Convenient and efficient access to tri-and tetra-substituted 4-fluoropyridines via a [3+2]/[2+1]cyclization reaction
Xuejio Tng,Kun Liu,Zeming Qu,Junyn Zhn,Rukui Zhu,Fn Teng,Lili Meng,Ynmin Hung,Chusheng Hung,Yimio He,∗,Qing Zhu,b
a Guangxi Key Laboratory of Natural Polymer Chemistry and Physics,College of Chemistry and Materials,Nanning Normal University,Nanning 530001,China
b Guangzhou Institutes of Biomedicine and Health,Chinese Academy of Sciences,Guangzhou 510530,China
c Key Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle,Nanchang Hangkong University,Nanchang 330063,China
Keywords:4-Fluoropyridine gem-Difluorocyclopropene[3+2]/[2+1]cyclization reaction DMSO as an oxidant Regioselectivity PKM2 modulator
ABSTRACT A [3+2]/[2+1]cycloaddition reaction of gem–difluorocyclopropenes is presented,offering a mild and efficient approach to accessing tri-and tetra-substituted 4-fluoropyridines in moderate to good yields with excellent regioselectivity.Multiple synthetic applications,including process-scale reactions,modification of bioactive molecules,derivatization reactions and synthesis of the analogue of the PKM2 modulator,are subsequently described.
Pyridine is one of the most prevalent heterocyclic skeletons in bioactive molecules.The fluorine atom embodies high electronegativity and a small atomic radius [1,2],and its incorporation into pyridine drastically improves the lipophilicity,bioavailability,metabolic stability and,in some cases,the potency of known biologically active molecules (Fig.1).Despite their increasing importance in pharmaceuticals and agrochemicals [3–8],developing a simple and practical method to prepare fluoropyridines,especially 4-fluoropyridines,still encounters great challenges.
The classical approach for the formation of 4-fluoropyridines is the Balz-Schiemann reaction (Scheme 1a) [9].Acidic conditions,toxicity of the reagents,and potential for explosions impede their synthetic applications.Nucleophilic substitution [10–20]of 4-halo or nitropyridines by inorganic or organic fluoride salts [21–31]offers an alternative route for accessing 4-fluoropyridines (Scheme 1b).However,the poor solubility of inorganic fluoride salts,stoichiometric usage of cost and toxic organic fluoride salts,make the reaction proceed under harsh conditions,leading to inferior conversion and functional group tolerance.
gem–Difluorocyclopropene is an intriguing framework,as it contains three important structural motifs: electrophilic difluoromethylene,alkene and the smallest carbocycle,thus possessing unusual electric and steric properties.Taking advantage of thegem–difluorocyclopropene as a valuable two-carbon donor,Waser and coworkers reported a [3+2]annulation of cyclopropenes with cyclopropylanilines to access to 6,6-difluorobicycle[3.1.0]hexanes[32].Furthermore,Cossy realized a concise,and efficient synthesis of 5-fluoropyridazines by a [3+3]cycloaddition reaction[33]using thegem–difluorocyclopropene as a three-carbon donor[34,35].Considering the importance of 4-fluoropyridines in pharmaceuticals and agrochemicals,we herein report that a convenient and effective [3+2]/[2+1]annulation reaction betweengem–difluorocyclopropenes [36–49]and isocyanides 2 [50–55]or aldimine esters 3 [56–59]to produce highly functionalized 4-fluoropyridines in moderate to good yields (Scheme 1c).Specifically,the whole transformation is characterized by the following points: i) Unlike the direct fluorination reactions of pyridines,this novel cycloaddition reaction originates from readily available substrates,and the construction of the pyridine ring and the introduction of a fluorine atom at specific position are completed simultaneously,thus providing a new pathway for the synthesis of 4-fluoropyridines;ii) In the cyclization reactions,the carboncarbonπbond ofgem–difluorocyclopropene is broken,while two carbon-carbon single bonds are formed;iii) The reactions are conducted under mild and air conditions,by which both tri-and tetrasubstituted 4-fluoropyridines are obtained in moderate to good yields with wide substrate applicability and pure regioselectivity;iv) In the synthesis of tetra-substituted 4-fluoropyridines,the reac-tion proceeds without transition metals,and DMSO is utilized as both a solvent and an oxidant.
