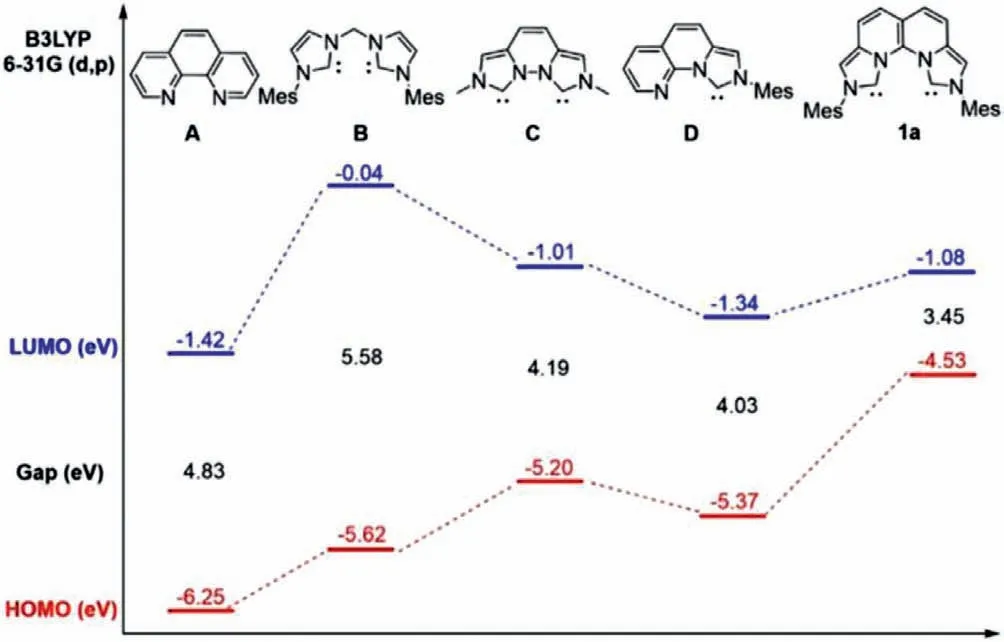
Fig.3.HOMO/LUMO energies of phen (A),methylene-bridged dicarbene (B),vegi(C),C,N-ligand (D) and rigid dicarbene (1a) at the B3LYP/6–31g(d,p) level.
Preparation of the free dicarbene 1a by deprotonation of 1a·2HCl with base in DMSO was conducted.The reaction system was darkened immediately and decomposed gradually in the presence of a strong base such astBuOK,tBuONa,KHDMS,NaHDMS,and LiHDMS.When a weak base (NaOAc or Et3N) was used,the reaction system remained unchanged over time.Anin situ1H NMR spectrum showed that the peak of the C1/C10 protons disappeared after 30 min from addition of NaH,indicating the formation of the free dicarbene 1a (see Supporting information for details).Unfortunately,decomposition of the free dicarbene occurred while13C NMR data were being collected.
To obtain more information about the free dicarbene 1a,DFT calculations was carried out at the B3LYP/6–31 g(d,p) level.The optimized structure of 1a is consistent with the crystal structure of the 1a·2HCl (Fig.S1 in the Supporting information).The distance between the two carbene atoms (C1 and C10) is shortened to 2.89 ˚A,which is smaller than that in the vegi ligand (2.97 ˚A)[16],but still larger than the N–N distance in phen (2.744 ˚A) [33].Moreover,the two imidazolium rings also twist less in 16.2°.The molecular orbital (MO) analysis showed that the carbeneσorbitals overlap with each other in HOMO-3,revealing high electron density between the two carbene sites.The HOMO/LUMO energies of 1a were calculated to be −4.53/−1.08 eV (Fig.S2 in Supporting information).In comparison,the HOMO/LUMO energies of phen,methylene-bridged dicarbene ligand,vegi [16],and C,N-ligand were also calculated to be −6.25/−1.42,−5.62/−0.04,−5.20/−1.01,and−5.37/−1.34 eV,respectively (Fig.3).It is clear to see that 1a has a much higher HOMO energy than the other ligands and a smallest energy gap between LUMO and HOMO.The significantly elevated HOMO energy level suggested the strong electron donating ability of 1a.