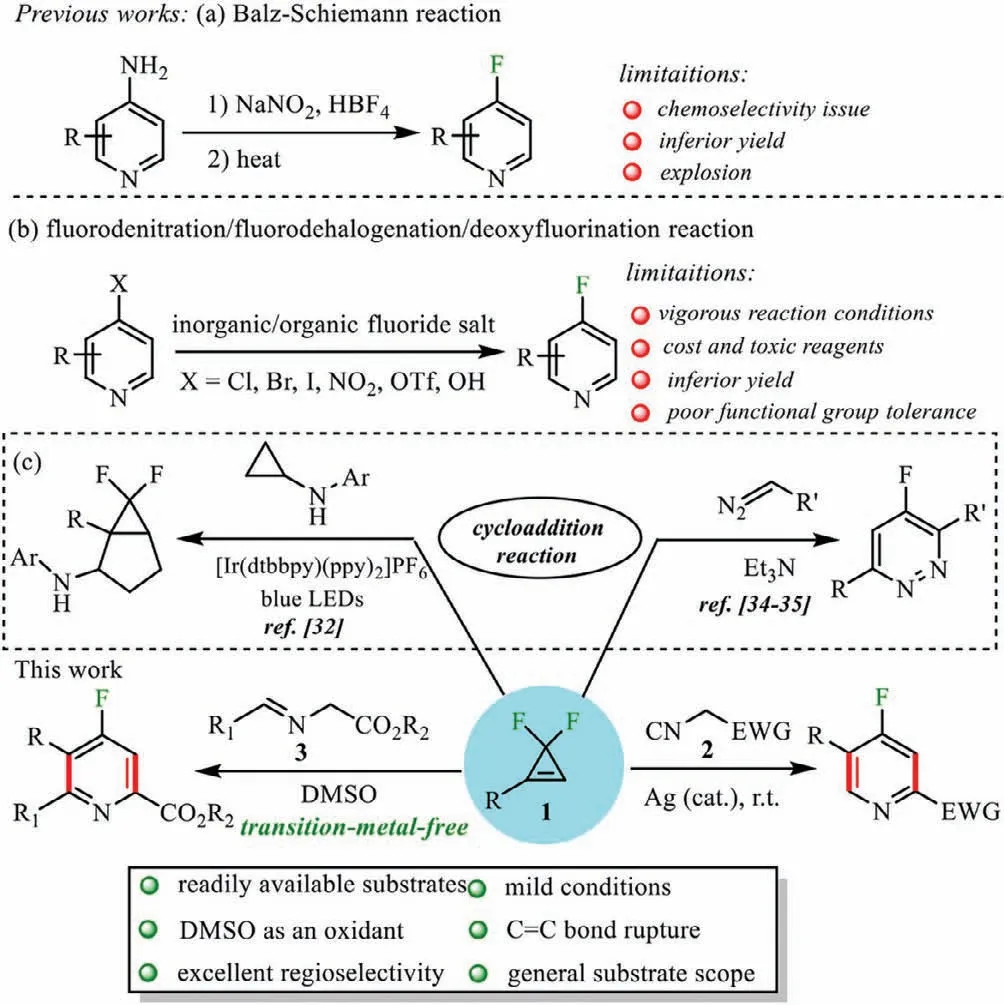
Scheme 1.Tactics for the construction of 4-fluoropyridines.