The coordination behavior of dicarbene 1 toward copper was then evaluated.The [Cu(I)(phen)2X]complexes are extensively investigated as emitters in OLEDs and photosensitizers in photocatalysis [34–39].We therefore wanted to use 1a·2HCl to mimic a similar Cu(I) complex.1a·2HCl did not react with CuCl in the absence of a base.When NaH was used,a new compound was isolated.However,the1H NMR spectrum showed no signal,indicating that it was a paramagnetic compound.Fortunately,single crystals were obtained in mixed DCM/methanol to show a structure of [Cu(II)1aCl2](2,68% yield).We also conducted a reaction of 1a·2HCl with CuCl2in the presence oftBuOK,and 2 was formed in 80% yield (Scheme 2).These results demonstrated the strong electron donating ability of 1a,which inevitably led to oxidation of the coordinated Cu(I) to Cu(II) during workup.Complex 2 adopts a distorted tetrahedral geometry with a bite angle (C1-Cu1-C10) of 90.7° and a dihedral angle between the C1-Cu1-C10 and Cl1-Cu1-Cl2 planes of 77°.The Cu center is slightly off the ligand plane.It is noted that the coordinated dicarbene ligand is almost planar as the dihedral angle between the two imidazolium rings is significantly reduced to only 7.6°,resulting in an even shortened C1-C10 distance of 2.762 ˚A,which is very close to the N–N distance in the phen ligand.It needs to point out that while three-and fourcoordinated Cu(I) complexes of phen ligands are common,fourcoordinate Cu(II) complexes are not typical [40,41].In our case,however,only a four-coordinate Cu(II) complex was formed no matter a Cu(I) or Cu(II) salt was used.This difference is probably related to their different electron-donating abilities.Therefore,ligand 1 can be considered as the C,C-analogues of the phen ligands with enhanced electron-donating ability.
To compare with the rigid phen N,N-ligand A and flexible dicarbene C,C-ligand B,coordination of Pd(OAc)2with the rigid dicarbene 1a-b was conducted in the absence of a base to directly afford the corresponding [Pd(II)1aCl2](3a) and [Pd(II)1bCl2](3b)in 90% and 95% yields,respectively.The structure of 3b was resolved by the single crystal X-ray diffraction analysis (Scheme 3).Complex 3b rests in a distorted square planar geometry with the dicarbene plane (C1-C13-C10) bent out of the coordinating plane(C1-Pd1-C10) in 34.9° due to the steric hindrance of the adjacent butyl groups,which resembles very much the corresponding phen[42,43]and flexible dicarbene complexes [44,45].The angle of Cl1-Pd1-Cl2 is 87.3°,similar to that in the corresponding phen complex(86.8°).However,the bite angle of C1-Pd1-C10 is 86.3°,larger than the bite angle of N-Pd-N (80.6°).In addition,a dihedral angle of 10.4° is found between the C1-Pd1-C10 plane and the Cl1-Pd1-Cl2 plane.The rigid skeleton is forced in a boat-shaped conformation,resulting in a dihedral angle of the two imidazolium rings of 29.6°and a further shortened C1-C10 distance of 2.704 ˚A from 2.The bond lengthens of C1-Pd and C10-Pd are 1.977 ˚A equally,which fall into the common range of NHC-Pd bonds.