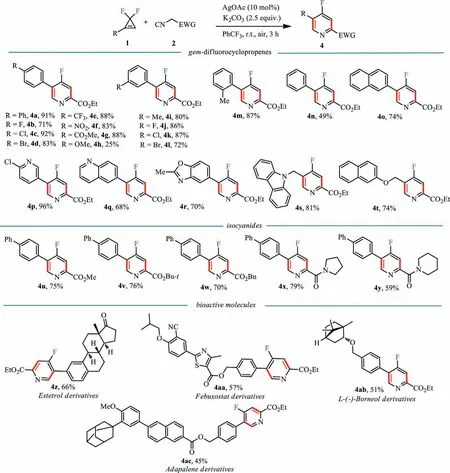
Scheme 2.Substrate scope for trisubstituted 4-fluoropyridines.Reaction conditions: 1 (0.2 mmol),2 (2.0 equiv.),AgOAc (10 mol%),K2CO3 (2.5 equiv.),PhCF3 (1 mL) at room temperature for 3 h in air,isolated yield.
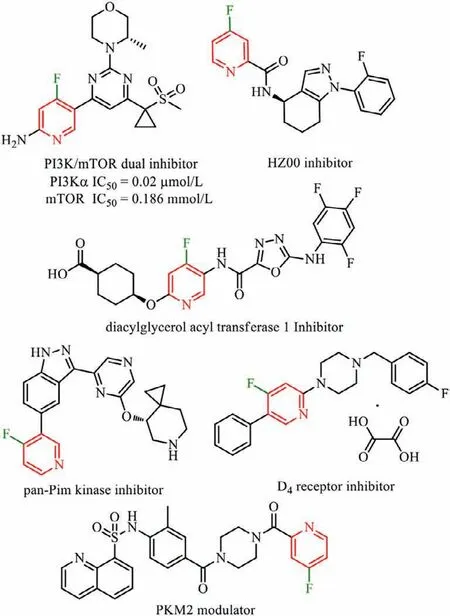
Fig.1.Selected biologically active 4-fluoropyridines.
The reaction was initially investigated usinggem–difluorocyclopropene 1a and ethyl isocyanoacetate 2a as model substrates,AgOAc as the catalyst and K2CO3as the base in PhCF3at room temperature;to our delight,the desired product 4a was obtained in 91% yield (Table 1,entry 1).Other catalysts such as Ag2CO3,Ag2O,AgBF4and Cu(OAc)2prevented the substrate 1a from being completely converted,and the reaction did not proceed without the catalyst (Table 1,entries 2–6).Replacing K2CO3with other bases made the reaction worse (Table 1,entries 7–9).Subsequent solvent screening revealed that PhCF3made better conversion (Table 1,entries 10–12).Then the reaction betweengem–difluorocyclopropene 1a and aldimine ester 3a in the conditions of AgOAc as the catalyst,K2CO3as the base,DDQ as an oxidant,and PhCF3as the solvent was detected.When the temperature was elevated to 70 °C,tetra-substituted 4-fluoropyridine 5a was produced in 22% yield (Table 1,entry 13).Substituting K2CO3with K3PO4increased the yield to 41% (Table 1,entry 14).The reaction still conducted smoothly without the catalyst and gave the product 5a in 54% yield (Table 1,entry 15).When the solvent was changed to DMSO,the yield of 5a was further promoted to 65% (Table 1,entry 16).Interestingly,the reaction was almost impervious even if no DDQ was added (Table 1,entries 17 and 18).Consequently,the optimal reaction conditions for the preparation of 4a were determined to be 1a (0.2 mmol) in the presence of 2a (2.0 equiv.),AgOAc (10 mol%),K2CO3(2.5 equiv.) and,PhCF3(1 mL) at room temperature for 3 h,while 5a was obtained in the presence of 1a (0.2 mmol),3a (1.5 equiv.),K3PO4(1.5 equiv.) and DMSO (1 mL) at 70 °C for 4 h.
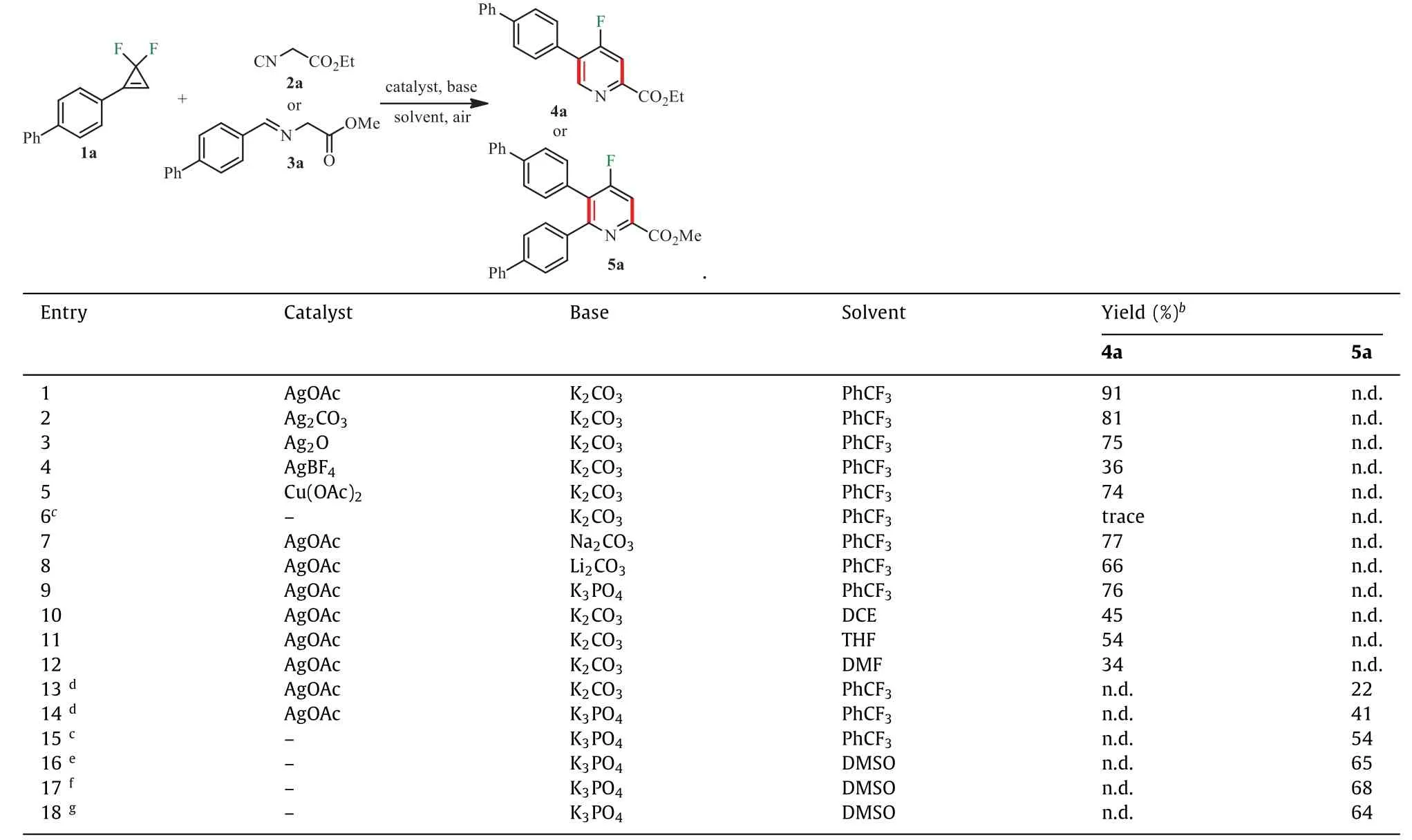
Table 1 Optimization of reaction conditionsa
With optimized conditions in hand,the reactions betweengem–difluorocyclopropenes 1 and isocyanides 2 were tested first.As shown in Scheme 2,thegem–difluorocyclopropene substrates bearing electron-donating substituents such as Me,Ph and electronwithdrawing substituents such as F,Cl,Br,CF3,NO2,CO2Me in thepara-ormeta-positions of the phenyl moiety worked smoothly under standard conditions,and gave the products 4a-4k in good yields.However,when theparaposition of the phenyl moiety in thegem–difluorocyclopropene substrate embedded strong electron-donating methoxy group,the yield of the product 4h was significantly reduced.Specifically,a 2-methyl-substituted substrate did not retard the process and gave product 4m in 87%yield.The substrates with aromatic heterocycles,for example,pyridine,quinolone and benzoxazole were also tolerant,producing the products 4p-4r in 68%−96% yields.Alkyl-substituted difluorocyclopropenes were also