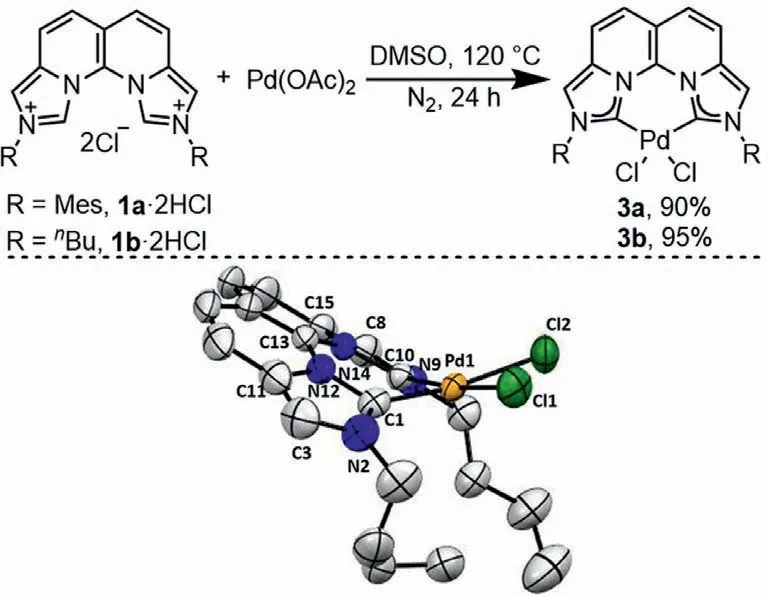
Scheme 3.Top: Synthesis of the Pd(II) complexes 3.Bottom: ORTEP plot (50% probability thermal ellipsoids) of 3b.Hydrogen atoms are omitted for clarity.
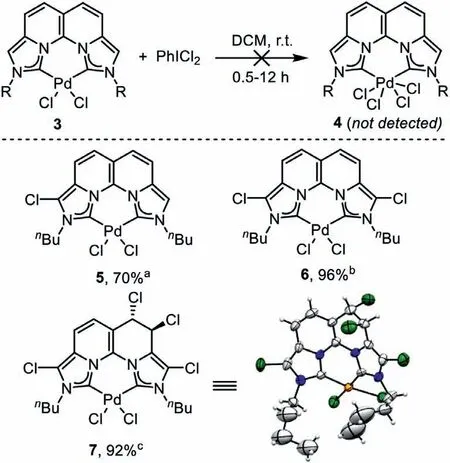
Scheme 4.Oxidation of the Pd(II) complex 3b and the corresponding products 5–7.Conditions: in the presence of (a) 1 equiv.,(b) 2 equiv.and (c) 4 equiv.of PhICl2.
In addition to the classic Heck,Negishi and Suzuki reactions involving a Pd(0)/Pd(II) catalytic cycle,reactions involving an alternative Pd(II)/Pd(IV) cycle have been actively pursued in the past decades.Well-defined Pd(IV) complexes are helpful to understand the catalytic process,but the synthesis is still elusive [46].In consideration of the rigid backbone and strong electron-donating ability of the dicarbene 1,possible access to a proposed Pd(IV)complex 4 by oxidation of the Pd(II) complex 3 was attempted(Scheme 4).The oxidation of 3a with 1 equiv.of PhICl2resulted in no reaction at all.However,the oxidation of 3b under the same conditions unexpectedly delivered a chloro–substituted product 5 in 70% yield.When 2 equiv.of PhICl2was used,a dichlorosubstituted product 6 was obtained in 96% yield.Increasing the amount of the oxidant to 4 equiv.led to further dearomatization of the naphthyridine backbone and gave a tetrachloro-substituted product 7 in 92% yield.Products 5–7 were fully characterized by NMR and HRMS,and the structure of 7 was also confirmed by the single crystal X-ray diffraction analysis.There was no Pd(IV) product identified in any reaction system.
Finally,the usefulness of the rigid chelating dicarbene 1 as a ligand in transition metal catalyzed reactions was preliminarily evaluated by the catalytic activity of 3a in the Suzuki coupling reactions of a series of representative aryl bromides with aryl boronic acids.Two Pd(II) complexes with a flexible dicarbene ligand (8)and a rigid phen ligand (9) were also prepared (see the Supporting information) and used as comparative catalysts.As can be seen in Table 1,in the presence of 0.5 mol% catalyst and 2 equiv.of Cs2CO3as a base,3a in general performed better than 8 and 9.When electron-deficient and sterically hindered substrates were employed,the reactions ran slower and 3a exhibited much improved catalytic activity than its counterparts (Table 1,entries 2,3 and 6–8).When weaker base NaOAc was employed,3a still worked fine while the performances of 8 and 9 dropped a lot (Table 1,entry 9).When the reactions were conducted with a lower catalyst loading,in water or at room temperature,the desired product was obtained in moderate yield by using 3a,but poor yield was attained by using 8 and 9 (Table 1,entries 10–12).For less activep-chlorotoluene,3a showed a lower efficiency under the standard conditions,while 8 and 9 were ineffective at all.The reaction was much improved when 5 mol% 3a was used (Table 1,entries 13 and 14).These results demonstrated the beneficial effect of the rigid and strongly electron-donating C,C-ligand 1 in the Pdcatalyzed Suzuki coupling reaction.