compatible with the reaction system.Methylene groups with nitrogen-centred linkages such as carbazole gave the product 4s in 81% yield,while oxygen-centred linkages such as naphthol afforded product 4t in 74% yield.The reaction still proceeded effectively with other esters such as methyl ester,tert–butyl ester,and benzyl ester,even amides in isonitriles (4u-4y),although the yields were slightly reduced.To further highlight the synthetic versatility of our method,several complex natural products and drugs,were subjected to the reaction conditions.Estetrol (1z) and L-(-)-borneol (1ab) derivatives gave the corresponding 4-fluoropyridines 4z and 4ab in 66% and 51% yield,respectively.Febuxostat 1aa and Adapalene 1ac also were converted to 4-fluoropyridine derivatives (4aa,57% and 4ac,45%).
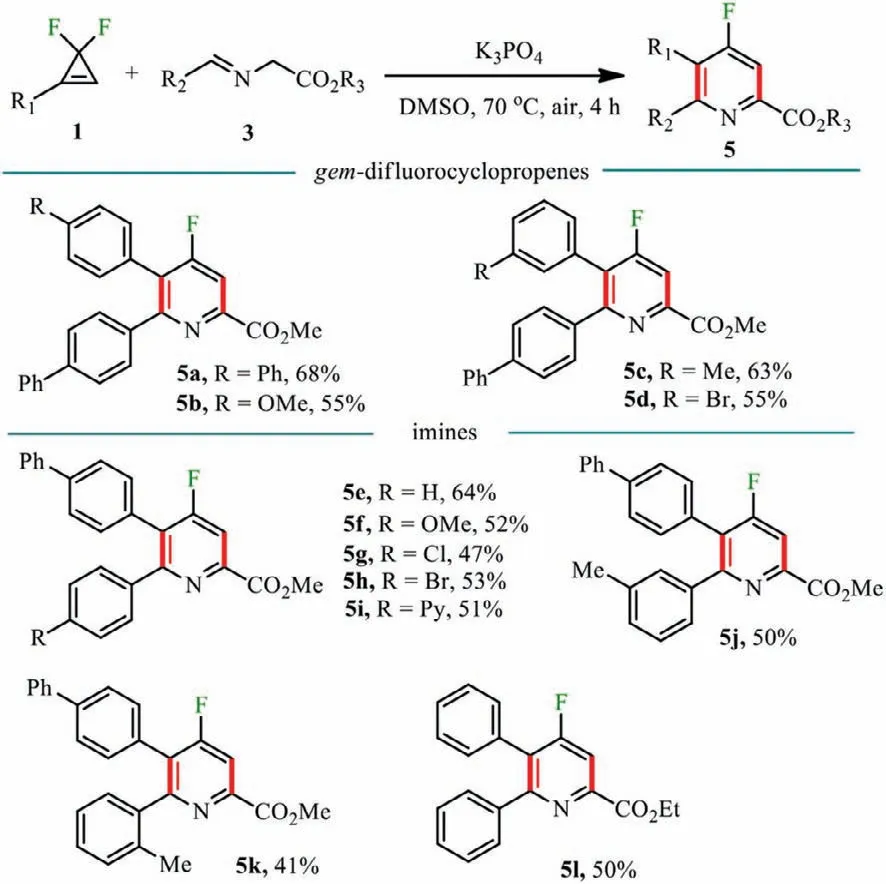
Scheme 3.Substrate scope for tetra-substituted 4-fluoropyridines.Reaction conditions: 1 (0.2 mmol),2 (1.5 equiv.),K3PO4 (1.5 equiv.),DMSO (1 mL) at 70 °C for 4 h in air.Isolated yields.
The substrate scope for tetra-substituted 4-fluoropyridines was then screened (Scheme 3).Thegem–difluorocyclopropene substrates with both electron-donating substituents such as Me,Ph,OMe and electron-withdrawing substituents such as Br in theparaormeta-positions of the phenyl moiety were utilized under standard conditions,and generated the products 5a-5d in moderate to good yields.Subsequent screening of the aldimine esters [60]revealed that electrical properties of the substituted groups in thepara-ormeta-positions of the benzene ring had little effect on the reaction (5f-5j).Other esters such as ethyl ester were also compatible with the reaction,producing the product 5l in 50% yield.Out of our expectation,aldimine esters with alkyl groups such as benzyl,n-pentyl,n–butyl,n-propyl,and even alkenyl and ester groups showed no reactivity in the protocol.
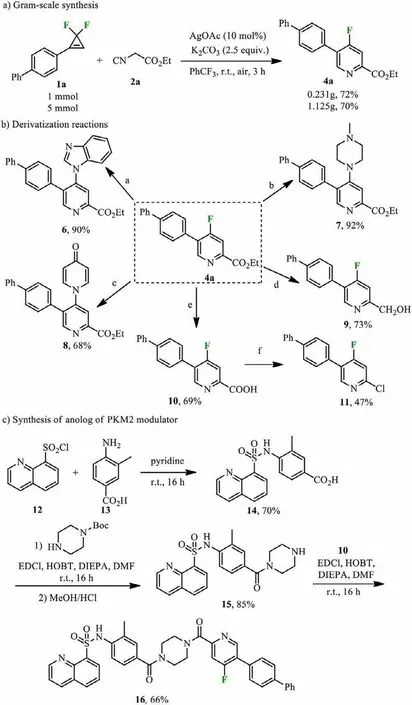
Scheme 4.Synthetic applications.Reaction conditions: (a) 4a (0.2 mmol),benzimidazole (0.4 mmol),MeCN (1 mL),80 °C,4 h,Ar;(b) 4a (0.2 mmol),1-methylpiperazine (0.4 mmol),MeCN (1 mL),r.t.,4 h,Ar;(c) 4a (0.2 mmol),4-hydroxypyridine (0.4 mmol),K2CO3 (0.4 mmol),NMP (1 mL),at 140 °C for 3 h;(d)4a (0.2 mmol),NaBH4 (0.6 mmol),ethanol (1 mL),0–25 °C,2 h;(e) 4a (0.2 mmol),KOH (0.4 mmol),methanol (1 mL),35 °C,2 h;(f) 4a (0.2 mmol),NaHCO3 (0.2 mmol),t-BuCl (0.3 mmol),CH2Cl2 (1 mL),60 °C.Isolated yield.
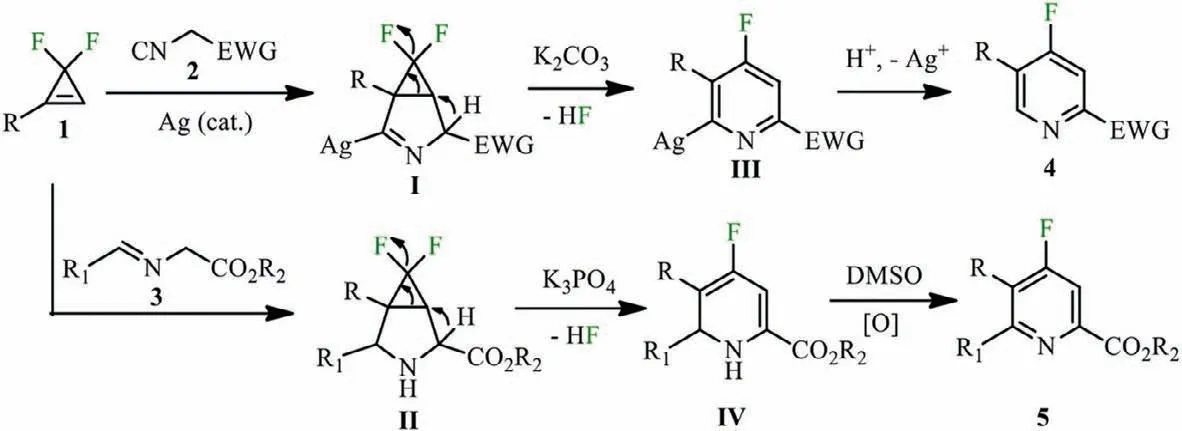
Scheme 5.Possible catalytic cycle.