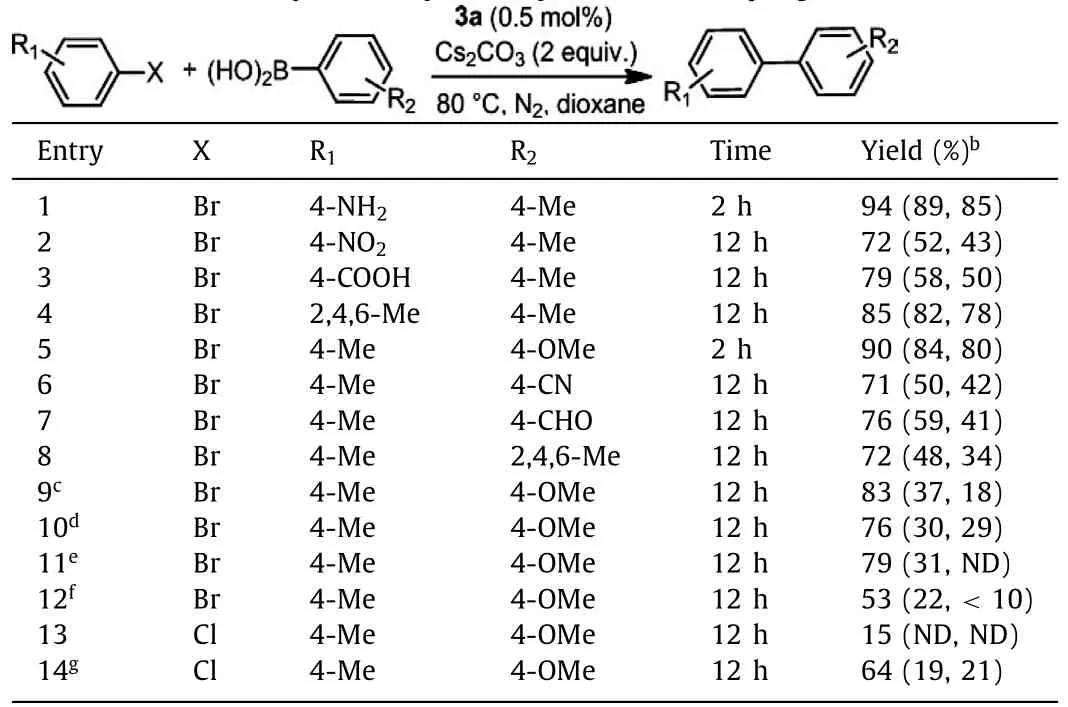
Table 1 Evaluation of the catalytic activity of 3a by the Suzuki coupling reaction.a
In summary,we have successfully prepared novel naphthyridine-fused bisimidazolium salts 1·2HCl by a straightforward three-step procedure.Although the whole molecules are nonplanar due to the aza[4]helicene skeleton,the deprotonated dicarbenes 1 acted as rigid C,C-chelating bidentate ligands formed very similar Cu(II) and Pd(II) complexes as the classic N,N-ligand phen.Differently,however,only a Cu(II) complex was obtained whenever a Cu(I) or Cu(II) source was used.Moreover,the Pd(II)complex exhibited higher catalytic activity than the Pd(II) complexes with a rigid phen ligand and a flexible dicarbene ligand as preliminarily evaluated by the Suzuki coupling reaction.These specific features are believed in related to the combination of a strong electron-donating ability and a rigid chelating skeleton of 1 as designed.These novel rigid dicarbene ligands are attractive in metal coordination for catalysis and optoelectronic materials [47].Further investigations are now going on in our laboratory.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
We appreciate the financial support from the National Natural Science Foundation of China (No.21772134) and the Fundamental Research Funds for the Central Universities (No.20826041D4117).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.10.069.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