To demonstrate the scalability of this methodology,the reaction of 1a with 2a was performed on 1.0 mmol and 5.0 mmol scales,affording 72% and 70% yields of the product 4a,respectively(Scheme 4a).The nucleophilic substitutions of 4-fluoropyridine 4a with benzimidazole,1-methylpiperazine and 4-hydroxypyridine released the corresponding products 6,7 and 8 in 90%,92% and 68%yield,respectively (Scheme 4b,paths a-c).NaBH4reduction of the carbonyl group in 4a afforded alcohol 9 in 73% yield (path d).Hydrolysis of the ester groups in 4a produced 10 in 69% yield (path e),which further conducted a chlorinated decarboxylation reaction to obtain the product 11 in 47% yield (path f).Next,total syntheses of the anolog of PKM2 modulator was investigated (Scheme 4c).The product 15 was formed after a two-step reaction sequence of the sulfamidation and condensation,which further condensed with 10 to finally obtain the anolog of PKM2 modulator 16.
On the basis of related literature reports [61,62]and our preliminary studies,a plausible mechanism is proposed in Scheme 5.A [3+2]cycloaddition reaction ofgem–difluorocyclopropenes 1 with isocyanides 2 or imines 3 to produce intermediates I and II.The synergy of the electron-pushing effect ofα-carbon of the ester group and the electron-pulling effect ofgem–difluoride stimulates the ring-opening defluorination reaction of intermediates I and II,releasing intermediates III and IV.Finally,the intermediate IV was oxidized by DMSO to generate tetrasubstituted-4-fluoropyridines 5.
In summary,we report a novel [3+2]/[2+1]cycloaddition reaction ofgem–difluorocyclopropenes with isocyanides or imines,providing a modular,concise and efficient approach for accessing tri-and tetra-substituted 4-fluoropyridines in moderate to good yields with high regioselectivity.This reaction starts from simple and readily available substrates,proceeding under mild and air conditions,and its substrate scope is general.In the synthesis of tetra-substituted 4-fluoropyridines,the reaction proceeds without transition metals,and DMSO is utilized as both a solvent and an oxidant.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.21861007,21702034),Natural Science Foundation of Guangxi Province (No.2021GXNSFAA075024),“BAGUI Scholar” Program of Guangxi Province of China,High-Level Innovation Team and Distinguished Scholar Program in Guangxi Colleges and Universities,the Jiangxi Province Science and Technology Project (No.20212BAB213024).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.02.031.
 Chinese Chemical Letters2022年6期
Chinese Chemical Letters2022年6期
- Chinese Chemical Letters的其它文章
- Photochemical defluorinative functionalization of α-polyfluorinated carbonyls via spin-center shift
- Methods of screening,monitoring and management of cardiac toxicity induced by chemotherapeutics
- Light-guided tumor diagnosis and therapeutics: From nanoclusters to polyoxometalates
- Nanofluidics for sub-single cellular studies:Nascent progress,critical technologies,and future perspectives
- Effective purification of oily wastewater using lignocellulosic biomass:A review
- Recent advances in microchip-based methods for the detection of pathogenic bacteria
